Phenolic profile and biological potential of Endopleura uchi extracts
2015-10-31LuSilvaRafaelaTeixeira
Luís R. Silva, Rafaela Teixeira
1CICS-UBI - Centro de Investigação em Ciências da Saúde, Universidade da Beira Interior, Av. Infante D. Henrique, 6201-506, Covilhã, Portugal2Instituto Politécnico de Castelo Branco, Escola Superior de Saúde Dr Lopes Dias, Avenida do Empresário, Campus da Talagueira, 6000-767, Castelo Branco, Portugal
3LEPABE, Departamento de Engenharia Química, Faculdade de Engenharia, Universidade do Porto, Rua Dr. Roberto Frias, s/n, 4200-465 Porto,Portugal
4REQUIMTE/Laboratório de Farmacognosia, Departamento de Química, Faculdade de Farmácia, Universidade do Porto, Rua de Jorge Viterbo Ferreira, 228, 4050-313 Porto, Portugal
Phenolic profile and biological potential of Endopleura uchi extracts
Luís R. Silva1,2,3*, Rafaela Teixeira4
1CICS-UBI - Centro de Investigação em Ciências da Saúde, Universidade da Beira Interior, Av. Infante D. Henrique, 6201-506, Covilhã, Portugal
2Instituto Politécnico de Castelo Branco, Escola Superior de Saúde Dr Lopes Dias, Avenida do Empresário, Campus da Talagueira, 6000-767, Castelo Branco, Portugal
3LEPABE, Departamento de Engenharia Química, Faculdade de Engenharia, Universidade do Porto, Rua Dr. Roberto Frias, s/n, 4200-465 Porto,Portugal
4REQUIMTE/Laboratório de Farmacognosia, Departamento de Química, Faculdade de Farmácia, Universidade do Porto, Rua de Jorge Viterbo Ferreira, 228, 4050-313 Porto, Portugal
ARTICLE INFO
Article history:
Received in revised form 20 September 2015
Accepted 15 October 2015
Available online 20 November 2015
Endopleura uchi
Bark
Phenolic compounds
Anti-diabetic activity
Biological potential
Objective: To improve the knowledge on the metabolite composition of Endopleura uchi bark, concerning phenolic compounds, and to evaluate some of its biological capacities for further possible exploitation in food and pharmaceutical industries associated to their healthpromoting qualities. Methods: Two different extracts (infusion and hydroethanolic) were studied concerning phenolic composition and biological potential. Results: Five compounds were determined by HPLC-DAD, being bergenin the major one. In general way, infusion presents a greater richness in these metabolites. The antioxidant, acetylcholinesterase,butyrylcholinesterase, α-glucosidase and antibacterial activity were checked by in vitro assays. A dose-dependent response was noticed against DPPH·, superoxide and nitric oxide radicals,acetylcholinesterase, butyrylcholinesterase and against α-glucosidase inhibitory assay. Antibacterial capacity of both extracts was investigated against Gram-positive and Gramnegative bacteria, being more effective against the first one. The concentrations of infusion extract tested here revealed that is non-toxic for intestinal (Caco-2) cells line. Conclusions:The results suggest that the extracts of Endopleura uchi may be interesting for incorporating in pharmaceutical preparations for human health, since it can suppress hyperglycaemia and inhibit cholinesteases, and or as food additive due to its antiradical and antibacterial activities.
Document heading doi: 10.1016/j.apjtm.2015.10.013
1. Introduction
Plants have been used for medicinal purposes for many centuries. Today, herbal medicines are being employed worldwide in a variety of health care settings and as home remedies. In some developing countries, communities rely heavily on traditional health practitioners and medicinal plants to meet their primary health care needs. In many industrialized countries herbal medicines are gaining popularity as alternative and complementary therapies. TheWorld Health Organization estimates that 65%-80% of the people in developing countries use traditional medicine for primary health care and 85% of that involves the use of plant extracts[1].
Among medicinal plants, some endemic species can be used for the production of raw materials or preparations containing phytochemicals with significant biological activities and consequent health benefits. Plants from tropical countries constitute a genuine biological source of natural metabolites, which are biosynthesized in response to abiotic and biotic stress for surviving in such extreme conditions, like excessive variations in temperature, desiccation,ultraviolet degradation, water stress and exposure to herbivores[2].
Endopleura uchi (E. uchi)(Huber) Cuatrec. (Humiriaceae) is a native tree of the Brazilian Amazon rainforest and is widely spread in the Amazon basin[1]. Popularly known as 'uxi-amarelo', 'cumatê','axuá', 'pururu', 'uxi-liso', 'uxi-ordinário' or 'uchi-pucu'. The erecttrees have pale gray bark and reach between 23 and 30 meters in height, with a stem diameter over one meter[3,4].
There are few studies of E. uchi and are concentrated in the fruits that are appreciated in the region[5,6]. The pulp of the fruit has a high content of fat, predominantly oleic acid[7] and carotenoids, mostly trans-β-carotene[5]. However, the bark is widely commercialized at fairs, markets and even drugstores, being prescribed in the form of tea, for arthritis, cholesterol, diabetes, diarrhea treatments, as an antiinflammatory, also against tumors and uterine infections[1,6,8,9].
The phytochemical screening of the bark revealed the predominance of tannins, coumarins and saponins as the main classes of secondary metabolites[10]. In a previous study, Luna[11] isolated two pentacyclic triterpenoids of the oleanane series (maslinic acid and methyl maslinate) and two coumarins (bergenin and dimethyl bergenin)from crude ethanolic extracts of the bark. Nunomura and coauthors[9] also isolated bergenin from E. uchi bark. Previously,bergenin has been isolated from E. uchi fruits[5], and another medicinal plants like Bergenial crassifolia, Mallotus philippinensis,Corylopsis spicata, Caesalpinia digyna, Mallotus japonicas, Humiria balsamifera and Sacoglottis gabonensis[9,12].
Bergenin alias Cuscutin is trihydroxybenzoic acid glycoside. It is a C-glucoside of 4-O-methyl gallic acid is a colouress crystalline polyphenol, it is hydrolysable tannin and an isocoumarin derivative with three hydroxyl (OH) groups and two phenolic OH groups[12]. Bergenin has been reported to have several biological activities, such as anti-inflammatory, antioxidant, hepatoprotective, neuroprotective,antidiabetic/antiobesity, antiulcer, antiarrhythmic, anti-HIV,antibacterial, antifungal, immunomodulatory and burn wound healing effects[1,5,6,12-14].
Thus, the aim of the present work was to improve the knowledge on the metabolite composition of E. uchi bark, concerning phenolic compounds, and to evaluate some of its biological capacities for further possible exploitation in food and pharmaceutical industries associated to their health-promoting qualities. For these purposes two different extracts (infusion and hydroethanolic) from E. uchi bark were tested for their antioxidant, cholinesteases, α-gucosidase and antibacterial capacities. The extract with greater biological potential was used to evaluate the cytotoxic against Caco-2 cell line. As far as we know, it is the first time this species is evaluated concerning these properties.
2. Materials and methods
2.1. Standards and reagents
All chemicals used were of analytical grade. The standard compounds were purchased from various suppliers: bergenin was from Selleckchem (Boston, USA).1,1-diphenyl-2-picrylhydrazyl(DPPH·), sodium pyruvate, thiazolyl blue tetrazolium bromide(MTT), β-nicotinamide adenine dinucleotide reduced form(NADH), phenazine methosulfate, nitrotetrazolium blue chloride, 5,5'-dithio-bis(2-nitrobenzoic acid), sulphanilamide,acetylcholinesterase (AChE) from electric eel (type Ⅵ-s, lyophilized powder), acetylthiocholine iodide, butyrylcholinesterase (BuChE)from equine serum (lyophilized powder), S-butyrylthiocholine chloride, α-glucosidase (type I from baker's yeast), 4-nitrophenyl α -D-glucopyranoside (PNP-G), sodium nitroprusside dehydrate,dimethyl sulfoxide (DMSO), dichloromethane, methanol (MeOH),acetonitrile and gentamicin were purchased from Sigma-Aldrich (St. Louis, MO, USA). N-(1-Naphthyl)ethylenediamine dihydrochloride,ethanol and potassium dihydrogen phosphate were obtained from Merck (Darmstadt, Germany). Hydrochloric and ortho-phosphoric acid were purchased from Panreac (Barcelona, Spain). Mueller Hinton Broth (MHB) and Mueller Hinton Agar (MHA) media were purchased from Liofilchem (Teramo, Italy). Dulbecco's modified Eagle medium (DMEM), phosphate-buffered saline, hepes buffered saline, nonessential amino acids, fetal bovine serum, antibiotic (10 000 U/mL penicillin, 10 000 μg/mL streptomycin), fungizone (250 μg/mL amphotericin B), human transferrin (4 mg/mL) and trypsin-EDTA were from Invitrogen (Gibco Laboratories; Lenexa, KS). Water was deionised using a Milli-Q water purification system(Millipore, Bedford, MA, USA).
2.2. Plant samples
Dried barks of E. uchi, corresponding to a mixture of different individuals, were from a medicinal plants distributor (Morais e Costa & Cª. Lda, Portugal). Voucher specimen was deposited at Laboratório de Farmacognosia, Faculdade de Farmácia, Universidade do Porto(ER-MC-022013).
2.3. Extracts' preparation
E. uchi barks were powdered and sieved in order to obtain a mean particle size lower than 910 μm and used for the preparation of two different extracts. The conditions used for extraction were adapted from those proposed by Teixeira and Silva[15].
Infusion: 1 g of dried material was boiled with 100 mL of water for three minutes, as indicated in the instructions provided in the packing. Thus, it mimics the way how it is usually prepared for human consumption. The resulting extract was filtered through a Büchner funnel, frozen and lyophilized. The extract was kept in a desiccator, in the dark, until analysis. The yield of E. uchi infusion extract was (8.10 ± 0.09) g from starting dry material.
Ethanolic/water: 1 g of dried material were extracted with ethanol: water (1:1) mixture, as follows: 30 min of sonication, 1 h of stirring (200 rpm) maceration at room temperature, plus 30 min of sonication. The obtained extract was filtered through a 0.45 μm polytetrafluoroethylene membrane (Millipore, Bedford, MA),evaporated under reduced pressure and kept at -20 ℃ until further analysis. The yield of E. uchi hydroethanolic extract was (7.00 ± 0.74) g from starting dry material.
Extractions were carried out in triplicate.
2.4. Phenolic compounds
Infusion and hydroethanolic extracts were redissolved in methanol(30 mg) and analysed in a HPLC/DAD (Gilson) as described before[16] for phenolic compounds determination. The obtained extracts were filtered through a 0.45 μm polytetrafluoroethylene membrane.
The phenolic compounds were analysed on an analytical HPLC unit (Gilson), using a previously described procedure[16]. Twenty microlitres of each aqueous extract were analysed on an analytical HPLC unit (Gilson), using a Spherisorb ODS2 (25.0 cm×0.46 cm; 5 μm, particle size; Waters, Milford, MA) column. The solvent system used was a gradient of water: formic acid (19:1) (A) and methanol (B), starting with 5% methanol and installing a gradient to obtain 15% B at 3 min, 25% B at 13 min, 30% B at 25 min, 35% B at 35 min, 45% B at 39 min, 45% B at 42 min, 50% B at 44 min,55% B at 47 min, 70% B at 50 min, 75% B at 56 min and 80% B at 60 min, at a solvent flow rate of 0.9 mL/min. Detection was achieved with a Gilson diode array detector. Spectral data from peaks were accumulated in the range 200—400 nm, and chromatograms were recorded at 280, 320 and 350 nm. The data were processed on Unipoint System software (Gilson Medical Electronics, Villiers-le-Bel, France). Phenolic compounds quantification was achieved by measuring the absorbance recorded in the chromatograms relative to external standards. Because standards of bergenin derivatives were not available, all compounds were quantified as bergenin. Bergenin and bergenin derivatives were determined at 280 nm. This procedure was performed in triplicate.
2.5. Antioxidant activity
The infusion and hydroalcoholic extracts were used for screening of the antioxidant activity. Spectrophotometric microassays were performed in a Multiskan Ascent plate reader (Thermo, Electron Corporation).
DPPH·assay: the disappearance of DPPH·was monitored spectrophotometrically at 515 nm, following a described procedure[17]. For each extract, a dilution series (five different concentrations) was prepared in a 96-well plate. Three experiments were performed in triplicate.
Nitric oxide assay: the scavenging activity was determined as before[17]. The chromophore formed with Griess reagent was read at 562 nm. For each extract, five different concentrations were tested. Three experiments were performed in triplicate.
Superoxide radical assay: the effect of the lyophilized extracts on superoxide radical-induced reduction of nitrotetrazolium blue chloride was monitored at 562 nm. Superoxide radicals were generated by the NADH/phenazine methosulfate system,as previously reported[17]. All components were dissolved in phosphate buffer (19 mM, pH 7.4). For each extract, five different concentrations were tested. Three experiments were performed in triplicate.
2.6. AChE and BuChE inhibitory activity
The inhibition of both AChE and BuChE activity was determined based on Ellman's method, as previously reported[17]. The absorbance was measured at 405 nm and the rates of reactions were calculated by Ascent Software version 2.6 (Thermo; electron corporation). Three experiments were performed in triplicate.
2.7. α-Glucosidase inhibitory activity
α-Glucosidase inhibitory activity was assessed according to the methods described by Silva et al[17]. Briefly, each well contained 100 μL of 2 mM PNP-G in 100 mM potassium phosphate buffer (pH 7.0)and 20 μL of the extract in buffer. The reaction was initiated by the addition of 100 μL of the enzyme solution (56.6 mU/mL). The plates were incubated at 37 ℃ for 10 min. The absorbance of 4-nitrophenol released from PNP-G at 400 nm was measured. The increase in absorbance was compared with that of the control (buffer instead of sample solution) to calculate the inhibitory activity.
2.8. Antibacterial activity
The study included nine bacteria species Staphylococcus aureus(S. aureus) (ATCC 20231), Staphylococcus epidermidis (ATCC 20044), Salmonella typhimurium (ATCC 43971), Proteus mirabilis(ATCC4479), Escherichia coli (E. coli) (ATCC 30083), Pseudomonas aeruginosa (P. aeruginosa) (ATCC 50071), Bacillus cereus (B. cereus)(ATCC 31), Enterococcus faecalis(ATCC20477) and Micrococcus luteus (M. luteus) (ATCC 20030), these species were selected due their great importance in foods and clinical[18,19]. Cultures were obtained from the Department of Microbiology, Faculty of Pharmacy, Porto University (Portugal). S. aureus (ATCC 25923) was used for quality control in antibacterial assays. All microorganisms were stored in broth medium with 20% glycerol at 70 ℃ and sub-cultured in MHA before each test, to ensure optimal growth conditions and purity. Minimum inhibitory concentration (MIC) was determined by employing broth microdilution methods based on the Clinical and Laboratory Standards Institute guidelines, reference documents M07-A8 and M100-S19, with minor modifications[18,20]. Briefly, the suspensions of bacteria cultures were prepared in ampoules containing 2 mL of NaCl 0.85% suspension medium(api®, Biomérieux, Marcy l'E'toile, France). After adjusting the turbidity to 0.5 McFarland, suspensions were diluted in MHB till the final bacterial density of 1.5×106CFU/mL. The MIC of extracts was determined by two-fold serial dilution method, in 96-well plates. The initial concentration was 8.0 mg/mL of dry matter, for each tested species. Briefly, 50 μL of the bacterial suspension was added in each well, which contained 50 μL of extract dilutions in MHB medium. The maximum DMSO concentration did not exceed 1.25%(v/v). The plates were incubated at 37 ℃ in a humidified atmosphere containing 5% CO2, without agitation for 18—24 h for both Gram positive and Gram negative bacteria. The MIC was determined as thelowest concentration of dried extracts inhibiting the visual growth of the test culture on the microplate. Gentamicin MIC for S. aureus(ATCC 25923) was determined as quality control, and the result was within the recommended limits[20]. Sterility and positive controls in MHB medium alone and with 1.25% of DMSO (v/v) were included. Positive control wells contained microorganisms without antibiotics. The experiments were performed in duplicate and repeated independently three times, yielding essentially the same results.
The determination of the minimum lethal concentration (MLC) of extracts was also accessed. The MLC was determined after 18—24 h of incubation for both Gram positive and Gram negative bacteria, by removing 20 μL of the contents from all wells showing no visible growth to MHA plates. The plates were incubated at 37 ℃. The MLC was defined as the lowest concentration showing 100% growth inhibition.
2.9. Cell culture conditions and treatments
Human colorectal adenocarcinoma cell line (Caco-2) from the American Type Culture Collection (LGC Standards S.L.U., Spain)was routinely cultured using DMEM supplemented with 10% fetal bovine serum, 1% nonessential amino acids, 1% antibiotic,1% fungizone and 6 μg/mL transferrin. Cells were cultured at 37℃ in a 5% CO2incubator as previously described[21]. Cells were washed with hepes buffered saline, trypsinized and sub-cultured in 48-well plates at a density of 25 000 cells/cm2. All the assays were performed after confluence. The infusion extract (16-1 000 μg/mL)was dissolved in medium containing 0.5% (v/v) DMSO. The final concentration of DMSO did not affect cellular viability. To determine the effect of the compounds/extracts on cells, viability was assessed 24 h after exposure.
MTT assay was performed by MTT reduction to formazan by viable cells was evaluated according to a described procedure[22]. After rejecting the cell culture supernatant, the cells were incubated with 1 ml of MTT solution (0.5 mg/mL in supplemented DMEM) for 30 min, at 37 ℃. After this period, the supernatant was eliminated and 1 mL of DMSO was added to each well in order to solubilise the purple crystals of formazan that was formed. The absorbance of the different solutions was then measured using a Multiskan Ascent plate reader (Thermo, Electron Corporation) working at 570 nm. The MTT assay was performed, in total of five experiments per extract. The lactate dehydrogenase assay was also performed to evaluate whether the tested extracts were cytotoxic to cells in the experimented concentrations. LDH converts pyruvate to lactate using NADH as a cofactor. The procedure was adapted from[22], and is based on the determination of NADH disappearance. This was monitored with a Multiskan Ascent plate reader (Thermo, Electron Corporation), in kinetic function, at 340 nm. A decrease in the absorbance is linked directly to the quantity of LDH released by the cells in the culture environment. The supernatant of every single well of the 48-well plates, in which the cells were treated with the extracts and the LDH assay was performed, in total of five experiments per extract.
2.10. Statistical analysis
All data were recorded as mean ± standard deviation of triplicate determinations. Mean values were compared using two way ANOVA and Bonferroni test, as post hoc test, were used to determine differences with statistical significance. Differences were considered significant for P<0.05. Statistical analysis was carried out using:Graphpad Prism 5 Software (San Diego, CA, USA).
3. Results
3.1. Phenolic compounds
The HPLC analysis of E. uchi bark extracts allowed the identification of five phenolic compounds, which comprised the bergenin (5) and four bergenin derivatives (1-4) (Figure 1 and Table 1). Among the identified compounds, bergenin (5) was previously reported by[9]. Both extracts showed a similar profile, some differences in quantitative composition were observed (Table 1).

Table 1 Phenolic compounds profile of E. uchi extracts (mg/kg).
The total amounts obtained for both extracts were 179 289.0 and 225 644.2 mg/kg of dried extract for hydroethanolic and infusion,respectively. The most representative compound in each extract was bergenin (5) with 87.6 and 88.7% for hydroethanolic and infusion,respectively.
3.2. Antioxidant activity
The evaluation of hydroethanolic and infusion extracts of E. uchi bark as scavenger was performed against DPPH·, O2·-and·NO radicals (Figure 2 and Table 2). The antioxidant activity of E. uchi infusion extract was previously reported against DPPH·, however the authors did not determine the IC50[1], being the scavenging activity of E. uchi against nitric oxide and super-oxide radicals reported for the first time in this work.
The both tested extracts exhibited a dose-dependent effect against DPPH·(Figure 2A), without significant differences among them,infusion being the most active (IC50=27 μg/mL), followed by
hydroethanolic extract (IC50=33 μg/mL) (Table 2).
The analysed extracts were particularly active against superoxide radical, being hydroethanolic extract more active than infusion(IC50=12 μg/mL and IC50=33 μg/mL, respectively) (Figure 2B, Table 2). Infusion was slightly more active against nitric oxide (IC50=24 μg/mL) comparatively hydroethanolic extract (IC50=29 μg/mL)(Table 2).

Table 2 IC25and IC50(μg/mL) values found in the antioxidant activity assays for E. uchi extracts.
3.3. AChE and BuChE inhibitory activity
Under the assays conditions to find inhibitors of ChE (acetyl and butyryl) a concentration-dependent inhibitory effect on the two enzymes were observed (Figure 3), being the AChE and BuChE activities of E. uchi bark extract reported in this work for the first time. The hydroethanolic extract was the most active against the both enzymes, with IC25=200 μg/mL and IC25=309 μg/mL for AChE and BuChE, respectively (Figure 3 and Table 2).
3.4. α-Glucosidase inhibitory activity
In the present study, we tested for the first time the α -glucosidase inhibitory effect of the hydroethanolic and infusion extracts of E. uchi bark, both were able to inhibit the α-glucosidase in a dose-dependent manner, with an IC50=2.2 μg/mL and IC50=2.4 μg/mL for hydroethanolic and infusion extracts, respectively (Figure 4 and Table 2). On the other hand, these results obtained for both extracts are much lower compared to positive control acarbose(IC50=284 μg/mL), revealing a great potential to inhibit this enzyme.
3.5. Antibacterial activity
The antibacterial activity of E. uchi extracts has been investigated against several Gram-negative and Gram-positive bacterial strains and MIC and MLC values were determined. By the analysis of the obtained results, there was no considerable variation between the MIC and MLC found among the different studied bacteria and extracts (Table 3). The infusion showed good bacteriostatic and bactericidal activity against Gram-negative, on the other hand,Gram-positive bacteria are more sensitive to hydroethanolic extract(Table 3).
3.6. Effect of E. uchi extracts in Caco-2 cell viability
As referred above, E. uchi bark infusions are commonly used in traditional medicine to treatment the diabetes[1]. Although the use is spread throughout the world, as far as we know there effect on human intestinal cells has not been scientifically evaluated before. Therefore, we have used a human colorectal adenocarcinoma cell line (Caco-2), in which cells were exposed to E. uchi bark infusion extract (0.016-1.000 mg/mL) for 24 h. Cell viability was evaluated by the MTT reduction and LDH release assays (Figure 5).
4. Discussion
The results obtained for phenolic compounds confirm that bergenin is the main one in E. uchi tea bark[9], this compound have been reported to exhibit various biological activities such as antiulcer,antihepatotoxic, anti-HIV, antiarrhythmic, neuroprotective,antifungal, anti-inflammatory, immunomodulatory and burn wound healing effects[23].
An antioxidant may be defined as 'any substance that when present at relatively low concentrations, compared with those of the oxidisable substrate, significantly delays or inhibits oxidation of that substrate'[24]. Reactive oxygen species (ROS) such as superoxide anion), hydroxyl (·OH), peroxyl (ROO·), and alkoxyl radicals(RO·), hydrogen peroxide (H2O2), and singlet oxygenmay attack biological macromolecules, giving rise to protein, lipid,and DNA damage, cell aging, oxidative stress-originated diseases(eg., cardiovascular and neurodegenerative diseases), and cancer. Antioxidants scavenge or quench ROS and reactive nitrogen species(RNS) products of respiration, including free radicals[24].
DPPH·is a stable free radical, which provides a good indication of a sample's anti-radical potential. The activity observed against this radical may be due in large part to phenolic compounds, namely bergenin and bergenin derivatives, whose anti-radical proprieties are known[25,26]. Additive or synergistic interactions might occur between phenolic compounds and other non-determined active compounds. These results are similar to those obtained by our research group with Satureja parvifolia decoction extract harvestedin the same region and tested under the same conditions, with IC50around 30 μg/mL[2].
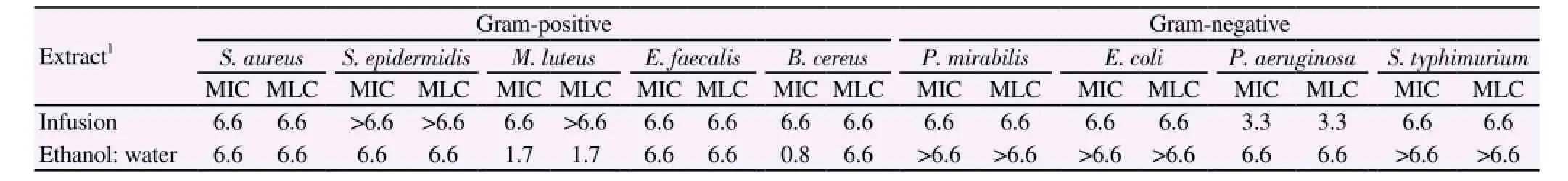
Table 3 MIC and MLC of E. uchi extracts against selected bacteria.
Subsequently, the extracts were also tested against other free radicals present in the human body, namely superoxide (O2·) and nitric oxide (·NO) radicals. Superoxide radical is one of the most effective free radicals, being implicated in cell damage as precursor of other important reactive oxygen species (ROS), such as hydroxyl radical and peroxy-nitrite, contributing to the pathological process of many diseases[27]. Hydroethanolic extract was more active than infusion (IC50=12 μg/mL and IC50=33 μg/mL, respectively). This activity may be associated with the presence of high level of phenolic compounds in both extracts, more specifically with the present of bergenin and bergenin derivatives[25,26,28]. However the presence of other non-determined bioactive compounds cannot be ignored, since the infusion showed the higher amounts in phenolic compounds and was less active.
Nitric oxide is a molecule with important biological functions,including blood pressure control, neural signal transduction and antimicrobial protection, which have an important role in the inflammation process[27]. Nevertheless, nitric oxide can be responsible for oxidative damage. Besides its own toxicity, this radical can further react with other species, instigating even more toxic radicals, such as peroxynitrite that results from its reaction with superoxide.
Concerning nitric oxide scavenging, infusion was slightly more active (IC50=24 μg/mL) comparatively hydroethanolic extract(IC50=29 μg/mL). The results obtained for all assays revealed that E. uchi extracts possess a strong antioxidant activity without great differences between the both analyzed extracts.
Alzheimer's disease (AD) is the most prevalent neurodegenerative disease without known cure, affects approximately 10% of the population older than 65 years of age. Clinical manifestations of AD are severe impairments in thought, learning, memory and language abilities[29].
Cholinesterases (ChE) are ubiquitous constituents of cholinergic pathways, they are responsible for the termination of the nerve impulse transmission at the cholinergic synapses by fast hydrolysis of acetylcholine (ACh)[30]. The inhibition of ChE is the major goal of cholinoactive drugs prescribed for AD, neurotoxins used as insecticides and nerve gases intended for chemical warfare. AChE-positive neurons project diffusely to the cortex, modulating cortical processing and responses to new and relevant stimuli. BuChE-positive neurons project specifically to the frontal cortex, and may have roles in attention, executive function, emotional memory and behavior[31]. Furthermore, BuChE activity progressively increases as the severity of dementia advances, while AChE declines, their inhibition may provide additional benefits[31].
The hydroethanolic extract was the most active against the both enzymes, with IC25=200 μg/mL and IC25=309 μg/mL for AChE and BuChE, respectively. Comparing the results obtained with literature data, the effect observed herein on both enzymes were interesting than that found with hydromethanolic extract of Spergularia rubra and Satureja parvifolia tested in the same concentrations with IC25=3 680 μg/mL and 1 360 μg/mL for AChE and IC25=4 290 μg/mL and 1 646 μg/mL for BuChE, respectively[2,32]. On the other hand, concerning AChE inhibition the extract revealed apparently to be more active than the positive control phytostigmine(IC50=1 770 μg/mL) tested before under the same conditions[33].
Additionally, in a previous work, Takahashi and co-authors[34]synthesized a series of bergenin derivatives and tested their effect on the viability in rat cortical neurons, some of them showed protection of neuronal death on the primary culture of rat cortical neurons,providing their neuroprotective effect.
The term diabetes mellitus describes a metabolic disorder of multiple etiologies characterized by chronic hyperglycaemia with disturbances of carbohydrate, fat and protein metabolism resulting from defects in insulin secretion, insulin action or both. The causes of type 2 diabetes are either a predominant insulin resistance with a relative insulin deficiency or a predominant insulin secretory defect with or without insulin resistance[35]. α-Glucosidase inhibitors(AGIs) are among the available glucose-lowering medications. The α-glucosidase enzyme is located in the brush border of the small intestine and is required for the breakdown of carbohydrates to absorbable monosaccharides. The AGI delay, but not prevent, the absorption of ingested carbohydrates, reducing the postprandial glucose and insulin peaks[36]. In recent years, several researchers have focused on the AGIs of medicinal plants[37].
The E. uchi bark extracts showed a considerable better inhibition than those obtained previously with other plants used in the treatment of the diabetes, namely Cecropia otusifolia (IC50=14 μg/mL), Malmea depresa (IC50=21 μg/mL), Acosmium panamense (IC50=109 μg/mL)[37] and Spergularia rubra (IC50=2 550 μg/mL)[32]. According to the results obtained herein, the inhibition of α-glucosidase may be one of the mechanisms associated with the use of this plant in the treatments of diabetes.
The results obtained may be attributed, at least partially, to the composition of the extracts concerning bergenin and bergenin derivatives. Kumar and co-authors[13] tested the type 2 antidiabetic activity of bergenin in diabetic rats, the results obtained allowed the authors to conclude that bergenin possesses significant antidiabetic,hypolipidemic and antioxidant activity in type 2 diabetic rats.
Concerning antibacterial activity, P. aeruginosa was the most sensitive species for infusion (MIC and MLC=3.3 mg/mL for both),this bacteria has been recognized as a frequent inhabitant of chronic non-healing wounds and is one of the most opportunistic bacteria isolated from wounds which cause high morbidity and mortality despite antimicrobial therapy[38].
Additionally, the hydroethanolic extract was more active against B. cereus (MIC=0.8 mg/mL and MLC=6.6 mg/mL) and M. luteus(MIC=1.7 mg/mL and MLC=1.7 mg/mL). B. cereus is ubiquitous organisms, present in virtually all environments, their spores and cells are common in soil and dust and can be readily isolated in soiland dust and can be readily isolated in small numbers from many foods, which include both raw and finished products such as milk and milk product. B. cereus may produce emetic toxin and diarrheal enterotoxins which cause two types of gastrointestinal diseases -emesis and diarrhea[39]. M. luteus has been implicated in a variety of infections, including meningitis, endocarditis, septic arthritis and central nervous system infections in immunocompromised hosts[40]. Silva and co-authors[6] evaluated the antibacterial activity of E. uchi aqueous extracts against several microorganisms, including E. coli,P. aeruginosa, E. faecallis and S. aureus, the authors reported that the extract had no effect; however the concentrations tested were much lower than in our study those reported. In another study, Politi and co-authors[1] reported a little inhibition haloes against five bacteria,being observed a little effect against S. aureus with 10% infusion extracts of E. uchi.
To check whether the observed antibacterial activity could be due to the presence of identified phenolic compounds, it is also possible that other bioactive compounds exists in the extracts, that interfere with the real antimicrobial potential, like tannins and organic acids[6,10,19].
The MTT assay evaluated the mitochondrial function, the mitochondrial dehydrogenase of viable cells reduce the yellow tetrazolium salt forms purple formazan crystals. Infusion extract was not shown to be toxic to Caco-2 cells accordingly to the MTT assay,revealing a significant increase in MTT reduction of cells treated with infusion in a dose-dependent manner, being obtained a MTT reduction higher than 118% at concentration of 1 mg/mL. As the experiments were carried out after cellular confluence, these results are hardly explained by an increase in cellular proliferation. It is possible that the cell stress caused by extract increased mitochondrial activity, and consequently MTT reduction[41].
LDH leakage is well known as a useful index for cell viabilities on the basis of loss of membrane integrity. Infusion extract has no effect on LDH leakage from Caco-2 cells.
Taking into account that the infusion of E. uchi extract showed no effect on human colorectal Caco-2 cell viability, and as described before also possess a great inhibitory effect against α-glucosidase enzyme in lower concentrations for the tested on cell viability, the results obtained herein proved that the E. uchi infusion may be used safely as antidiabetic.
The study reported herein describes, for the first time, some biological properties of E. uchi bark, as well as phenolics quantification. The results indicate that E. uchi bark extracts are a rich source of bergenin and bergenin derivatives showing α-glucosidase, antibacterial and cholinesterases (AChE and BuChE) inhibitory properties and radical scavenging capacity. This study adds value knowledge to the species and represents a basis for the quality control of this plant drug. Therefore, E. uchi bark may be considered as one plant part with great antidiabetic potential. However, further investigations are required to incite the use of its extracts in human health applications in food and pharmaceutical industries.
Conflict of interest statement
We declare that we have no conflict of interest.
Acknowledgments
Luís R. Silva acknowledges FCT the financial support for the Postdoc grant (SFRH/BPD/105263/2014).
[1] Politi FAS, Mello JCP, Migliato KF, Nepomuceno ALA, Moreira RRD,Pietro RCLR. Antimicrobial, cytotoxic and antioxidant activities and determination of the total tannin content of bark extracts Endopleura uchi. Int J Mol Sci 2012; 12: 2757-2768.
[2] Cabana R, Silva LR, Valentão P, Viturro CI, Andrade PB. Effect of different extraction methodologies on the recovery of bioactive metabolites from Satureja parvifolia (Phil.) Epling (Lamiaceae). Ind Crops Prod 2013; 48: 49-56.
[3] Cuatrecasas JA. A taxonomic revision of Humiriaceae, contributions from the United States National Herbarium. Smithsonian Institute: Washington;1961.
[4] Schultes R. De plantis toxicariis e mundo novo tropicale commentationes XXXVI. Justicia (Acanthaceae) as a source of an hallucinogenic snuff. Econ Bot 1990; 44: 61-70.
[5] Magalhães LAM, Lima MP, Marinho HA, Ferreira AG. Identificação de bergenina e carotenóides no fruto de uchi (Endopleura uchi,Humiriaceae). Acta Amazonica 2007; 37: 447-450.
[6] Silva SL, Oliveira VG, Yano T, Nunomura RCS. Antimicrobial activity of bergenin from Endopleura uchi (Huber) Cuatrec. Acta Amazonica 2009;39: 187-191.
[7] Marx F, Andrade EA, Zoghbi MGB, Maia JS. Studies of edible Amazonian plants. Part 5: Chemical characterisation of Amazonian Endopleura uchi fruits. Eur Food Res Technol 2002; 214: 331-334.
[8] Manoel Pio Corrêa LAP. Dicionário das plantas úteis do Brasil e das exóticas cultivadas. Rio de Janeiro: Imprensa nacional; 1984.
[9] Nunomura RCS, Oliveira VG, Da Silva SL, Nunomura SM. Characterization of bergenin in Endopleura uchi bark and its antiinflammatory activity. J Braz Chem Soc 2009; 20, 1060-1064.
[10] Politi FAZ. Estudos farmacognósticos e avaliação de atividades biológicas de extratos obtidos das cascas pulverizadas de Endopleura uchi (Huber)Cuatrec. (Humiriaceae). Araraquara: Universidade Estadual Paulista;2009.
[11] Luna JS. Estudo dos constituintes químicos de Endopleura uchi (Huber)Cuatrec. (Humiriaceae). Maceió: Universidade Federal de Alagoas; 2000.
[12] Patel DK, Patel K, Kumar R, Gadewar M, Tahilyani V. Pharmacological and analytical aspects of bergenin: a concise report. Asian Pac J Trop Dis 2012; 2: 163-167.
[13] Kumar R, Patel DK, Prasad SK, Laloo D, Krishnamurthy S, Hemalatha S. Type 2 antidiabetic activity of bergenin from the roots of Caesalpinia digyna Rottler. Fitoterapia 2012; 83: 395-401.
[14] Bento JF, Noleto GR, Petkowicz CLO. Isolation of an arabinogalactan from Endopleura uchi bark decoction and its effect on HeLa cells. Carbohydr Polym 2014; 101: 871-877.
[15] Teixeira R, Silva LR. Bioactive compounds and in vitro biological activity of Euphrasia rostkoviana Hayne extracts. Ind Crops Prod 2013; 50: 680-689.
[16] Silva LR, Pereira MJ, Azevedo J, Goncalves RF, Valentao P, de Pinho PG, et al. Glycine max (L.) Merr., Vigna radiata L. and Medicago sativa L. sprouts: A natural source of bioactive compounds. Food Res Int 2013; 50:167-175.
[17] Silva LR, Azevedo J, Pereira MJ, Carro L, Velazquez E, Peix A, et al. Inoculation of the nonlegume Capsicum annuum (L.) with Rhizobium strains. 1. Effect on bioactive compounds, antioxidant activity, and fruit ripeness. J Agric Food Chem 2014; 62: 557-564.
[18] Lopes G, Sousa C, Silva LR, Pinto E, Andrade PB, Bernardo J, et al. Can phlorotannins purified extracts constitute a novel pharmacological alternative for microbial infections with associated inflammatory conditions? PLoS ONE 2012; 7: e31145.
[19] Taveira M, Silva LR, Vale-Silva LA, Pinto E, Valentão P, Ferreres F,et al. Lycopersicon esculentum Seeds: An industrial byproduct as an antimicrobial agent. J Agric Food Chem 2010; 58: 9529-9536.
[20] CLSI. Methods for dilutionantimicrobial susceptibility tests for bacteria that grow aerobically, Approved Standard. 8rd ed. Wayne: PA; 2009.
[21] Silva R, Carmo H, Dinis-Oliveira R, Cordeiro-da-Silva A, Lima S,Carvalho F, et al. In vitro study of P-glycoprotein induction as an antidotal pathway to prevent cytotoxicity in Caco-2 cells. Arch Toxicol 2011; 85: 315-326.
[22] Silva LR, Valentao P, Faria J, Ferreres F, Sousa C, Gil-Izquierdo A, et al. Phytochemical investigations and biological potential screening with cellular and non-cellular models of globe amaranth (Gomphrena globosa L.) inflorescences. Food Chem 2012; 135: 756-763.
[23] Nazir N, Koul S, Qurishi MA, Najar MH, Zargar MI. Evaluation of antioxidant and antimicrobial activities of Bergenin and its derivatives obtained by chemoenzymatic synthesis. Eur J Med Chem 2011; 46: 2415-2420.
[24] Halliwell B, Gutteridge JMC. Free radicals in biology and medicine. Oxford: Oxford University, Press; 1989.
[25] Rana VS, Rawat MSM, Pant G, Nagatsu A. Chemical constituents and antioxidant activity of Mallotus roxburghianus Leaves. Chem Biodivers 2005; 2: 792-798.
[26] De Abreu HA, Lago IAS, Souza GP, Pilo-Veloso D, Duarte HA, Flávio A, et al. Antioxidant activity of (+)-bergenin: a phytoconstituent isolated from the bark of Sacoglottis uchi Huber (Humireaceae). Org Biomol Chem 2008; 6: 2713-2718.
[27] Pacher P, Beckman JS, Liaudet L. Nitric oxide and peroxynitrite in health and disease. Physiol Rev 2007; 87: 315-424.
[28] Rice-Evans C, Miller N, Paganga G. Antioxidant properties of phenolic compounds. Trends Plant Sci 1997; 2: 152-159.
[29] Choi DY, Lee YJ, Hong JT, Lee HJ. Antioxidant properties of natural polyphenols and their therapeutic potentials for Alzheimer's disease. Brain Res Bull 2012; 87: 144-153.
[30] Mesulam MM, Guillozet A, Shaw P, Levey A, Duysen EG, Lockridge O. Acetylcholinesterase knockouts establish central cholinergic pathways and can use butyrylcholinesterase to hydrolyze acetylcholine. Neurosc 2002; 110: 627-639.
[31] Lane RM, Potkin SG, Enz A. Targeting acetylcholinesterase and butyrylcholinesterase in dementia. Int J Neuropsychopharmacol 2006; 9:101-124.
[32] Vinholes J, Grosso C, Andrade PB, Gil-Izquierdo A, Valentão P, Pinho PG, et al. In vitro studies to assess the antidiabetic, anti-cholinesterase and antioxidant potential of Spergularia rubra. Food Chem 2011; 129:454-462.
[33] Pereira DM, Ferreres F, Oliveira JMA, Gaspar L, Faria J, Valentão P,et al. Pharmacological effects of Catharanthus roseus root alkaloids in acetylcholinesterase inhibition and cholinergic neurotransmission. Phytomedicine 2010; 17: 646-652.
[34] Takahashi H, Kosaka M, Watanabe Y, Nakade K, Fukuyama Y. Synthesis and neuroprotective activity of bergenin derivatives with antioxidant activity. Bioorg Med Chem 2003; 11: 1781-1788.
[35] WHO. Definition, diagnosis and classification of diabetes mellitus and its complications. Report of WHO consultation. Geneva: WHO; 1999.
[36] Ross SA, Gulve EA, Wang M. Chemistry and Biochemistry of Type 2 Diabetes. Chem Rev 2004; 104: 1255-1282.
[37] Andrade-Cetto A, Becerra-Jiménez J, Cárdenas-Vázquez R. Alfaglucosidase-inhibiting activity of some Mexican plants used in the treatment of type 2 diabetes. J Ethnopharmacol 2008; 116: 27-32.
[38] Lau GW, Hassett DJ, Britigan BE. Modulation of lung epithelial functions by Pseudomonas aeruginosa. Trends Microbiol 13: 389-397.
[39] Arslan S, Eyi A, Küçüksarı R. Toxigenic genes, spoilage potential, and antimicrobial resistance of Bacillus cereus group strains from ice cream. Anaerobe 2014; 25: 42-46.
[40] Sterry W, Paus R, Burgdorf W. Dermatology.5th ed. Stuttgard: Thieme Medical Publishers; 2006.
[41] Costa VM, Silva R, Tavares LC, Vitorino R, Amado F, Carvalho F, et al. Adrenaline and reactive oxygen species elicit proteome and energetic metabolism modifications in freshly isolated rat cardiomyocytes. Toxicology 2009; 260: 84-96.
15 August 2015
Luís R. Silva, CICS-UBI - Centro de Investigação em Ciências da Saúde, Universidade da Beira Interior, Av. Infante D. Henrique, 6201-506, Covilhã, Portugal.
Tel: + 351 220 428 654
Fax: +351 226 093 390
E-mail: lmsilva@ff.up.pt
It is supported by Post-doc grant (SFRH/BPD/105263/2014).
杂志排行
Asian Pacific Journal of Tropical Medicine的其它文章
- Demographic, socioeconomic and environmental changes affecting circulation of neglected tropical diseases in Egypt
- Roots extracts of Adenophora triphylla var. japonica improve obesity in 3T3-L1 adipocytes and high-fat diet-induced obese mice
- Anti TB drug resistance in Tanga, Tanzania: a cross sectional facility base prevalence among pulmonary TB patients
- In vitro inhibitory effects of plumbagin, the promising antimalarial candidate, on human cytochrome P450 enzymes
- Vibrio spp. from Macrobrachium amazonicum prawn farming are inhibited by Moringa oleifera extracts
- Inhibition effect of miR-577 on hepatocellular carcinoma cell growth via targeting β-catenin
