Abnormal expression of WIF1 in hepatocellular carcinoma cells and its regulating effect on invasion and metastasis factors of TIMP-3 and caveolin-1 of hepatocellular carcinoma
2015-10-31GuangSongHongXiaCaoShaoXinYaoCangTuoLi
Guang Song, Hong-Xia Cao, Shao-Xin Yao, Cang-Tuo Li
Department of Interventional Therapy, Affiliated Tangshan Workers Hospital of Hebei Medical University, Tangshan 063000, Hebei, China
Abnormal expression of WIF1 in hepatocellular carcinoma cells and its regulating effect on invasion and metastasis factors of TIMP-3 and caveolin-1 of hepatocellular carcinoma
Guang Song, Hong-Xia Cao, Shao-Xin Yao, Cang-Tuo Li*
Department of Interventional Therapy, Affiliated Tangshan Workers Hospital of Hebei Medical University, Tangshan 063000, Hebei, China
ARTICLE INFO
Article history:
Received in revised form 20 September 2015
Accepted 15 October 2015
Available online 20 November 2015
WIF1
Hepatocellular carcinoma
TIMP-3
Caveolin-1
Objective: To discuss the abnormal expression of Wnt inhibitory factor (WIF1) in hepatocellular carcinoma cells and its regulating effect on the hepatocellular carcinoma invasion and metastasis factors of tissue inhibitor of matrix metalloproteinases-3 (TIMP-3)and caveolin-1. Methods: RT-PCR and Western blot were employed to detect the expression of WIF1 in six hepatocellular carcinoma cell lines of HepG2, Hep3B, Huh7, PLC/PRF/5,SMMC-7721 and MHCC97 and the immortalized human liver cell line THLE-3. Besides,Lipofectamine 2000 was employed to transfect the eukaryotic expression vector pcDNA3.1-WIF1 and blank plasmid pcDNA3.1 into hepatocellular carcinoma cell lines. Transwell assay was used to detect the effect of WIF1 on the invasion ability of hepatocellular carcinoma cells;Western blot was used to detect the effect of WIF1 on the expression of TIMP-3 and caveolin-1 in hepatocellular carcinoma cells, it also discussed the effect on the expression of β-catenin. Results: The expression of WIF1 in hepatocellular carcinoma cell lines was lower than that in the normal liver cell lines (P<0.01); while there was basically no expression of WIF1 in the human highly metastatic cell line MHCC-97 and moderate expression in HepG2 and SMMC-7721. Therefore, HepG2 and SMMC-7721 were chosen as the further experimental cell lines. After transfecting the eukaryotic expression vector pcDNA3.1-WIF1 and blank plasmid pcDNA3.1 into hepatocellular carcinoma cell lines, compared with the blank plasmid group, the cell viability and invasion ability in the WIF1 group were all reduced (P<0.01),the expression of TIMP-3, caveolin-1 and mRNA were all down-regulated (P<0.01), and the expression ofβ-catenin was decreased (P<0.01). Conclusions: Because of down-regulation or missing of expression of WIF1 in hepatocellular carcinoma cell lines, the up-regulation of WIF1 expression can significantly inhibit the invasion and metastasis of HepG2 and SMMC-7721 of hepatocellular carcinoma cell lines, which are related to the up-regulated expression of TIMP-3 and down-regulated expression of caveolin-1 and may be realized through the Wnt/ β-catenin signaling pathway.
Document heading doi:10.1016/j.apjtm.2015.10.007
1. Introduction
A total of 90% of the malignant tumor in the primary liver cells or intrahepatic duct cells is the hepatocellular carcinoma (HCC),as the common solid tumor that ranks the fifth place in the world. It has the bad prognosis and difficult clinical prevention andtreatment[1,2]. Most of HCC patients who received the hepatectomy had a recurrence after the operation, leading to the mortality at a high level. Its main reason is the invasion and metastasis of HCC[3]. Accordingly, the in-depth research on the mechanism of invasion and metastasis of HCC will be of critical importance for the more effective prevention and treatment of HCC and the improvement on the treatment of HCC.
Wnt gene was found by Nusse et al in 1982 during the induction of mouse mammary carcinoma using the papilloma-virus. Wnt signaling pathway is some kind of highly conserved signaling pathway in the multicellular eukaryotes to regulate the process ofmany life activities. Wnt signaling pathway is in the abnormally active state in HCC and most of HCC cells. Besides, more and more researches have proved that the silenced expression of inhibitory factors in the Wnt signaling pathway might be the key cause for the abnormal activation of Wnt signal[4-6]. As the secretory antagonist of Wnt signaling pathway, Wnt inhibitory factor-1 (WIF1) was found on the human retina, showing the high affinity with Wg and XWnt8. Previous researches had proved that the expression of WIF1 protein and mRNA had the lower expression in HCC tissue than the one in the neighboring tissue, while the low expression of WIF1 was related to the low overall survival rate. In addition, its expression was reduced in HCC cell lines such as Huh7, HepG2, SMMC-7721,MHCC97-L, Bel-7402 and PLC/PRF/5[7,8], which indicated that WIF1 might have the close relationship with the occurrence and development of HCC. Furthermore, by up-regulating the expression of WIF1, it could regulate the expression of related proteins through Wnt/β-catenin signaling pathway to significantly inhibit the invasion and metastasis abilities of cervical carcinoma cells and prostate cancer cells[9,10]. It is presumed that WIF1 may affect the invasion and metastasis abilities of HCC cells through the related signaling pathways.
The invasion and metastasis of tumors refer to the complicated and multi-level biological process, while the degradation of basement membrane or extracellular matrix is the precondition for the infiltration and metastasis of tumors. The tissue inhibitor of matrix metalloproteinases-3 (TIMP-3) is the member of TIMPs,which can inhibit the degradation of extracellular matrix by the matrix metalloproteinases (MMPs) to play the role of anti tumor growth, invasion and metastasis. TIMP-3 had the low expression in the plasma and tissues of patents with HCC and its expression was even lower in the metastatic lesions[11], which indicated that the low expression of TIMP-3 was closely related to the invasion and metastasis abilities of HCC. The ways of tumor metastasis include the lymphatic metastasis, hematogenous metastasis and implantation metastasis, where over 50% tumors were metastasized through the lymphatic channel. More and more researches have proved the close relationship between the caveolin-1 and lymphatic metastasis of tumors. The high expression of caveolin-1 in HCC patients might be closely related to the tissue differentiation, infiltration of portal vein, infiltration of hepatic vein, intrahepatic metastasis and recurrence[12], which indicated the close relationship between the expression of caveolin-1 and the occurrence and development of HCC. In consequence, this study would further detect the expression of WIF1 in HCC cell lines. Besides, by transfecting the eukaryotic expression vector pcDNA3.1-WIF1, it was to discuss its effect on the invasion and metastasis abilities of HCC cell lines and the expression of TIMP-3 and caverolin-1 after the over-expression of WIF1. In addition, the possible mechanism would also be discussed.
2. Materials and methods
2.1. Reagents and instruments
The human HCC cell lines of HepG2, Hep3B, Huh7, PLC/PRF/5 and SMMC-7721 and immortalized human liver cell line THLE-3 were purchased from the Cell Bank of Type Culture Collection of Chinese Academy of Sciences, with the registered number TCHu72,TCHu106, TCHu182, TCHu119, TCHu52, and GNHu40. MHCC97 was purchased from Institute of Hepatocellular Carcinoma, Medical College of Fudan University; the rabbit anti TIMP-3, caveolin-1 and β-catenin antibodies from Epitomics; the mouse anti GADPH antibody from Beyotime Biotechnology; transwell chamber from Corning; the fetal bovine serum, DMEM culture medium and trypsin from Gbico; the crystal violet from Sigma; the biosafety cabinet from Thermo Scientific; mini vertical electrophoresis tanks and mini Trans-blot electrophoresis tank from Beijing Liuyi Instrument Factory; and ChemiDocTMXRS gel imaging system from Bio-Rad.
2.2. RT-PCR
The total RNA was extracted by referring to the instruction manual of trizol kit (Invitrogen) and the extraction was performed in the condition without RNAase. Primers were shown in Table 1. RNA was reversely transcripted into cDNA through one-stop RTPCR kit and then PCR amplification was performed. A total of 5 μL amplification product was used for further detection using 2% agarose gel. Primers were added into 25 μL PCR reaction system respectively, with the reaction conditions of degeneration at 94 ℃for 45 s, renaturation at 59 ℃ for 45 s and extension at 72 ℃ for 60 s, with 35 cycles.
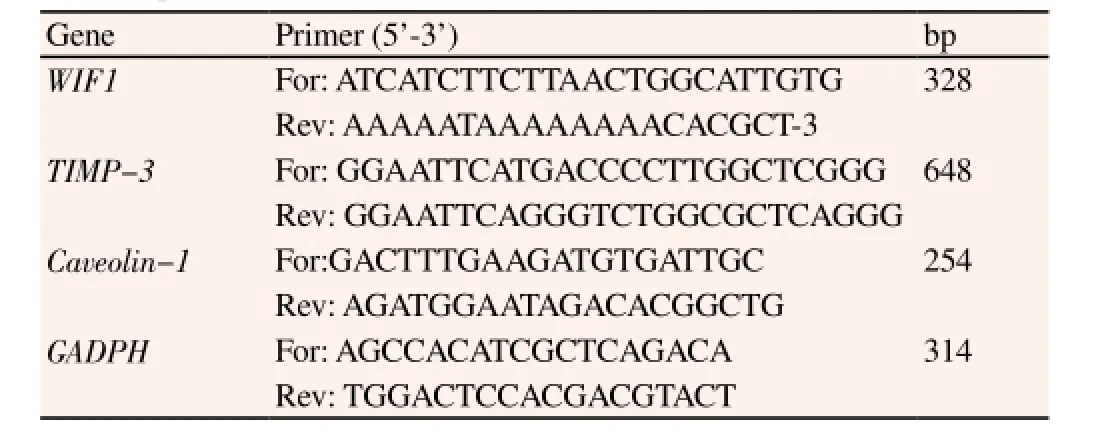
Table 1 RT-PCR primers.
2.3. Construction of WIF1 expression vector
Taking the cDNA of human placenta tissue as the template, RTPCR amplification was performed and then cloned into pGEM-T Easy (Promega) vector. It was sequenced to obtain the complete coding sequence of WIF1. The pcDNA3.1 plasmid (Invitrogen) was connected with the fragments of target gene after the amplification of WIF1. The double endonuclease restriction of BamH Ⅰ and Xho Ⅰ was performed and purified respectively. The plasmid and fragments of target gene were recovered, which were connected with T4 DNA ligase and then cultured in LB flat plate that contained the ampicillin. The positive clone was selected and sequenced to prove the successful construction of eukaryotic expression vector pcDNA3.1-WIF1.
2.4. MTT method
The HCC cell lines were seeded onto 96-well plate. When theconfluency of cells reached to 50%, it was to transfect pcDNA3.1-WIF1 and blank plasmid with the transfection concentration of 50 ng and 50 ng using Lipofectamine 2000 (Invitrogen) respectively. A total of 48 h after the transfection, 20 μL 5 mg/mL MTT was added for the continuous culture of 4 h and then the culture medium was sucked out. Afterwards, 150 μL DMSO was added in each well and then it was shaken to fully dissolve the crystals. CD value was measured at 560 nm of analyzer of enzyme-linked immunosorbent assay.
2.5. Cell invasion assay
The Matrigel was evenly paved on the micromembrane of Transwell chamber to be prepared as the gel for further use. The HCC cell lines were seeded onto a 6-well plate. When the confluency of cells reached to 50%, it was to transfect pcDNA3.1-WIF1 and blank plasmid pcDNA3.1 with the transfection concentration of 1 μg and 1 μg using Lipofectamine 2000 respectively. A total of 48 h after the transfection, cells were digested and added in the upper chamber of Transwell, while the lower chamber contained DEME medium with 5% fetal bovine serum for further 24 h culture. Then the Transwell chamber was taken out and washed, using the paraformaldehyde for the fixation. It was then stained with the crystal violet. The number of membrane-penetrating cells in five fields was counted under the inverted optical microscope and the mean number of cells for each field was calculated to represent the invasion ability of cells.
2.6. Western blot assay
The HCC cell lines were seeded onto a 6-well plate, with 100 μL in each well. When the confluency of cells reached 50%, it was to transfect pcDNA3.1-WIF1 and blank plasmid pcDNA3.1 with the transfection concentration of 1 μg and 1 μg using Lipofectamine 2000 respectively. 48 h after the transfection, cells were scraped and centrifuged. Then the proper RIPA lysis buffer was added in the collected cells. It was put in Vortex instrument for 30 s of shaking every 10 min. After 40 min, it was centrifuged at 4 ℃ and 10 000 rpm for 10 min. The supernatant was sucked carefully to obtain the total protein. The protein concentration was measured by BCA kit. The protein loading buffer was treated with SDS gel electrophoresis and then transferred by the wet method. Then the film was immersed into the primary antibody solution for the incubation at 4 ℃overnight. After being washed, it was immersed into the secondary antibody solution for the incubation at the room temperature for 1-2 h. Afterwards, the film was taken out and washed, while ECL reagent was added on the film for exposure in the gel imaging system. Statistics was performed on the gray value of each antibody band using ‘Quantity One' software.
2.7. Data analysis
The results were expressed as mean±SD, with three repeats for each set of data at least. The t test was employed and P<0.05 was meant to have significant difference. All data were treated using SPSS 17.0.
3. Results
3.1. Expression of WIF1 in HCC lines and normal liver cells
According to results of RT-PCR (Figure 1) and Western blot(Figure 2), the expression of WIF1 in HCC cell lines of HepG2,Hep3B, Huh7, PLC/PRF/5, SMMC-7721 and MHCC97 was all significantly higher than the one in the immortalized human liver cell line THLE-3 (P<0.01); it had basically no expression in the highly metastatic MHCC97 and moderate expression in HepG2 and SMMC-7721. Therefore, these two cell lines were chosen as the further research subjects.
3.2. Effect of WIF1 on viability of HCC cell lines
As shown in Figure 3, after transfecting pcDNA3.1-WIF1 into HepG2 and SMMC-7721 cell lines, the results of MTT assay showed that the cell viability of HepG2 and SMMC-7721 was significantly reduced (P<0.01).
3.3. Effect of WIF1 on invasion ability of HCC cell lines
As shown in Figure 4, compared with the control group, after the up-regulated expression of WIF1, the number of HepG2 cells into subarachnoid space was significantly reduced 213.47±25.69 vs. 58.97±6.45 (P<0.01). As shown in Figure 5, compared with the control group, after the up-regulated expression of WIF1, the number of SMMC-7721 cells into the subarachnoid space was significantly reduced 178.43±21.08 vs. 39.60±3.83 (P<0.01).
3.4. Effect of WIF1 on expression of TIMP-3 and caveolin-1 mRNA in HCC cells
As shown in Figure 6, the increased expression of WIF1 could lead to the up-regulated expression of TMP-3 mRNA and down-regulated expression of caveolin-1 mRNA in HepG2 and SMMC-7721 cells,with significant difference (P<0.01). 3.5. Effect of WIF1 on expression of TIMP-3 and caveolin-1 protein in HCC cells
As shown in Figure 7, the increased expression of WIF1 could lead to the up-regulated expression of TMP-3 protein and down-regulated expression of caveolin-1 protein in HepG2 and SMMC-7721 cells,with significant difference (P<0.01).
3.6. Effect of WIF1 on Wnt/β-catenin signaling pathway of HCC cells
As shown in Figure 8, the increased expression of WIF1 could lead to the down-regulated expression of β-catenin in HepG2 and SMMC-7721 cells 0.875±0.072 vs. 0.279±0.043, 0.915±0.102 vs. 0.302±0.055, with significant difference (P<0.01).
4. Discussion
HCC is the malignant tumor with the bad prognosis, bringing the great pain to patients and great inconvenience to medical staff. Its pathogenesis has not been clear, but it is regulated by many signaling pathways, in which Wnt signaling pathway is some kind of highly conserved signaling pathway in the multicellular eukaryotes to regulate the process of many life activities. Wnt signaling pathway is in the abnormally active state in 60%-70% of HCC cells. The silenced expression of inhibitory factors in the Wnt signaling pathway might be the key cause for the abnormal activation of Wnt signal[4-6]. As the typical inhibitory factor of Wnt signalingpathway, WIF1 was firstly found on the human retina and then proved in the bodies of rat, toad, and zebra. Such gene is located on 12q14. The function of WIF1 is mainly mediated by WIF domain,which can compete with Wnt ligand for being bound with fizzled receptor to block the Wnt signaling pathway and thus inhibit the formation of tumors. According to researches, the expression of WIF1 was reduced or absent in many human tumors. Huang et al[8]adopted RT-PCR to detect 105 HCC samples. The results showed the decreased expression of WIF1 mRNA, which might be related to the low overall survival rate. It also had the low expression in SMMC-7721, Bel-7402, PLC and HepG2. Deng et al[7] performed RT-PCR detection and found the gradually decreased expression of WIF1 in Huh7, HepG2, SMMC-7721 and MHCC97-L and then no such expression, which were all lower than the ones in the normal tissues. Besides, qRT-PCR was performed to detect 15 HCC tissues and neighboring ones. Results also showed the lower expression in the cancer tissues than the ones in the neighboring ones, which could fully indicate the low expression of WIF1 in HCC tissue and HCC cell lines and it might be closely related to the occurrence and development of HCC. Accordingly, in this study, RT-PCR and Western blot were employed to detect the expression of WIF1 in six HCC cell lines of Huh7, HepG2, SMMC-7721, MHCC97, PLC/ PRF/5 and Hep3B and one immortalized human liver cell line of THLE-3. Results showed the expression in HCC cell lines than the one in THLE-3 and normal liver cell line. WIF1 had basically no expression in the highly metastatic MHCC97 and moderate expression in HepG2 and SMMC-7721, which were in accordance with findings of Deng et al[7]. Therefore, these two cell lines were chosen as the further research subjects. Deng et al[7] transfected the eukaryotic expression vector pcDNA3.1-WIF1 into HepG2 and SMMC-7721 and found that the over-expression of WIF1 could significantly inhibit the number of clones and the proliferation of tumor cells. Hu et al[4] found that the transfection of recombinant adenoviral vector Ad-WIF1-Fc into HCC cells could significantly down-regulate the expression of E2F1, cyclin D1 and c-myc and thus promote the apoptosis of tumor cells, reduce the microvessel density, inhibit the expression of vascular endothelial growth factor and stromal cell-derived factor-1 and thus inhibit the angiogenesis and migration of microvascular endothelial cells. But its effect on the invasion and metastasis abilities of HCC cells has not been clear. The invasion and metastasis is the key cause to affect the prognosis and overall survival rate of patients with HCC. Therefore, this study is by constructing the eukaryotic expression vector pcDNA3.1-WIF1 and transfecting it into the HCC cell lines of HepG2 and SMMC-7721 through the liposome. The results showed that the increased expression of WIF1 could significantly inhibit the migration and invasion abilities of HCC cells. Thus it further discussed the mechanism.
The invasion and metastasis of tumors refer to the multi-step and multi-factor biological process, where the degradation of extracellular matrix is the precondition for the infiltration and metastasis of tumors. The degradation of extracellular matrix is mediated by MMPs, while TIMP is the specific inhibitor of MMP that can maintain the physiological function of normal extracellular matrix. TIMP-3 is the member of TIMPs, which is located at the human chromosome 22q12.3. It is bound with MMPs to form the complex of MMP-TIMP, which can thus inhibit the activity of MMPs, keep from the degradation of extracellular matrix and inhibit the metastasis and spread of tumors. The previous researches had proved its low expression in the HCC tissue and cell lines. Besides, the expression of TIMP-3 was gradually decreased in the in-situ HCC tissue, portal vein thrombosis tissue and metastatic lymph node lesions, which indicated that the low expression of TIMP-3 might be related to the invasion and metastasis abilities of HCC[11]. Furthermore, some research also proved that the increased expression of TIMP-3 could significantly inhibit the proliferation,invasion and metastasis of HepG2 and SMMC-7721 cells[13]. In addition, Lin et al[14] transfected TIMP-3 into CT26 colon cancer cells through the adenoviral vector, which could significantly inhibit the growth of tumor and hepatic metastasis. Anania et al[15] proved that the recovered expression of TIMP3 in the thyroid carcinoma NIM1 cells could significantly inhibit the adhesion, migration and invasion abilities of tumor cells and also inhibit the angiogenesis and infiltration of macrophages. It fully indicated that the up-regulated expression of TIMP-3 could significantly inhibit the invasion ability of tumor cells. Accordingly, in this study, after transfecting the eukaryotic expression vector pcDNA3.1-WIF1 into HCC cell lines,it is found that, compared with the blank plasmid group, it could significantly increase the expression of TIMP-3, which could be regarded as one of mechanisms that it could significantly inhibit the invasion ability of tumor cells after the increased expression of WIF1.
Caverolin-1 is the important structural protein of Caveolae. It is the flask-like caveolate structure that was found by Yamada on the cell membrane. With diverse biological functions, it plays the key role in the life activities of membrane transport, cholesterol transport and signal transduction. Tang et al[12] proved the high expression of caveolin-1 in HCC patients, which might be closely related to the tissue differentiation, infiltration of portal vein, infiltration of hepatic vein, intrahepatic metastasis and recurrence. The transfection of eukaryotic expression vector pcDNA3.1-caveolin-1 into HepG2 cells could not only inhibit the apoptosis, but also up-regulate the expression of MMP-2, MMP-9 and vascular endothelial growth factor to enhance the migration and invasion abilities of tumor cells. Cokakli et al[16] performed the Immunohistochemistry and Western blot and found that the expression of caveolin-1 in the normal liver tissue, liver cirrhosis and HCC was gradually increased, namely 5%,45% and 66%. Patients with the high expression of caveolin-1 had the high infiltration of portal vein. The high expression of caveolin-1 in HepG2 and Huh7 cells enhanced the motion and invasion ability of tumor cells and regulated the expression of related proteins. It fully indicated the close relationship between the expression of caveolin-1 and the occurrence and development of HCC. The high expression of Caverolin-1 was closely related to the highly metastatic HCC. By down-regulating its expression, the metastasis of tumor would be inhibited. Therefore, in this study, after transfecting the eukaryotic expression vector pcDNA3.1-WIF1 into HCC cell lines,it is found that, compared with the blank plasmid group, it could significantly increase the expression of TIMP-3, which could be regarded as one of the mechanisms that it could significantly inhibitthe invasion ability of tumor cells after the increased expression of WIF1.
By up-regulating the expression of WIF1, it could inhibit the Wnt signaling pathway and thus inhibit the TGF-β1-induced epithelialmesenchymal transition[17]. The increased expression of WIF1 could significantly inhibit the expression of target genes E-cadherin and vascular endothelial growth factor of Wnt/β-catenin signaling pathway and the expression of Wnt1 and TCF-4 in the cervical carcinoma, which could thus inhibit the proliferation, angiogenesis and invasion of tumor cells[9]. It indicated that WIF1 could inhibit the Wnt signaling pathway to inhibit the invasion and metastasis abilities of tumor cells. Accordingly, in this paper, after transfecting the eukaryotic expression vector pcDNA3.1-WIF1 into HCC cell lines,it is found that, compared with the blank plasmid group, it could significantly inhibit the expression of β-catenin. In addition, TIMP-1[18] and TIMP-2[19] could affect the biological behavior of normal cells and malignant tumor cells through the Wnt/β-catenin signaling pathway, which indicated the certain relationship between TIMP-3 and Wnt/β-catenin signaling pathway. The over-expression of caveolin-1 in the zebra fish could interfere the nucleus transcription of β-catenin[20]. Caveolin-1 had the over-expression in the highly invasive HCC cell lines and the over-expression could enhance the invasion ability of HCC and thus promote the tumor formation and lung metastasis of mice. It induced the formation of epithelialmesenchymal transition through Wnt/β-catenin signaling pathway and thus promoted the HCC metastasis[21], which also showed the close relationship between caveolin-1 and Wnt/β-catenin signaling pathway. It thus indicated that the increased expression of WIF1 in HCC cell lines could significantly up-regulate the expression of TIMP-3 and down-regulate the expression of caveolin-1 to inhibit the invasion and metastasis of tumors, which might be realized through the Wnt/β-catenin signaling pathway.
Conflict of interest statement
We declare that we have no conflict of interest.
Acknowledgements
This work was carried out with the support of ‘Cooperative Research Program for Agriculture Science &Technology Development (Project No.PJ01134801)' Rural Development Administration, Republic of Korea.
[1] Chen KW, Ou TM, Hsu CW. Current systemic treatment of hepatocellular carcinoma: A review of the literature. World J Hepatol 2015; 7(10): 1412-1420.
[2] Attwa MH, El-Etreby SA. Guide for diagnosis and treatment of hepatocellular carcinoma. World J Hepatol 2015; 7(12): 1632-1651.
[3] Klasser GD, Echandi L, Shannon M. Hepatocellular carcinoma metastasis to the condyle: a case report and review of the literature. J Am Dent Assoc 2014; 145(10): 1063-1067.
[4] Hu J, Dong A, Fernandez-Ruiz V. Blockade of Wnt signaling inhibits angiogenesis and tumor growth in hepatocellular carcinoma. Cancer Res 2009; 69(17): 6951-6959.
[5] Lachenmayer A, Alsinet C, Savic R. Wnt-pathway activation in two molecular classes of hepatocellular carcinoma and experimental modulation by sorafenib. Clin Cancer Res 2012; 18(18): 4997-5007.
[6] Suarez MI, Uribe D, Jaramillo CM. Wnt/beta-catenin signaling pathway in hepatocellular carcinomas cases from Colombia. Ann Hepatol 2015;14(1): 64-74.
[7] Deng Y, Yu B, Cheng Q. Epigenetic silencing of WIF-1 in hepatocellular carcinomas. J Cancer Res Clin Oncol 2010; 136(8): 1161-1167.
[8] Huang L, Li MX, Wang L. Prognostic value of Wnt inhibitory factor-1 expression in hepatocellular carcinoma that is independent of gene methylation. Tumour Biol 2011; 32(1): 233-240.
[9] Ramachandran I, Thavathiru E, Ramalingam S. Wnt inhibitory factor 1 induces apoptosis and inhibits cervical cancer growth, invasion and angiogenesis in vivo. Oncogene 2012; 31(22): 2725-2737.
[10] Yee DS, Tang Y, Li X. The Wnt inhibitory factor 1 restoration in prostate cancer cells was associated with reduced tumor growth, decreased capacity of cell migration and invasion and a reversal of epithelial to mesenchymal transition. Mol Cancer 2010; 9: 162.Y.
[11] Zhou Q, Wang YF, Zhou XB. Relationship between expression level of TIMP-3 and invasion/metastasis of hepatocellular carcinoma. Chin J Pathophysiol 2011; 27(9): 1762-1766.
[12] Tang Y, Zeng X, He F. Caveolin-1 is related to invasion, survival, and poor prognosis in hepatocellular cancer. Med Oncol 2012; 29(2): 977-984.
[13] Chen YR, Shen B, Li SY. The effects of TIMP-3 on the proliferation,apoptosis, invasiveness and migration of human hepatocarcinoma cell line. Guangdong Med J 2014; 35(1): 29-32.
[14] Lin H, Zhang Y, Wang H. Tissue inhibitor of metalloproteinases-3 transfer suppresses malignant behaviors of colorectal cancer cells. Cancer Gene Ther 2012; 19(12): 845-851.
[15] Anania MC, Sensi M, Radaelli E. TIMP3 regulates migration, invasion and in vivo tumorigenicity of thyroid tumor cells. Oncogene 2011; 30(27):3011-3023.
[16] Cokakli M, Erdal E, Nart D. Differential expression of Caveolin-1 in hepatocellular carcinoma: correlation with differentiation state, motility and invasion. BMC Cancer 2009; 9: 65.
[17] Xu JH, Yang HP, Zhou XD. Role of Wnt inhibitory factor-1 in inhibition of bisdemethoxycurcumin mediated epithelial-to-mesenchymal transition in highly metastatic lung cancer 95D cells. Chin Med J (Engl) 2015;128(10): 1376-1383.
[18] Bijakowski C, Vadon-Le GS, Delolme F. Sizzled is unique among secreted frizzled-related proteins for its ability to specifically inhibit bone morphogenetic protein-1 (BMP-1)/tolloid-like proteinases. J Biol Chem 2012; 287(40): 33581-33593.
[19] Xia Y, Wu S. Tissue inhibitor of metalloproteinase 2 inhibits activation of the beta-catenin signaling in melanoma cells. Cell Cycle 2015; 14(11): 1666-1674.
[20] Mo S, Wang L, Li Q. Caveolin-1 regulates dorsoventral patterning through direct interaction with beta-catenin in zebrafish. Dev Biol 2010;344(1): 210-223.
[21] Yu H, Shen H, Zhang Y. CAV1 promotes HCC cell progression and metastasis through Wnt/beta-catenin pathway. PLoS One 2014; 9(9):e106451.
15 August 2015
Cang-Tuo Li, M.M., Attending Physician, Affiliated Tangshan Workers Hospital of Hebei Medical University, Tangshan, Hebei, China.
E-mail: nzhongj@126.com
Foundation project: The project was supported by National Science & Technology Pillar Program during the 12th Five-year Plan Period under the research and development of new trisacryl gelatin microspheres (No.: 2012BAI15B06).
杂志排行
Asian Pacific Journal of Tropical Medicine的其它文章
- Demographic, socioeconomic and environmental changes affecting circulation of neglected tropical diseases in Egypt
- Phenolic profile and biological potential of Endopleura uchi extracts
- Roots extracts of Adenophora triphylla var. japonica improve obesity in 3T3-L1 adipocytes and high-fat diet-induced obese mice
- Anti TB drug resistance in Tanga, Tanzania: a cross sectional facility base prevalence among pulmonary TB patients
- In vitro inhibitory effects of plumbagin, the promising antimalarial candidate, on human cytochrome P450 enzymes
- Vibrio spp. from Macrobrachium amazonicum prawn farming are inhibited by Moringa oleifera extracts
