缩二脲钴配合物的合成、表征与热分解(英文)
2015-10-20王美玲臧晴钟国清
王美玲 臧晴 钟国清
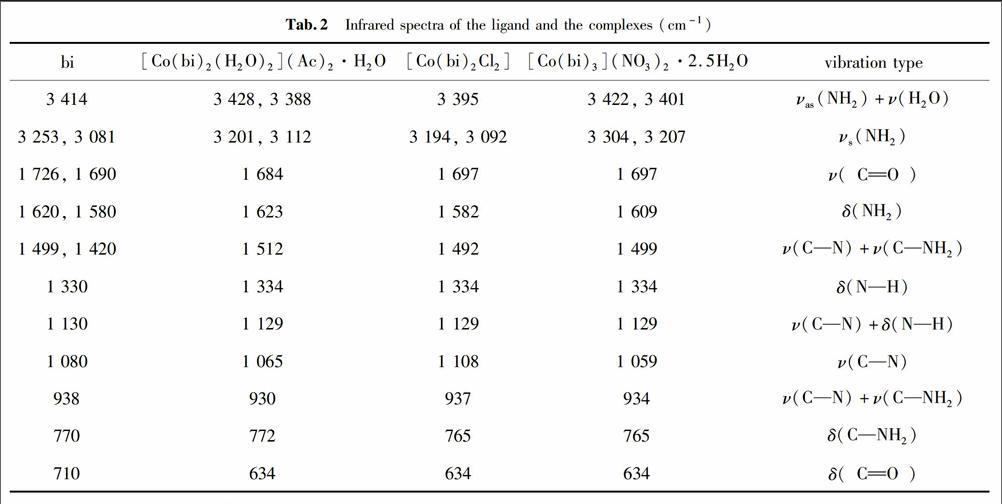
摘要在甲醇溶液中,以乙酸钴、氯化钴、硝酸钴和缩二脲为原料合成了3种结构不同的缩二脲钴配合物.通过元素分析、红外光谱、X射线粉末衍射和热分析对产物进行了表征,其化学组成为[Co(bi)2(H2O)2](Ac)2·H2O (1),[Co(bi)2Cl2] (2)和[Co(bi)3](NO3)2·2.5H2O (3) (bi = NH2CONHCONH2).配合物1中每个Co2+与2个缩二脲分子中的4个羰基氧原子和2个水分子中的氧原子配位,配合物2中每个Co2+与2个缩二脲分子中的4个羰基氧原子和2个氯原子配位,而配合物3中每个Co2+与6个全部来自缩二脲分子的羰基氧原子配位,均形成了配位数为6的配合物.配合物1和3的热分解过程包括失水和配体的分解,而配合物2的热分解过程只是配体的分解过程,最后完全分解形成氧化钴.
关键词缩二脲;钴配合物;表征;热分解
Biuret is a kind of important chelating ligand, and can act as an O,O′bidentate neutral ligand or N,N′bidentate anionic ligand binding to metal ions, such as scandium(Ⅲ)[1], copper(Ⅱ)[2], samarium(Ⅲ)[3], thorium(IV)[4] and so on. In recent years the chemistry of biuret and related compounds attracts increasing attention. A novel biscyclometalated complex named [Ir2(mbiuretato N,N′:O,O′)(ptpy)4] was obtained and studied[5]. Whats more, a research in thermal degradation kinetics of biuretformaldehyde polymeric ligand was done lately[6]. In animal husbandry, biuret is an excellent feed additive for ruminants, it has the advantages of good palatability, low toxicity and easy to digest, and is safer than other non protein nitrogen feed additives, such as urea. In medicine, biuret can be used for the preparation of hypnotics and sedatives and it is also good at lowering the blood pressure. In chemical industry, biuret can be utilized as raw materials for synthesis of paints, lubricants, and coatings. At home, the complexes of biuret ligand have been rarely reported[79], especially in the comparison of different anions in the synthesis of Co(Ⅱ) complexes. The biuret complexes of trace elements as feed additives for ruminants can play a dual role of essential trace elements and non protein nitrogen nutrition supplements. Here we report three Co(Ⅱ) complexes synthesized with biuret and three different cobalt salts.
1Experimental
1.1Materials and physical measurements
All chemicals purchased were of analytical reagent grade and used without further purification. Cobalt acetate tetrahydrate, cobalt chloride hexahydrate, cobalt nitrate hexahydrate and biuret were purchased from Sinopharm Chemical Reagent Co. Ltd. of Shanghai.
Elemental analyses for C, H and N in the complexes were measured on a Vario EL CUBE elemental analyzer, and the content of cobalt was determined by EDTA complexometric titration with murexide as indicator. IR spectra were obtained with KBr pellets on a Nicolet 5700 FTIR spectrophotometer in the range of 4 000~200 cm-1. The powder Xray diffraction measurements were recorded on a D/maxⅡ Xray diffractometer in the diffraction angle range of 3~80°. The thermogravimetric analysis data were obtained using a SDT Q600 thermogravimetry analyzer in the air atmosphere in the temperature range of 20~800 ℃ with a heating rate of 10 ℃ min-1.