Annexin A2 silencing enhances apoptosis of human umbilical vein endothelial cells in vitro
2015-10-31ShuLeJiangDongYanPanChaoGuHaiFengQinShiHongZhao
Shu-Le Jiang, Dong-Yan Pan, Chao Gu, Hai-Feng Qin, Shi-Hong Zhao
Department of Ophthalmology, Affiliated Changhai Hospital of Second Military Medical University, Shanghai, 200433, China
Annexin A2 silencing enhances apoptosis of human umbilical vein endothelial cells in vitro
Shu-Le Jiang, Dong-Yan Pan, Chao Gu, Hai-Feng Qin, Shi-Hong Zhao*
Department of Ophthalmology, Affiliated Changhai Hospital of Second Military Medical University, Shanghai, 200433, China
ARTICLE INFO
Article history:
in revised form 20 September 2015
Accepted 15 October 2015
Available online 20 November 2015
Retinal neovascularization
Annexin A2
RNA interference
Gene silence
Cell apoptosis
Objective: To study the effects of inhibited Annexin A2 (ANXA2) on human umbilical vein endothelial cells (HUVECs) in vitro. Methods: Short hairpin RNA (shRNA) targeting ANXA2 was designed and cloned into double marked lentvirial vector GV248 for RNAi to generate the recombinant expression plasmids, which were stably transfected into HUVECs. The protein and mRNA expression levels of ANXA2 were analyzed by western blotting and realtime polymerase chain reaction, respectively. Cell proliferation (cell counting kit-8 assay),apoptosis (flow cytometry analysis), the expression (western blotting) and the activity of caspases (enzyme-linked immunosorbent assay) were used to assess the effects of silencing ANXA2 on HUVECs in vitro. Results: The plasmids to express ANXA2-specific shRNA were constructed and were infected into HUVEC resulting in the stably transfected experimental(ANXA2-shRNA), control (control-shRNA) and mock (no plasmid) cell lines, which were verified with western blot and real-time PCR. HUVEC/ANXA2-shRNA showed an inhibition rate 91.89% of ANXA2 expression compared to the mock HUVEC. ANXA2 silencing cell strain obviously presented a lower cell proliferation activity compared to the control and mock HUVECs, with an inhibition rate 82.35% on day 7 in vitro. FACS analysis indicated that the HUVEC/ANXA2-shRNA cells undergoing apoptosis increased by 102.61% compared to the mock HUVECs (P<0.01). Moreover, the activity levels of caspase-3, caspase-8 and caspase-9 in HUVEC/ANXA2-shRNA cells were increased and the activated cleaved caspase-3, cleaved caspase-8 and cleaved caspase-9 were upregulated evidently compared with that of the control and mock HUVECs by 56.29%, 89.59% and 144.58% (P<0.01). Conclusions: shRNA-mediated silencing of ANXA2 could not only be able to suppress HUVECs proliferation but to upregulate the enzyme activity of caspases, which bring to an increase of cell apoptosis. This work suggested that ANXA2 may represent a useful target of future molecular therapies.
Document heading doi:10.1016/j.apjtm.2015.10.006
1. Introduction
Annexin A2 (ANXA2), a kind of calcium depended membrane phospholipids binding protein, is one of the important members of Annexins family which widely expressed in cell membrane,cytoplasm and extracellular matrix[1,2]. ANXA2 can function inmany life activities, such as formation of cell membrane structure,membrane fusion, signal transduction, cell migration, DNA synthesis, cell proliferation and cell apoptosis. It is also reported that ANXA2 may play a key role in the retinal neovascularization. With its partner S100A10 (p11), ANXA2 is able to form a heterotetrameric complex[3-10], which binds both plasminogen and its activator (tissue plasminogen activator, tPA) to accelerate the generation of plasmin[11-17]. The isolated endothelial cells from the mice deficient in annexin A2 (ANXA2-/-) are unable to support tPA-dependent plasminogen activation in vitro[18,19]and the ANXA2-/-mice exhibit reduced angiogenesis in growth factor-stimulated assays in adulthood. Retinal neovascularizationis a common pathological change of many eye diseases, which results in wide injury to eyes to get blindness[20]. We assumed that ANXA2 may affect the process of neovascularization of the retina,so the objectives of the present study were to focus on the effects of silencing ANXA2 by small hairpin RNA (shRNA) on human umbilical vein endothelial cells (HUVECs) in vitro that is usually used in the study of angiogenesis. We found that shRNA-mediated silencing of ANXA2 could not only be able to suppress HUVECs proliferation but to upregulate the enzyme activity of caspases,which bring to an increase of cell apoptosis.
2. Materials and methods
2.1. Plasmid construction
The shDNAs encoding ANXA2-specific shRNA targeting ANXA2 nucleotides (GeneBank: NM_001002858) (Table 1), which were designed with the free software OPtiRNA(http://optirna.unl.edu/)and synthesized as by GeneChem, China, were used to construct experimental plasmids (pGV248/ANXA2-shRNA) by inserting into an Age I and EcoR I linearized pGV248, an shRNA shuttle expression vector containing a green fluorescent protein reporter gene (GeneChem, China). A negative control vector (pGV248/ control-shRNA) was constructed similarly with an unrelated shRNA sequence that does not suppress the expression of genes expressed in humans (GeneChem). All inserted sequences were verified by DNA sequencing.
2.2. Cell culture
HUVEC cells were obtained from ATCC, USA. The cells were cultured in Ham's-F12K medium (Zhongqiaxinzhou Biotechnologies, China), supplemented with 5% fetal calf serum,100 U/mL penicillin, and 0.1 mg/mL streptomycin in a humidified atmosphere with 5% CO2at 37 ℃.
2.3. Cell transfection
For experimentation, HUVEC cells were in vitro seeded into 24-well plates, allowed to grow to 80%-90% confluence, and transfected by plasmids (pGV248/control-shRNA and pGV248/ ANXA2-shRNA) with DNA Transfection Reagent (Invitrogen,USA) according to the manufacturer's instruction. At 48 h after transfection, cells were selected by culturing in the presence of 400 μg/mL of G418 (Sangon Biotech, China) for 2 wk. Individual G418-resistant monoclonals were obtained by performing a limiting dilution with subsequent proliferation in medium supplemented with 200 μg/mL of G418 to generate the stably transfected experimental(HUVEC/ANXA2-shRNA), control (HUVEC/control-shRNA)and mock (no plasmid) cell lines. The efficiency of infection was monitored by fluorescence microscopy.
2.4. Real-time PCR analysis
Total RNA was isolated from cells using an RNeasy kit(Invitrogen, USA). Single-stranded cDNA was synthesized from 1 μg total RNA using an oligo (dT) 18-mer as primer, and reverse transcription (Invitrogen, USA) in a final reaction volume of 25 μL. Quantitative real-time PCR was performed using the TaqMan method and Probe Real Master Mix (Tiangen Biotech, Beijing,China) in a 7500 real-time PCR System (Applied Biosystems Inc,CA). GAPDH was used as control. The following primers were used: i) ANXA2 forward, 5'-GTGAAGAGGAAAGGAACCGA-3' and reverse, 5'-CTTGATGCTCTCCAGCATGT-3'; and ii) GAPDH forward, 5'-GCCTTCCGTGTTCCTACC-3' and reverse, 5'-AGAGTGGGAGTTGCTGTTG-3'. The analysis was performed using the 2-△△Ctmethod and the relative value of ANXA2 mRNA expression was normalized to GAPDH gene expression.
2.5. Western blot analysis
Total protein was isolated from cultured cells with the use of an extraction kit (Sangon Biotech, China) according to the manufacturer's instructions. Each 20 mg of protein was separated by 15% SDS-PAGE and then transferred onto PVDF membranes and blocked with 3% BSA in Tris-buffer. Mouse anti-human monoclonal antibody (Cell Signaling Technology, USA) was used. Detection was performed with HRP-conjugated goat anti-mouse immunoglobulins and enhanced chemiluminescence (Clinx, China). Bands were subsequently visualised and analyzed by a ChemiScope5600 integrated chemiluminescence imaging system (Clinx, China). ANXA2 levels were presented as relative ratio (RR) and calculated using the formula for signal intensity (SI) of ANXA2 and GAPDH:RR = SIANXA2/SIGAPDH.
2.6. Cell proliferation assay
Cell proliferation was evaluated using a cell counting kit-8(Beyotime Institute of Biotechnology, China). Cells and blank controls were seeded in 96-well plates (2×103cells /well with 100 μL medium) and for 24 h. Next, 10 μL CCK-8 solution was added to the culture medium for 2 h and the absorbance (A490) was recorded by a microplate reader (BioTek, USA). This was repeated 3 times at various time points.
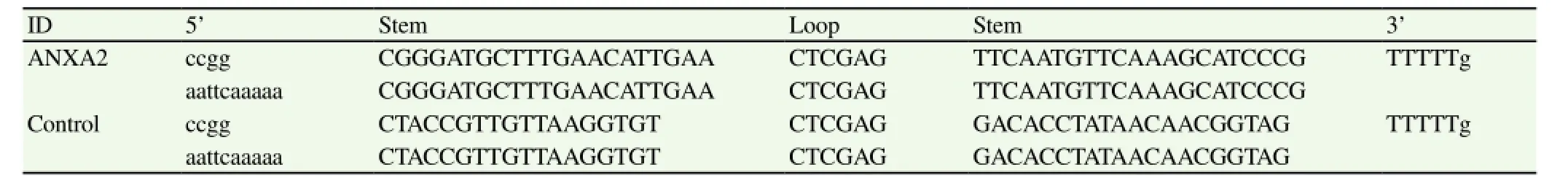
Table 1 Synthesized shDNAs encoding ANXA2-specific shRNA.
2.7. Enzyme-linked immunosorbent assay
HUVEC caspase-8, caspase-9 and caspase-3 levels were estimated with the use of enzyme-linked immunosorbent assay kits (Innovative Research, USA) according to the manufacturer's instructions. Colorimetric analysis was performed on a microplate reader (Bio-Rad Laboratories, USA) and the absorbance (A405) of the samples was measured to calculate the protein concentrations according to the standard curve generated.
2.8. FCM analysis of cell apoptosis
HUVEC/ANXA2-shRNA, HUVEC/control-shRNA and the mock HUVECs were stained relatively with FITC-conjugated annexin Ⅴand propidium iodide (PI) as supplied by an apoptosis detection kit(Beyotime, China). Cells were analyzed using a FACSCalibur (BD Biosciences, USA).
2.9. Statistical analysis
Statistical significance was assessed by the 2-tailed Student t test. P values of <0.05 were considered statistically different.
3. Results
3.1. ANXA2 expression in HUVEC cells is inhibited by shRNA in vitro
The silencing efficiency of ANXA2-specific shRNA in HUVEC cells reached approximately 82%. As shown in Figure 1b, the ratio of ANXA2 to GAPDH demonstrated that the expression levels of ANXA2 protein were significantly lower in the HUVEC/ANXA2-shRNA cells than in the HUVEC/control-shRNA cells and in the mock HUVECs (P<0.01). The levels detected in the HUVEC/ control-shRNA cells and HUVECs were not significantly different(P>0.05). It was suggested that the ANXA2 mRNA was markedly downregulated following transfection with stably expressing shRNA at the protein level.
3.2. ANXA2 mRNA expression after RNA interference in vitro
Quantitative PCR showed that the expression of ANXA2 mRNA in HUVECs transfected with shANXA2 was significantly inhibited(Figure 2), and the reduction of the ANXA2 mRNA expressions was 85% down (P<0.01). The mRNA relative levels were measured in sample rate/cutoff rate (S/CO).
3.3. Suppression of HUVEC proliferation
The effect of ANXA2 suppression on the proliferation following transfection with specific shRNA is shown in Figure 3. At 72 h after transfection, the cell proliferation ability in HUVEC/ANXA2-shRNA cells was significantly decreased (P<0.01 or P<0.05)compared with that in the HUVEC/control-shRNA cells and in the mock HUVECs. The result indicated that the growth of HUVEC/ ANXA2-shRNA cells was markedly inhibited in vitro.
3.4. Silencing ANXA2 enhances apoptosis of HUVECs
Cells were analyzed using an FACS by the staining method with FITC-conjugated annexin Ⅴand PI (Figure 4). The number of HUVEC/ANXA2-shRNA cells undergoing apoptosis was 41.9% and increased by 102.61% compared to the mock HUVECs(P<0.01), which suggests that ANXA2 silencing enhances apoptosis in HUVECs.
3.5. Silencing ANXA2 affects the levels of caspase-3,-8 and -9
Since the above results suggest that silencing of ANXA2 upregulates apoptosis, we intended to investigate the activity of apoptosis-related caspases. The activity levels of HUVEC caspase-3,-8 and -9 were estimated with the use of ELISA kits (Table 2). The levels of caspase-3, -8 and -9 in HUVEC/ANXA2-shRNA cells was 0.112, 0.061 and 0.053, respectively compared with 0.063, 0.021 and 0.035 in the mock HUVECs (P<0.01). It suggests that ANXA2 silencing increased the activity levels of HUVEC caspase-3,caspase-8 and caspase-9. Furthermore, the total protein was isolated from cultured cells and was analyzed by means of western blotting(Figure 5). The cleaved caspase-3, cleaved caspase-8 and cleaved caspase-9 were upregulated by 56.29%, 89.59% and 144.58% compared to that in mock HUVECs (P<0.01).

Table 2 Activity levels of caspase-3, -8 and -9 in HUVECs.
4. Discussion
ANXA2 is a member of the annexin family of proteins and exists either in monomeric form or as a heterotetramer containing two light chains of S100A10/p11 and two chains of ANXA2, which has recently drawn attention for its ability to regulate multiple key processes in cells[21-23]. In the present study, we focus on the effects of silencing ANXA2 by shRNA on human umbilical vein endothelial cells (HUVECs) in vitro.
shRNA is a sequence of RNA that makes a tight hairpin turn that can be used to silence target gene expression via RNA interference (RNAi)[24,25]. Expression of shRNA in cells is typically accomplished by delivery of plasmids or through viral or bacterial vectors[26]. In this work, the shRNA targeting ANXA2 was designed and cloned into double marked lentivirial vector GV248 for RNAi to generate the recombinant expression plasmids, which were infected into HUVEC resulting in the stably transfected experimental(ANXA2-shRNA) cell line, HUVEC/ANXA2-shRNA, with an inhibition rate 91.89% of ANXA2 expression compared to the mock and the control HUVECs.
Our data showed that ANXA2 silencing cell strain obviously presented a lower cell proliferation activity compared to the control and mock HUVECs. In addition, FACS analysis indicated that the HUVEC/ANXA2-shRNA cells undergoing apoptosis increased by 102.61% compared to the mock HUVECs (P<0.01), which is in agreement with previous research. How ANXA2 affects cell apoptosis? Huang[27] reported that ANXA2 was involved in P53-mediated apoptosis in that ANXA2 levels decreased significantly in P53 induced cell apoptosis. Madureira et al[28-31] revealed that ANXA2 could protect DNA from degradation. These results ggested that ANXA2 may affect the P53 expression and its tivity, and the down proteins such as Bcl2, Bax, and caspase mily to inhibit cell apoptosis. In this paper, we have also confirmed is viewpoint. The activated cleaved caspase-3, cleaved caspase-8 d cleaved caspase-9 of the HUVEC/ANXA2-shRNA cells were pregulated evidently compared with that of the control and mock UVECs by 56.29%, 89.59% and 144.58% (P<0.01).
Collectively, the results revealed a link between the level of ANXA2 expression and cell apoptosis, which suggested that the potential utility of ANXA2 as a predictive biomarker for detecting angiogenesis and that ANXA2 might be used in the treatment of neovascularization disorders as a therapeutic target of molecularbased strategies.
Conflict of interest statement
We declare that we have no conflict of interest.
[1] Makris GC, Makris MC, Wilmot VV, Geroulakos G, Falagas ME. The role of infection in carotid plaque pathogenesis and stability: the clinical evidence. Curr Vasc Pharmacol 2010; 8(6): 861-872.
[2] Waisman DM. Annexin Ⅱ tetramer:structure and function. Mol Cell Biochem 1995; 149(1): 301-322.
[3] Hitchcock JK, Katz AA, Schäfer G. Dynamic reciprocity: the role of annexin A2 in tissue integrity. J Cell Commun Signal 2014; 8(2): 125-133.
[4] Luo M, Hajjar KA. Annexin A2 system in human biology: cell surface and beyond. Seminars Thrombosis Hemostasis 2013; 39(4). Doi: 10.1055/ s-0033-1334143
[5] Zhang X, Liu S, Guo C, Zong J, Sun MZ. The association of annexin A2 and cancers. Clin Transl Oncol 2012; 14(9): 634-640.
[6] Madureira PA, O'Connell PA, Surette AP, Miller VA, Waisman DM. The biochemistry and regulation of S100A10: a multifunctional plasminogen receptor involved in oncogenesis. J Biomed Biotechnol 2012; 2012:353687.
[7] Hedhli N, Falcone DJ, Huang B, Cesarman-Maus G, Kraemer R, Zhai H,et al. The annexin A2/S100A10 system in health and disease: emerging paradigms. J Appl Biomed 2012; 10(1155): 406273.
[8] Flood EC, Hajjar KA. The annexin A2 system and vascular homeostasis. Vasc Pharmacol 2011; 54(3): 59-67.
[9] Madureira PA, Surette AP, Phipps KD, Taboski MA, Miller VA, Waisman DM. The role of the annexin A2 heterotetramer in vascular fibrinolysis. Blood 2011; 118(18): 4789-4797.
[10] Surette AP, Madureira PA, Phipps KD, Miller VA, Svenningsson P,Waisman DM . Regulation of fibrinolysis by S100A10 in vivo. Blood 2011; 118 (11): 3172-3181.
[11] Lokman NA, Ween MP, Oehler MK, Ricciardelli C. The role of annexin A2 in tumorigenesis and cancer progression. Cancer Microenvironment 2011; 4(2): 199-208.
[12] Zhai H, Acharya S, Gravanis I, Mehmood S, Seidman RJ, Shroyer KR, et al. Annexin A2 promotes glioma cell invasion and tumor progression. J Neurosci 2011; 31(40): 14346-14360.
[13] Ohno Y, Izumi M, Kawamura T, Nishimura T, Mukai K, Tachibana M. Annexin Ⅱ represents metastatic potential in clear-cell renal cell carcinoma. Brit J Cancer 2009; 101(2): 287-294.
[14] Mohammad HS, Kurokohchi K, Yoneyama H, Tokuda M, Morishita A, Jian G, et al. Annexin A2 expression and phosphorylation are upregulated in hepatocellular carcinoma. Int J Oncol 2008; 33(6): 1157-1163.
[15] Chan MS , Chen SF , Felizola SJ , Wang L , Nemoto N , Tamaki K, et al. Correlation of tumor-infitrative lymphocyte subtypes alteration with neoangiogenesis before and after neoadjuvant chemotherapy treatment in breast cancer patients. Int J Biol Markers 2014; 3: e193-e203.
[16] O'Connell PA, Surette AP, Liwski RS, Svenningsson P, Waisman DM. S100A10 regulates plasminogen-dependent macrophage invasion. Blood 2010; 116(7): 1136-1146.
[17] Sharma M, Ownbey RT, Sharma MC. Breast cancer cell surface annexinⅡ induces cell migration and neoangiogenesis via tPA dependent plasmin generation. Exp Mol Pathol 2010; 88(2): 278-286.
[18] Krone KA, Allen KL, McCrae KR. Impaired fibrinolysis in the antiphospholipid syndrome. Curr Rheumatol Rep 2010; 12(1): 53-57.
[19] Jacovina AT, Deora AB, Ling Q, Broekman MJ , Almeida D , Greenberg CB, et al. Homocysteine inhibits neoangiogenesis in mice through blockade of annexin A2-dependent fibrinolysis. J Clin Invest 2009;119(11): 3384-3394.
[20] Chen J, Smith LEH. Retinopathy of prematurity. Angiogenesis 2007;10(2): 133-140.
[21] Hajjar KA, Guevara CA, Lev E, Dowling K, Chacko J. Interaction of the fibrinolytic receptor, annexin Ⅱ, with the endothelial cell surface. Essential role of endonexin repeat 2. J Biol Chem 1996; 271(35): 21652-21659.
[22] He KL, Sui G, Xiong H, Broekman M J, Huang B, Marcus AJ, et al. Feedback regulation of endothelial cell surface plasmin generation by PKC-dependent phosphorylation of annexin A2. J Biol Chem 2011;286(17): 15428-15439.
[23] Peterson EA, Sutherland MR, Nesheim ME, Pryzdial EL. Thrombin induces endothelial cell-surface exposure of the plasminogen receptor annexin 2. J Cell Sci 2003; 116(Pt 12): 2399-2408.
[24] Kim JY. Regulation of short-distance transport of RNA and protein. Curr Opin Plant Biol 2005; 8(1): 45-52.
[25] Fire A, Xu S, Montgomery M, Kostas S, Driver S, Mello C. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature 1998; 391(6669): 806-811.
[26] Bernstein E, Hammond SM, Beach D, Hannon GJ. An RNA-directed nuclease mediates post-transcriptional gene silencing in Drosophila cells. Nature 2000; 404(6775): 293-296.
[27] Huang Y, Jin Y, Yan CH, Yu Y, Bai J, Chen F, et al. Involvement of annexin A2 in p53 induced apoptosis in lung cancer. Mol Cell Biochem 2008; 309(1-2):117-123.
[28] Madureira PA, Hill R, Lee PW, Waisman DM. Genotoxic agents promote the nuclear accumulation of annexin A2: role of annexin A2 in mitigating DNA damage. PLoS One 2012; 7(11): e50591.
[29] Wang YX, Lv H, Li ZX, Li C, Wu XY. Effect of shRNA mediated down-regulation of annexin A2 on biological behavior of human lung adencarcinoma cells A549. POR 2012; 18(2): 183-190.
[30] Zhang J, Guo B, Zhang Y, Cao J, Chen T. Silencing of the annexin Ⅱgene down-regulates the levels of S100A10, c-Myc, and plasmin and inhibits breast cancer cell proliferation and invasion. Saudi Med J 2010;31(4): 374-381.
[31] Sharathchandra A, Lal R, Khan D, Das S. Annexin A2 and PSF proteins interact with p53 IRES and regulate translation of p53 mRNA. RNA Biol 2012; 19(12): 1429-1439.
15 August 2015
Shi-Hong Zhao, Ph.D, Chief Physician, Department of Ophthalmology, Affiliated Changhai Hospital of Second Military Medical University,Shanghai, 200433, China.
Tel: +86-2181873555
E-mail: zhaosh2000@sina.com
Foundation project: This project has been funded by the National Natural Science Foundation of China (No.81271017, No.81470652).
杂志排行
Asian Pacific Journal of Tropical Medicine的其它文章
- Demographic, socioeconomic and environmental changes affecting circulation of neglected tropical diseases in Egypt
- Phenolic profile and biological potential of Endopleura uchi extracts
- Roots extracts of Adenophora triphylla var. japonica improve obesity in 3T3-L1 adipocytes and high-fat diet-induced obese mice
- Anti TB drug resistance in Tanga, Tanzania: a cross sectional facility base prevalence among pulmonary TB patients
- In vitro inhibitory effects of plumbagin, the promising antimalarial candidate, on human cytochrome P450 enzymes
- Vibrio spp. from Macrobrachium amazonicum prawn farming are inhibited by Moringa oleifera extracts
