Effect of propofol on generation of inflammatory mediator of monocytes
2015-10-31YunNieYanXiLuLiHongLv
Yun Nie, Yan-Xi Lu, Li-Hong Lv
Operating Room, First People's Hospital of Ji'nan, Shandong 250011, China
Effect of propofol on generation of inflammatory mediator of monocytes
Yun Nie*, Yan-Xi Lu, Li-Hong Lv
Operating Room, First People's Hospital of Ji'nan, Shandong 250011, China
ARTICLE INFO
Article history:
in revised form 20 September 2015
Accepted 15 October 2015
Available online 20 November 2015
Propofol
LPS
IL-6
IL-8
TNF-α
Anti-inflammation
Objective: To evaluate the effect of propofol with different concentrations on the expression of inflammatory mediators of interleukin and tumor-necrosis factor-α (TNF-α) by stimulating the mouse primary monocytes and human monocytic cell line with lipopolysaccharide (LPS)and also discuss the effect of propofol on the secretion of inflammatory mediator and its possible molecular mechanism. Methods: The mononuclear cells of mouse spleen were separated and then purified to obtain the primary monocytes. The dose-effect relationship of production of pro-inflammatory cytokines by monocytes which were stimulated by LPS,namely the monocytes were stimulated by the dose of 0-500 ng/mL for 24 h. ELISA was employed to detect the concentration of IL-6, IL-8 and TNF-α. The effect of propofol on the secretion of above pro-inflammatory cytokines by the monocytes was observed. Cells were divided into the control group, the 0.1% DMSO group, the LPS group and the treatment group with LPS + different dose of propofol (propofol 1-100 μg/mL). ELISA was employed to detect the concentration of IL-6, IL-8 and TNF-α. The change in the expression of important signaling molecules in Toll-like receptor and NF-κB signaling pathway was detected after THP-1 cells were treated with propofol. Results: The concentration of TNF-α was (3 863±153)pg/mL after 12 h of stimulation by LPS and then its concentration was decreased gradually. But the concentration of IL-6 and IL-8 was relatively high after 24 h of stimulation by LPS,(5 627±330) pg/mL and (1 626±200) pg/mL, respectively. The propofol could inhibit the expression of IL-6, IL-8 and TNF-α caused by LPS. After the intervention treatment of 50 μg/ mL propofol, the concentration of IL-6, IL-8 and TNF-α was significantly decreased (P<0.01). Conclusions: The propofol can inhibit the expression of TLR-4 and NF-κB to inhibit the activation of p38 and the expression of pro-inflammatory cytokines.
Document heading doi:10.1016/j.apjtm.2015.10.008
1. Introduction
The propofol, namely 2,6-diisopropylphenol, has the similar structure with α-tocopherol and butylated hydroxyanisole as some kinds of rapid and short-acting anesthetic drug. It has been widely applied in the clinical anesthesia and ICU because of the rapid onset,short lasting duration, rapid awaking and fewer side effects[1,2]. In addition to the general anesthesia and sedation, the propofol can also be used to lower the blood pressure and inhibit the inflammatoryresponse in the clinical practice. According to previous researches,the anti-inflammation of propofol involves many aspects and many signaling pathways. The inhibition against the generation of inflammatory mediator and chemotactic factors is the key feature of its anti-inflammation[3,4]. The lipopolysaccharide (LPS) is the main chemical component of endotoxin and the promoter of inflammatory response. As the main effecter cell of LPS, the monocyte can release many pro-inflammatory cytokines after being stimulated by LPS,including IL-6, IL-8 and TNF-α. Therefore, the monocyte is a key link in the inflammatory response[5-7].
The objective of this study is to study the molecular mechanism of inhibitive effect of propofol on inflammatory mediator of monocytes. The mouse primary monocytes and human monocytic cell line were chosen as the research subjects. By exploring the stimulation dose and acting time of LPS, the inflammatoryenvironment at the cellular level was simulated. Cells were treated with propofol under the stimulation of LPS. ELISA was employed to detect the expression of pro-inflammatory cytokines of IL-6, IL-8 and TNF-α and then discuss the dynamic effect of propofol on the generation of inflammatory mediator of monocytes under the stimulation of LPS. LPS was bound with LPS binding protein on the monocytes to activate the downstream signaling pathway. Real-time PCR was employed to detect and analyze the expression of node molecules in the related signaling pathways, in order to discuss the possible anti-inflammation molecular mechanism of propofol.
2. Materials and methods
2.1. Materials and reagents
The human monocyte THP-1 was purchased from the Cell Bank of Type Culture Collection of Chinese Academy of Sciences. A total of 15 specific pathogen free Balb/c mice, male or female,with the weight of (15±5) g were purchased from Shanghai SLAC Laboratory Animal Co., Ltd. Mice were fed with the standard pellet diet in the standard animal cage, with 5 mice in each cage. During the experiment, mice could eat and drink freely. The feeding room had the good ventilation and natural lighting day and night. The room temperature was maintained at 18-25 ℃.
The cell strainer was purchased from BD Falcon (the USA)-REF352350; the mouse percoll from Dakewe Biotech Company;RPMI-1640 culture medium from Hyclone (the USA); the fetal bovine serum from GIBCO (the USA); the propofol from SIGMA(the USA)-D126608; the lipopolysaccharide from SIGMA (the USA)-L2630; IL-6 ELISA kit from Abcam (the USA): ab100713(mouse) / ab178013 (human); IL-8 ELISA kit from Abcam (the USA): ab46103 (mouse) / ab185986 (human); TNF-α ELISA kit from Abcam (the USA): ab100747 (mouse) / ab181421 (human);total RNA extraction kit from Ambion (the USA)-12183-555; reverse transcription kit from Applied Biosystems (the USA)-4366597;Real-time PCR fluorescent quantitative kit from Bio-Rad (the USA)-172-5264; Anti-p38/Anti-p38 (phospho T180) monoclonal antibody from Abcam (the USA)-ab7952/178867; horseradish peroxidase(HRP) labeled secondary antibody from Beijing Zhongshan Jinqiao Biotechnology.
The auto ELISA detector was Shanghai Utrao-SM600; RNA quantitative analyzer: Qubit Fluorometer; optical microscope:Olympus BX53; CO2incubator: Thermo Scientific Series 8000;Fluorescent quantitative PCR detection system: Bio-Rad-CFX96 Touch.
2.2. Methods
2.2.1. Separation of primary cells and cell culture and treatment
After 7 d of adaptive feeding, the mononuclear cells were separated from the spleen of Balb/c mice (lymphocytes and monocytes). Mice were executed by cervical vertebra luxation and then immersed into 75% ethanol for 1-2 min. The mouse spleen was separated under the sterile condition. It was washed with PBS twice and then the tissues were shredded. They were ground in the cell strainer until only the connective tissue left. The separation liquid with the spleen cells was transferred into the centrifuge tube. The 500 μL RPMI-1640 culture medium was added and 800 g of it was centrifuged for 30 min. The layer of mononuclear cells was sucked out gently. The 1640 culture medium that contained 10% fetal bovine serum was added and it was incubated at 37 ℃ and 5% CO2. After 12 h of incubation, the suspended lymphocytes were gently removed to obtain the adherent monocytes. The nonspecific esterase staining (monocytes were positive) was adopted to prove its purity.
THP-1 cells were maintained in the liquid nitrogen. After the cell thawing, The RPMI-1640 culture medium that contained 10% fetal bovine serum was added and it was incubated at 37 ℃ and 5% CO2.
2.2.2. ELISA detection and analysis
The cell culture supernatant was collected. The standard well,sample well and blank well were set respectively. The standard substance with the different concentrations was added in the standard 7-well one time. Then the serum sample was added. The ELISA plate was covered by the film and it was incubated at 37 ℃for 2 h. A total of 100 μL biotinylated primary antibody was added in each well and it was incubated at 37 ℃ for 1 h. The liquid in the well was removed. Each well was washed with 350 μL cleaning solution. It was immersed for 1-2 min. The plate was washed three times. A total of 100 μL HRP-labeled secondary antibody was added in each well. The ELISA plate was covered by the film and it was incubated at 37 ℃ for 30 min. A total of 90 μL TMB substrate was added in each well. The ELISA plate was covered by the film. It was placed in a dark place to be colored at 37 ℃ for 15-25 min. When the first 3-4 of the standard wells appeared to be the obvious gradient blue, the reaction was stopped and then 50 μL 2M H2SO4was added. Afterwards, the ELISA was used to measure OD value of each well at 492 nm. X axis referred to OD value and Y axis to the log of concentration. The standard curve was drawn and the log of corresponding concentration was obtained from the standard curve according to OD value of the sample. The concentration value was calculated according to the log of concentration. Finally, the actual concentration of the sample would be the product of concentration value and dilution factor.
2.2.3. Real-time PCR
The collected cells were washed with PBS (RNase free). The total RNA extraction kit was used to extract RNA. Qubit Fluorometer system was to detect the concentration and purity of RNA according to the instruction manual of reverse transcription kit. After the reverse transcription of cDNA to total RNA, Real-time PCR was employed to detect the expression of related genes. The mRNA sequence of related gene was queried in NCBI database to design Real-time PCR primer. All primers were synthesized by Shanghai Generay Biotech Co., Ltd., with the specific sequences shown inTable 1. The double Ct method was employed to calculate the relative expression of target gene: the mean of three parallel repeated tests was treated as the Ct value of each sample, namely △Ct =Ct(target gene)-Ct(reference), △△Ct= △Ct(sample) -△ Ct (control). Therefore, the relative expression of target gene =2-△△Ctand the relative expression for the control group was 20=1. The PCR reaction system was shown in Table 2, where the concentration of primers was decided by the different conditions of gene amplification.

Table 2 PCR reaction system.
2.2.4. Western blot
The collected cells were washed with PBS twice. After being digested with pancreatin, the supernatant was removed. The precipitated cells were lysed with RIPA lysis buffer (including the protease inhibitor cocktail) for blowing and mixing. Cells were lysed using the ultrasound. The lysis mixture was centrifuged at 4 ℃ and 1 3000 r/min for 20 min. The supernatant was transferred to the new centrifuge tube. Protein Assay kit was employed to detect the protein concentration.
SDS-PAGE electrophoresis was performed on 15-20 μg total protein samples. The gel was soaked in the transfer buffer for 10 min of equilibrium. It was installed with the transfer 'sandwich', 100 V and 45-60 min. After the transfer, PVDF film was washed with TBS for 10-15 min. The film was placed in TBS/T blocking buffer containing 5% (w/v) skimmed milk powder and shaken at the room temperature for 1 h. Then the primary antibody with the appropriate degree of dilution was added (diluted with TBST containing 1% (w/ v) skimmed milk powder). It was incubated at the room temperature for 2 h and then the film was washed with TBST for 3 times and 5-10 min each time. The film was incubated with the secondary antibody (1:10 000, HRP-labeled) that was diluted with TBST containing 0.05% (w/v) skimmed milk powder. It was incubated at the room temperature for 1h and then the film was washed with TBST for 3 times and 5-10 min each time. It was exposed and then photographed to save the experimental results. Quantity one v4.62 was used to measure the gray value of molecular band (trace tracking method). The optical density curve was drawn according to the optical density of different bands. The ratio of target protein and reference was chosen as the basis of relative quantification. The statistical analysis was then performed.
2.3. Statistical analysis
The experimental data were treated using SPSS 11.5. Results were expressed by mean±SD. The t-test was performed for the comparison between two groups, while P<0.05 indicated the statistical difference.
3. Results
3.1. Separation of monocytes
The principle for the separation of mononuclear cells is that the specific gravity of Ficoll solution was smaller than that of granulocyte and erythrocyte, but larger than that of mononuclear cell. After the separation, it would be the plasma, platelet,mononuclear cell, Ficoll solution, granulocyte and erythrocyte from top to bottom, as shown in Figure 1. The layer of mononuclear cells was sucked and RPMI-1640 culture medium was used for the resuspension and culture. The separated mononuclear cells mainly included the lymphocytes and monocytes, where the lymphocytes belonged to the suspension cells. According to such characteristic, 2 h after the culture of mononuclear cells, the suspended lymphocytes were removed and the adherent monocytes were remained. The nonspecific esterase was stained (monocytes were positive). After the staining, the gray flocculent precipitate could be found in the cytoplasm. According to the observation under the microscope, the positive cells occupied over 90%, which fully proved that the purity of separated monocytes was relatively high.
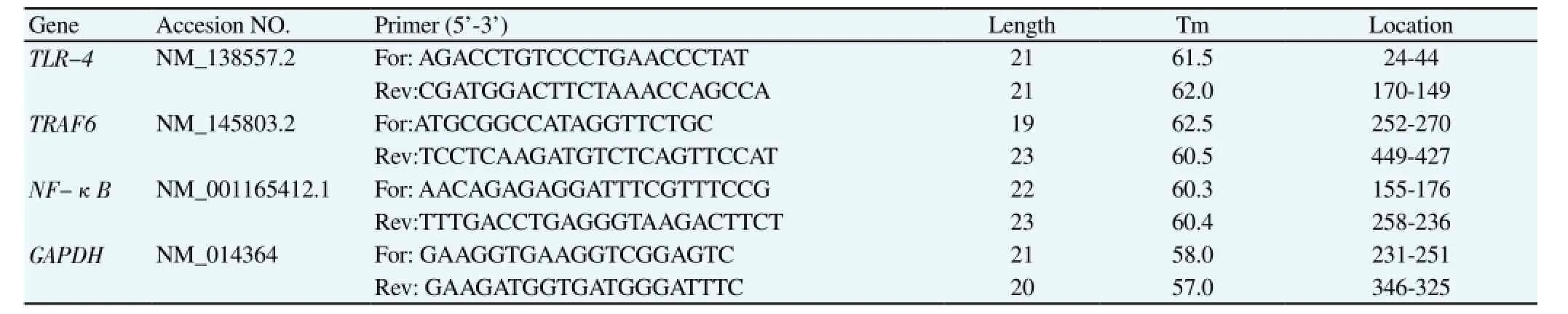
Table 1 Specific primers of Real-time PCR.
3.2. Change in expression of pro-inflammatory cytokines in monocytes under stimulation of LPS
To study the dose-effect relationship for the expression of proinflammatory cytokines in monocytes under the stimulation of LPS,the LPS with the gradient concentration (0, 1, 10 and 50 ng/mL) was added in the separated primary monocytes culture medium. A total of 24 h after the culture, ELISA was employed to detect the content of IL-6, IL-8 and TNF-α in the supernatant of culture medium. It could be seen that (Figure 2A), the content of IL-6, IL-8 and TNF-α in the supernatant in the control group (0 ng/mL LPS) was quite low and such content showed the dose-dependent relationship with the concentration of LPS. After the stimulation of 10-50 ng/mL LPS,the change in the content of IL-6, IL-8 and TNF-α was relatively sensitive. Twenty-four hours after the stimulation of 50 ng/mL LPS,the content of IL-6, IL-8 and TNF-α in the culture medium was(6 027.0±330.1) pg/mL, (1 436±200) pg/mL and (2 776±101) pg/ mL, respectively. Therefore, the concentration of LPS stimulation in this study was determined as 50 ng/mL. To further confirm the timeeffect relationship for the expression of pro-inflammatory cytokines in monocytes under the stimulation of LPS, after the stimulation of 50 ng/mL LPS, the content of IL-6, IL-8 and TNF-α in the culture medium was detected at the time point after 0, 12, 24 and 48 h respectively. The results showed that, 12 h after the stimulation of 50 ng/mL LPS, three pro-inflammatory cytokines were all significantly up-regulated (P<0.05 vs. Ctrl). Twelve hours after the stimulation of LPS, the concentration of TNF-α was (863±153) pg/mL and it was gradually decreased, but still at a high level. However, 24 h after the stimulation of LPS, the concentration of IL-6 and IL-8 was relatively high, namely (5 627.0±330.0) pg/mL and (1 626±200) pg/mL. According to the dose-effect and time-effect relationship for the expression of pro-inflammatory cytokines in monocytes under the stimulation of LPS, 50 ng/mL LPS with the treatment for 24 h were chosen in this study.
Figure 2C/D referred to the dynamic change of inflammatory factors after the intervention of propofol. According to Figure 2C,24 h after the treatment of 50 ng/mL LPS in the mouse primary monocytes, it showed the change in the concentration of IL-6,IL-8 and TNF-α after the intervention of 0.1% DMSO and 1-100 μg/mL propofol respectively. Compared with the control group(0.1% DMSO), the concentration of IL-6, IL-8 and TNF-α after the intervention of 5 μg/mL propofol was significantly decreased(P<0.05), and resulted in the significant difference with the group treated with 50 μg/mL propofol (P<0.01). In Figurea 2D, after the same treatment in THP-1 cells, it was to study the effect of propofol on the concentration of IL-6, IL-8 and TNF-α. Results were in accordance with findings of the experiment of mouse primary monocytes. To be specific, the propofol could inhibit the expression of IL-6, IL-8 and TNF-α caused by LPS; after the intervention of 50 μg/mL propofol, the concentration of IL-6, IL-8 and TNF-α was significantly decreased (P<0.01).
3.3. Effect of propofol on key molecules in signaling pathway
LPS was bound with the receptor to activate the signaling pathway and then regulate the expression of many pro-inflammatory cytokines through the Toll-like MyD88-dependent pathway. TLR4,TRAF6, NF-κB and p38 are all important node molecules. Realtime PCR was employed to detect the effect of propofol on the expression of above signaling molecule gene.
As shown in Figure 3, the stimulation of LPS could significantly up-regulate the expression of TLR4, TRAF6, NF-κB and p38 in THP-1 cells (P<0.01). As the negative control group, after the stimulation of LPS, DMSO (0.1%) with the same concentration of the propofol group was given in the DMSO group. According to Figure 3A/C, the propofol could significantly reduce the upregulation of TLR4 and NF-κB that was caused by the stimulation of LPS; while according to Figure 3B/D, the propofol did not downregulate the expression of TRAF6 and p38 directly, but indicated that TRAF6 and p38 might not be the molecules under the direct action of propofol.
The Western blot assay was employed to detect the activation of p38. As shown in Figure 3E, the stimulation of LPS could upregulate the expression of p38 gene, while the effect of propofoltreatment on the expression of p38 was not significant (P>0.05),but it could significantly inhibit the phosphorylation of p38 protein(P<0.01).
4. Discussion
The propofol is a common anesthetic and sedative drug used in the clinical practice. In recent years, its immunoregulation has been increasingly focused, as well as the in-depth researches on its anti-inflammation mechanism. The monocytes are from the hematopoietic stem cell of bone marrow and they are developed in the bone marrow. Because of the non-specific esterase in the cells, it has the strong phagocytosis, as the main cell to join in the immune regulation and inflammatory response[8,9]. As the main component of outer membrane of Gram-negative bacteria, LPS consists of O-antigen polysaccharide, core polysaccharide and highly conserved lipid A, as the main cause of sepsis, septic shock and even death. As the main effector cell of LPS, the monocyte can release many kinds of pro-inflammatory cytokines, such as IL-6, IL-8 and TNF-α after the stimulation of LPS[10-13]. Therefore, LPS was added in the cell culture medium to simulate the inflammatory environment at the cellular level.
To study the immune regulation mechanism of propofol, the separation medium of mononuclear cells was used to separate the mononuclear cells from the spleen of mice. The main components of separation medium are Ficoll-Urografin, with the specific gravity of about 1.077±0.001. Due to the large specific gravity, the erythrocyte would be deposited at the bottom of tube after being centrifuged. The specific gravity of lymphocytes and monocytes was the same as the separation medium and it would be aggregated above the layer of separation medium after being centrifuged[14]. According to such principle, a great number of mononuclear classes could be separated from the spleen or blood. But due to the limited samples of blood and the monocytes only occupied about 5% of mononuclear cells,the mononuclear cells were separated from the spleen tissue in this study. Furthermore, the adherence of monocytes was used to purify the monocytes. The nonspecific esterase was stained (monocytes were positive). After the staining, the gray flocculent precipitate could be found in the cytoplasm. According to the experiment, the positive cells occupied over 90%, which proved that the purity of separated monocytes was relatively high. To eliminate the effect of species or cell specificity on the results of the experiment, the human monocyte THP-1 was also chosen as the research subject to discuss the immune regulation mechanism of propofol.
LPS was firstly bound with LPS binding protein on the cell membrane. Then it recognized LPS receptor molecule CDl4 and then was bound with it to form the compound. Such compound was bound with Toll-like receptor TLR-4 for the activation. After activating IL-1 receptor associated kinase-4 and IL-1 receptor associated kinase-4 by MyD88, the signal was transmitted to NF-κ B[15,16]. After being activated, NF-κB was entered into the nucleus to activate the transcription and translation of pro-inflammatory factors such as IL-6, IL-8 and TNF-α[17,18]. LPS was added during the culture of monocytes. Twenty-four hours later, ELISA was employed to detect the content of IL-6, IL-8 and TNF-α in the supernatant of culture medium. Results showed that the content of IL-6, IL-8 and TNF-α was dependent with the concentration of LPS. Twenty-four hours after the stimulation of 50 ng/mL LPS,the content of IL-6, IL-8 and TNF-α in the culture medium was(6 027.0±330.1) pg/mL, (1 436±200) pg/mL and (2 776±101) pg/ mL, respectively.
To study the effect of propofol on the inflammatory factors, 24 h after the treatment of 50 ng/mL LPS, the mouse primary monocytes were given the intervention of 0.1% DMSO and 1-100 μg/mL propofol and then the change in the concentration of IL-6, IL-8 and TNF-α was observed. Compared with the control group, the concentration of IL-6, IL-8 and TNF-α after the intervention of 5 μg/mL propofol was significantly decreased (P<0.05) and had the significant difference with the group treated with 50 μg/mL propofol (P<0.01). The same experiment was performed on THP-1 cells. Results were in accordance with findings of the experiment of mouse primary monocytes. To be specific, the propofol could inhibit the expression of IL-6, IL-8 and TNF-α caused by LPS; after the intervention of 50 μg/mL propofol, the concentration of IL-6,IL-8 and TNF-α was significantly decreased (P<0.01). All above results indicated that the propofol could significantly inhibit the upregulation of inflammatory factors caused by LPS. To specify the molecular mechanism of such process, real-time PCR was employed to detect the change in the expression of node molecules of above signaling pathway after the treatment of propofol. LPS was boundwith the receptor to activate the signaling pathway and then regulate the expression of pro-inflammatory factors through the Toll-like and MyD88-dependent pathway. TLR4, TRAF6, NF-κB and p38 are all important node molecules in the signaling pathway. Real-time PCR was adopted to detect the effect of propofol on the gene expression of above signaling molecules. As the dissolution of propofol requires DMSO as the dissolvent, DMSO with the same concentration was chosen as the negative control. According to the experimental results, the propofol could significantly reduce the up-regulation of TLR4 and NF-κB caused by the stimulation of LPS, but did not directly down-regulate the expression of TRAF6 and p38, which indicated that TRAF6 and p38 might not have the direct action molecules of propofol. However, in the study of signaling pathway,it is seldom to be involved in the signaling pathway by directly regulating the expression of target genes, because many signaling molecules in the signaling pathway relied on the post-translational modification or conformational change to achieve the regulation of its inhibitory or active state[19,20]. In consequence, Western blot was employed to detect the activation of p38 in this study. Results also proved the expectation, namely that the stimulation of LPS could up-regulate the expression of p38. There was no significant effect of propofol treatment on the expression of p38 (P>0.05), but it could significantly inhibit the phosphorylation of p38 protein (P<0.01). Such result indicated that the propofol could inhibit the expression of TLR-4, inhibit the activation of downstream molecules and then inhibit the expression and secretion of inflammatory molecules to play its role of immune regulation. However, the involvement in the immune regulation through such signaling pathway was just the part of anti-inflammatory action of propofol, while the in-depth study of molecular mechanism would be focused in our further studies.
Conflict of interest statement
We declare that we have no conflict of interest.
[1] Thiry JC, Hans P, Deby-Dupont G, Mouythis-Mickalad A, Bonhomme V, Lamy M. Propofol scavenges reactive oxygen species and inhibits the protein nitration induced by activated polymorphonuclear neutrophils. Eur J Pharmacol 2004; 499(1-2): 29-33.
[2] Huang CJ, Slovin PN, Nielsen RB, Skimming JW. Diprivan attenuates the cytotoxicity of nitric oxide in cultured human bronchial epithelial cells. Intensive Care Med 2002; 28(8): 1145-1150.
[3] Zhao LL, Hu GC, Zhu SS, Li JF, Liu GJ. Propofol pretreatment attenuates lipopolysaccharide-induced acute lung injury in rats by activating the phosphoinositide-3-kinase/Akt pathway. Braz J Med Biol Res 2014; 47(12): 1062-1067.
[4] Ma L, Wu XY, Zhang LH, Chen WM, Uchiyama A, Mashimo T, et al. Propofol exerts anti-inflammatory effects in rats with lipopolysaccharideinduced acute lung injury by inhibition of CD14 and TLR4 expression. Braz J Med Biol Res 2013; 46(3): 299-305.
[5] Adaramoye OA, Akinwonmi O, Akanni O. Effects of propofol, a sedative-hypnotic drug, on the lipid profile, antioxidant indices, and cardiovascular marker enzymes in wistar rats. ISRN Pharmacol 2013;2013: 230261.
[6] Ma L, Wu X, Chen W, Fujino Y. Propofol has anti-inflammatory effects on alveolar type II epithelial cells. Acta Anaesthesiol Scand 2010; 54(3):362-369.
[7] Li GF, Tong X, Luan T, Zang B. The effects of postconditioning with propofol on Toll-like receptor 4 expression in the lung tissue of rat with acute lung injury. Zhongguo Wei Zhong Bing Ji Jiu Yi Xue 2012; 24(10):620-623.
[8] Gjevestad GO, Holven KB, Ulven SM. Effects of exercise on gene expression of inflammatory markers in human peripheral blood cells: A systematic review. Curr Cardiovasc Risk Rep 2015; 9(7): 34.
[9] Tang WH. Effect of propofol on the secretion of cytokines from human monocytes stimulated with LPS. Master's Thesis of The Second Military Medical University; 2008.
[10] Zhou D, Huang C, Lin Z, Zhan S, Kong L, Fang C, et al. Macrophage polarization and function with emphasis on the evolving roles of coordinated regulation of cellular signaling pathways. Cell Signal 2014;26(2): 192-197.
[11] Rossol M, Heine H, Meusch U, Quandt D, Klein C, Sweet MJ, et al. LPS-induced cytokine production in human monocytes and macrophages. Crit Rev Immunol 2011; 31(5): 379-446.
[12] Askar B, Ibrahim H, Barrow P, Foster N. Vasoactive intestinal peptide(VIP) differentially affects inflammatory immune responses in human monocytes infected with viable Salmonella or stimulated with LPS. Peptides 2015; 71: 188-195.
[13] Petropoulou PI, Berbée JF, Theodoropoulos V, Hatziri A, Stamou P,Karavia EA, et al. Lack of LCAT reduces the LPS-neutralizing capacity of HDL and enhances LPS-induced inflammation in mice. Biochim Biophys Acta 2015; 1852(10): 2106-2115.
[14] Zhang SH, Lin DD, Zhang MJ, Chen XS, Xu JM, Chan H, et al. An exploration of the conditions for isolating peripheral blood mononuclear cells from pig's blood using Ficoll density gradient centrifugation. Chin J Schisto Control 2007; 19(3): 192-195.
[15] Li J, Csakai A, Jin J, Zhang F, Yin H. Therapeutic developments targeting Toll-like receptor-4-mediated neuroinflammation. Chem Med Chem 2015;doi: 10.1002/cmdc.201500188.
[16] Zou ZY, Hu YR, Ma H, Wang YZ, He K, Xia S, et al. Coptisine attenuates obesity-related inflammation through LPS/TLR-4-mediated signaling pathway in Syrian golden hamsters. Fitoterapia 2015; 5: 139-146.
[17] Teng X, Wei N, Chen H, Zhai K. TN-2 Exerts anti-inflammatory effects on LPS-induced rat dorsal root ganglion neurons by inhibiting TLR4-mediated NF-κB and MAPK pathways. J Mol Neurosci 2015; 2: 315.
[18] Xu E, Chen J, Wang Y, Ke Z, Luo S, Zou Z. A phosphoproteomic study reveals shp-1 cleavage reprograms LPS signaling via a PI-3K/NF-κB and mTORC1 related mechanism. J Proteomics 2015; 128: 30-38.
[19] van Griensven M. Preclinical testing of drug delivery systems to bone. Adv Drug Deliv Rev 2015; doi:10.1016/j.addr.2015.07.006.
[20] Barhoumi A, Lui Q, Kohane DS. Ultraviolet light-mediated drug delivery: Principles, applications, and challenges. J Control Release 2015;doi:10.1016/j.jconrel.2015.07.018
15 August 2015
Yun Nie, M.M., First People's Hospital of Ji'nan, Ji'nan,Shandong, China.
Tel.: 15806601555
E-mail: m15806601555@163.com
Foundation project: The project was supported by Natural Science Foundation of Shandong Province (No.: ZR2013HM061).
杂志排行
Asian Pacific Journal of Tropical Medicine的其它文章
- Demographic, socioeconomic and environmental changes affecting circulation of neglected tropical diseases in Egypt
- Phenolic profile and biological potential of Endopleura uchi extracts
- Roots extracts of Adenophora triphylla var. japonica improve obesity in 3T3-L1 adipocytes and high-fat diet-induced obese mice
- Anti TB drug resistance in Tanga, Tanzania: a cross sectional facility base prevalence among pulmonary TB patients
- In vitro inhibitory effects of plumbagin, the promising antimalarial candidate, on human cytochrome P450 enzymes
- Vibrio spp. from Macrobrachium amazonicum prawn farming are inhibited by Moringa oleifera extracts
