微/纳米ZnO绒球的制备及其在自然光下催化降解有机染料性能
2015-09-03佟拉嘎刘金艳王岑晨
佟拉嘎 刘金艳 王岑晨 荣 华 李 巍
(北京石油化工学院化学工程学院,北京 102617)
微/纳米ZnO绒球的制备及其在自然光下催化降解有机染料性能
佟拉嘎 刘金艳 王岑晨 荣 华 李 巍*
(北京石油化工学院化学工程学院,北京 102617)
·以谷氨酸氟硼酸(GluBF4)离子液体水溶液为反应介质,以物质的量比为1∶6的二水合醋酸锌[Zn(Ac)22H2O]和氢氧化钠为原料,室温下制备前驱体,再微波辅助加热制备了纳米氧化锌粉体,获得了纳米结构微米尺寸纳米ZnO绒球.利用场发射扫描电镜(FESEM)、X射线衍射(XRD)、比表面(BET)、能量色散·谱(EDS)等对产物进行了表征.所得产物为六方晶系纤锌矿结构,粉体粒径20.4 nm,绒球比表面积为28.3 m2g–1,产物纯度较高,收率95.3%.同时探讨了纳·米ZnO绒球生成的可能机理.该纳米材料在日光下显示较高的光催化活性和稳定性.分别配制浓度为10 mgL–1的100 mL甲基橙(MO)和甲基紫(MV)水溶液,30 mg纳米氧化锌为光催化降解催化剂,太阳光激发下5 h脱色率分别达到74.3%和96.9%;溶液总有机碳(TOC)含量随光降解的进行缓慢下降;光催化剂重复利用5次,催化剂形貌不变、颜色不变,质量基本未发生变化.
氨基酸离子液体; 微波辅助加热; 微/纳米ZnO; 有机污染物; 光催化降解
1 Introduction
Nano-ZnO is a novel semiconductor material with a wide bandgap(3.37 eV) and large exciton binding energy(60 meV).1This material has attracted much attention as it shows promise in a variety of applications,including photocatalysis,cosmetics,rubbers,paints,ceramics,and optoelectronic devices because of its unique optical,electrical and magnetic behavior.2–9The preparation and control of particle size and morphology of these powders are critical as they exert significant effects on the properties of these materials.There are numerous methods to prepare nano-ZnO,which yields a variety of morphologies.10,11However,there are drawbacks to these synthesis methods,including the requirement of specialized instruments or expensive starting materials,causing a lot of pollution,agglomeration of the products,or low yields.These problems have limited the applications of nano-ZnO materials.Research in this field remains focused on the exploration of stable and repeatable synthetic methods that are amenable to large-scale preparation.
Ionic liquids(ILs) are highly polarizable and can therefore absorb microwave radiation,resulting in homogeneous heating.Thus,microwave-heated ILs can be used to synthesize nano-ZnO materials.This method combines high reaction speeds,mild reaction conditions,high yields,and environmentally friendly solvents.Wang and Zhu12reported the microwave-assisted preparation of flower- and needle-like nano-ZnO in imidazolium IL solution at high temperatures.Imidazolium ILs are known to perform emulsification,directional induction,and nucleation in the preparation of micro/nano ZnO powder with different morphologies.13–21However,the synthetic and post treatment processes of imidazolium ILs are complex and expensive.Amino acid-based ILs show potential in the preparation of nano-ZnO22as they can be obtained using simple and green synthetic routes from readily available raw materials.23Additionally,amino acid ILs possess excellent thermal and chemical stability.
Nano-ZnO materials are excellent photocatalysts,24
particularly for the photodegradation of organic contaminants.Yu and coworkers25used nano-ZnO in the photodegradation of Rhodamine B in solution.Recently,Zhu2and Venkatesha10et al.reported the catalytic effects of rod- and flower-like nano-ZnO in the photodegradation of dye solutions,with the mechanism being explored in detail.ZnO has been shown to have both a higher photocatalytic reactivity and quantum yield when compared with TiO2in the degradation of organic contaminants that are difficult to biodegrade.10,26,27
In this work we report the preparation of nano-ZnO pompons in glutamic acid fluoborate IL solutions.The nano-ZnO-mediated photocatalytic degradation of methyl orange(MO) and methyl violet(MV) solutions under sunlight was examined.Additionally,the stability of the nano-ZnO pompons was examined.
2 Experimental
2.1 Reagents
Glutamic acid(purity>99%,Beijing Biodee Biotechnology Co.,Ltd.,China),fluoboric acid(40% aq.,Tianjin Fuchen Chemical Reagent Factory,China),zinc acetate dehydrate(AR,Sinopharm Chemical Reagent Beijing Co.,Ltd.,China),sodium hydroxide and absolute ethyl alcohol(AR,Beijing Chemical Works,China),MO and MV(industrial grade,Beijing Chemical Works,China).All these chemicals were used without further purification.Deionized water was used in all experiments.
2.2 Characterization
Both amino acid ILs and nano-ZnO were prepared using a XH-100A microwave reactor(Beijing Xianghu Science and Technology Development Limited Company,China).The morphology and elemental composition of nano-ZnO were determined using a Quanta400F scanning electron microscope(FEI,USA) and Apollo 10+ energy spectrometer(EDAX,USA).The specific area of nano-ZnO was determined using an ASAP2020 specific surface area determination meter(Micromeritics,USA).The crystal structure of nano-ZnO was examined using XD-2 X-ray diffractometer(Beijing Puxitongyong Instrument Co.,Ltd.,China).The photodegradation of organic dyes,using nano-ZnO pompons,was examined using a UV-2700 ultraviolet-visible(UV-Vis) spectrophotometer(Shimadzu,Japan),a DGY-1 photochemical reaction instrument(Nanjing Duozhu Science and Technology Development Limited Company,China) and a LIQUI TOC/TN analyzer(Analytik Jena AG,Germany).
2.3 Preparation of nano-ZnO
Sodium hydroxide(6.0 g) was dissolved in deionized water(40 mL) then maintained at 25°C in a water bath.Glutamic acid fluoborate ionic liquid(2.0 g) was dissolved in deionized water(20 mL) then added to the sodium hydroxide solution while stirring.Zinc acetatedihydrate(5.5 g) was dissolved in deionized water(40 mL) then added to the mixture and kept at 25°C for 20 min.The resulting white emulsion was added to the microwave reactor and heated to 80°C for 10 min.The reaction mixture was allowed to cool then left for 20 h.The supernatant was removed and the white precipitate was washed with 30 mL deionized water(three times) and absolute ethyl alcohol(four times).The precipitate was dried at 120°C for 5 h to yield white powder(1.94 g,95.3%).
2.4 Photocatalytic degradation of MO and MV using ZnO
Nano-ZnO(30 mg) w·as added to separate Erlenmey·er flasks containing MO(10 mgL–1,100 mL) and MV(10 mgL–1,100 mL).The flasks were sealed and left in direct sunlight at ambient temperature(~15°C,mid-November in Beijing) without agitation.Samples(3 mL) were taken every 1 h and centrifuged immediately to remove the catalyst.The UV-Vis absorption profile of the supernatant was recorded.The catalytic activity was determined by plotting the change in intensity(Dt) of the absorption of the dye solution over time.Dtwas calculated as follows:Dt=(A0– At)/A0× 100%,where A0is initial absorbance value of dye solution,and Atis absorbance value of dye solution after time t.
3 Results and discussion
3.1 Morphology and structure of nano-ZnO
The morphology of the nano-ZnO was examined using scanning electron microscopy(Fig.·1).These images indicated that the reaction of NaOH,Zn(Ac)22H2O,and glutamic acid fluoborate ionic liquid yielded micro/nano ZnO with a morphology that resembles pompons.The particle diameters were distributed between 1.6 and 3.0 μm.Small spherical particles were observed at low magnification,consistent with the foliated packing observed at higher magnification.Flocculent nano-ZnO was observed among the foliated nano structure,resulting in a porous structure with a dense core and a loose surface.Th·ese clusters gave a specific surface area as large as 28.3 m2g–1.Razali et al.28reported spherical micro/nano ZnO powder by heating the precursors for extended times under high temperatures(150°C) in a diethanolamine solution.They reported microspheres with a loose core and a dense surface,suggesting that the preparation conditions used can lead to distinct differences in the growth mechanisms and accumulations of the nanoparticles.
The XRD profile of the nano-ZnO pompons is shown in Fig.2.The quantity and location of the peaks are identical to those of ZnO in standard JCPDS card(No.80-0075),revealing the hexagonal wurtzite structure.The average diameter of the particles was calculated using the Scherrer equation and was found to be 20.4 nm.This indicated that micron-sized pompons consisted of aggregations of nanostructures.The energy spectrum of the nano-ZnO pompons did not contain peaks of other elements,indicating that the purity of the material was high(Fig.2,inset).
As a comparison,we synthesized nano-ZnO under identical conditions(Section 2.3) without the addition of the GluBF4IL.The product was found to be unifor·mly needle-like(Fig.3) with a specific surface area of 5.33 m2g–1.The needle length was 900–950 nm with a root diameter of 90–100 nm and a tip diameter of 5–10 nm.The XRD results showed that the nano needles also displayed a hexagonal wurtzite structure.
3.2 Growth mechanism of nano-ZnO pomponinduced by GluBF4ionic liquid
The resultant morphology of hot-solution preparations of nano-ZnO is dependent upon a number of parameters.These include type of solvent,solution composition,concentration,pH value,agitation speed,reaction time,and temperature.The removal of the GluBF4IL from the synthesis we have reported,and subsequent generation of a uniform needle-like morphology,indicated the key role played by the ionic liquid in the formation of the pompons.

Fig.1 Scanning electron microscopy(SEM) images of nano-ZnO pompons at different magnifications

Fig.2 XRD and energy spectrum diagram of ZnO pompons

Fig.3 SEM image of nano-ZnO needles
A possible growth mechanism involves the Zn2+formingunder the conditions of high pH value,which then rapidly decomposes into ZnO when heated to 80°C.ZnO molecules may then agglomerate to form seeds of different sizes under stirring.Directional induction of the cations in the ionic liquid may cause the seeds to grow into foliated nanostructures of different sizes.Foliated ZnO particles and seeds may thencongregate in large quantities around cations in the IL by the Coulomb interaction to eventually form ZnO pompons.This is shown schematically in Fig.4.
Thus,the role of the IL in the preparation of nano-ZnO pompons can be summarized as follows:(i) the high concentration of ions in solution enables nucleation of crystals and orients the ZnO seeds,facilitating their organized aggregation;(ii) the low surface tension of the IL helps emulsification of particles in the solution,allowing the ZnO seeds to aggregate evenly and generate a structure with consistent morphology;and(iii) the weak Coulomb interaction between ions leads to weak restrictive power of nuclear ions on the outer and dermal particles,attributing to the pompon structure with high particle density in the core area and low on the surface,and so the outside surface of the pompon shows loose and porous structure inlayed with slices.The topography of the grains in broken pompons,and a half pompon that grew along the vessel wall are shown in Fig.5 and Fig.6,respectively.These images demonstrate that the particles were gathered densely at the core while only loosely on the surface.
3.3 Photocatalyzed degradation of MO and MV catalyzed by nano-ZnO pompons
The UV-Vis spectrum of the nano-ZnO pompons displayed a strong absorption around 370 nm(Fig.7).The band gap of the material was calculated to be 3.27 eV from its absorption band edge,which is approximately 0.1 eV lower than the literature value.1
The time-dependent changes in the absorption profiles of the dyes(MO and MV),when exposed to nano-ZnO pompons in sunlight,are shown in Fig.8.The absorption maxima for MO and MV occurred at 463 and 577 nm,respectively.The high energy peaks(227 and 272 nm for MO and 207,245,and 298 nm for MV) are attributed to the aromatic ring structures.Dilute solutions of MO and MV containing a catalytic amount of nano-ZnO did not change significantly when kept in the dark or low light at ambient temperature.Additionally,dilute solutions of MO and MV that did not contain nano-ZnO were stable long-term when stored in sunlight at ambient temperature,showing no significant change in the absorption profile.However,following addition of a small amount of nano-ZnO into the solution,decoloration occurred rapidly in direct sunlight,demonstrating that nano-ZnO catalyzed the degradation of these organic contaminants.The decrease of the main absorption peak of MO reached 74.3% after 5 h while that of MV reached 96.9%.These experiments were performed under sunlight.However,when UV light was used,the rate of degradation increased dramatically.The changes observed in the UVVis absorption spectrum of MO over time,when exposed to UV light in the presence of nano-ZnO pompons,are shown in Fig.9.The decrease in absorbance reached 97.0% after only 30-min exposure.

Fig.4 Formation of micro/nano ZnO pompons

Fig.5 SEM image of grains at the core and on the surface of pompons

Fig.6 SEM image of half pompon along the vessel wall
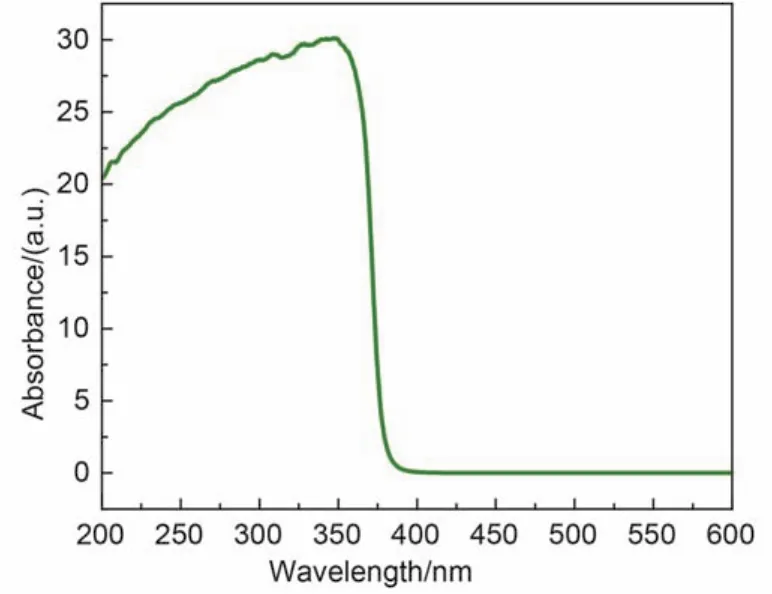
Fig.7 Ultraviolet-visible spectrum of nano-ZnO pompons
The rate of degradation of MO was slower than that of MV(Fig.10).The large overlap in the absorption profile of MO with nano-ZnO in the near-UV and blue region lowered the amount of shortwave photons absorbed by nano-ZnO per unit time that were required to stimulate its catalytic activity.
Comparison experiments between the degradation catalyzed by nano-ZnO pompons and nano-ZnO needles showed that thedegradation efficiency by the former was higher than the latter,which was in turn higher than that of commercially available bulk ZnO(Fig.10).These results showed the significant influence of the morphology of the ZnO on its photocatalytic reactivity.The morphology of the nano-ZnO remained unchanged with no signs of adsorption of other species even after 5 cycles of photodegradation.The mass of the catalyst remained constant after 5 cycles in 5 different MO solutions and lost 3.2% mass in MV solution,proving that this nano-ZnO catalyst was reusable.Zhu and co-workers2have studied the photodegradation of various dyes under UV irradiation using rod-like nano-ZnO.They reported that the catalyst required at least 7 h to see satisfactory degradation.Venkatesha et al.10studied the catalytic effects of flower-like nano-ZnO and reported a degradation time above 6 h.The nano-ZnO pompons we have reported display higher photocatalytic reactivity than nano-ZnO powders with different morphologies.

Fig.8 Ultraviolet-visible spectra of MO(a) and MV(b) with the change of photodegradation time catalyzed by nano-ZnO pompons

Fig.9 Ultraviolet-visible absorption spectra of MO catalyzed by nano-ZnO pompons under ultraviolet light

Fig.10 Relationship between the decoloration rate and time when ZnO with different morphologies used to degrade MO or MV solutions

Fig.11 Variation of the TOC content in the dye solutions along with the degradation time
Yu et al.25studied the degradation mechanism of organic dyes catalyzed by nano-ZnO using a 250-W high-voltage mercury lamp.According to Yu's results,electrons within ZnO particles were excited from the valence band to the conduction band to give photoelectrons and photoholes.These highly active photoexcited species were captured by other molecules including dye molecules,which triggered a series of chain reactions predominated by radical transfer.Hydroxyl radicals,peroxy radical anions,hydrogen peroxide radicals,and other highly active radicals were generated,which upon reaction with the dye molecules turned them into nontoxic and colorless small molecules.This was consistent with the observed decrease in intensity of all absorption peaks in the dye solutions and the fact that there were no new peaks observed in the short wavelength region.This indicated that the multi-aromatic ring structures of the dye molecules were not degraded into single ring aromatic fragments such as benzene and substituted benzene.That is,the stable dye molecules were degraded into small molecular products that showed no optical activities in the wavelength region observed.
The pH values of the dye solutions before and after the pho-todegradation were 7.55 and 6.73 for MO,and 7.32 and 6.76 for MV,which indicated that acidic small molecules were generated,including CO2.To follow the variation of the organic components during the degradation process,a total organic carbon(TOC) analysis was performed,the results of which are shown in Fig.11.This analysis indicated that the large organic molecules were gradually degraded into small inorganic molecules.
4 Conclusions
Nano-ZnO particles,resembling pom·pons,were synthesized from zinc acetatedihydrate [Zn(Ac)22H2O] and sodium hydroxide in a glutamic acid fluoborate(GluBF4) IL with microwave heating.The highly pure particles were obtained in high yields and possessed a uniform morphology and size.Addit·ionally,they possessed a high specific surface area of 28.3 m2g–1.The nano-ZnO was found to have a hexagonal wurtzite structure.The nano-ZnO pompons displayed high photocatalytic reactivity and photostability when exposed to sunlight.When exposed to sunlight for 5 h,the decrease in absorbance values for methyl orange and methyl violet aqueous solutions containing nano-ZnO reached 74.3% and 96.9%,respectively.After 5 cycles,the morphology of the catalyst remained unchanged while its mass was not significantly affected.This work shows the potential of these nano-ZnO pompons for the treatment of environmental pollutants.
(1)Wu,J.J.;Liu,S.C.Adv.Mater.2002,14,215.
(2)Zhu,Z.F.;Yang,D.;Liu,H.Adv.Powder Technol.2011,22,493.doi:10.1016/j.apt.2010.07.002
(3)Krishnakunar,T.;Jayaprakash,R.;Pinna,N.;Singh,V.N.;Mehta,B.R.;Phani,A.R.Mater.Lett.2009,63,242.doi:10.1016/j.matlet.2008.10.008
(4)Zhang,L.X.;Zhao,J.H.;Zheng,J.F.;Li,L.;Zhu,Z.P.Appl.Surf.Sci.2011,258,711.doi:10.1016/j.apsusc.2011.07.116
(5)Hamedani,N.F.;Mahjoub,A.R.;Khodadadi,A.A.;Mortazavi,Y.Sensors Actuators B 2011,156,737.doi:10.1016/j.snb.2011.02.028
(6)Li,X.Q.;Fan,Q.F.;Li,G.L.;Huang,Y.H.;Gao,Z.;Fan,X.M.;Zhang,C.L.;Zhou,Z.W.Acta Phys.-Chim.Sin.2015,31(4),783. [李湘奇,范庆飞,李广立,黄瑶翰,高 照,范希梅,张朝良,周祚万.物理化学学报,2015,31(4),783.] doi:10.3866/ PKU.WHXB201502062
(7)Du,H.Y.;Wang,J.;Qiao,Q.;Sun,Y.H.;Shao,Q.;Li,X.G.Acta Phys.-Chim.Sin.2015,31(4),800. [杜海英,王 兢,乔 俏,孙炎辉,邵 强,李晓干.物理化学学报,2015,31(4),800.] doi:10.3866/PKU.WHXB201501283
(8)Han,X.G.;He,H.Z.;Kuang,Q.;Zhou,X.;Zhang,X.H.;Xu,T.;Xie,Z.X.;Zheng,L.S.J.Phys.Chem.C 2009,113,584.doi:10.1021/jp808233e
(9)Huang,J.F.;Xia,C.K.;Cao,L.Y.;Zeng,X.R.Mater.Sci.Eng.B 2008,150,187.doi:10.1016/j.mseb.2008.05.014
(10)Venkatesha,T.G.;Nayaka,Y.A.;Viswanatha,R.;Vidyasagar,C.C.;Chethana,B.K.Powder Technol.2012,225,232.doi:10.1016/j.powtec.2012.04.021
(11)Shi,R.X.;Yang,P.;Dong,X.B.;Ma,Q.;Zhang,A.Y.Appl.Surf.Sci.2013,264,162.doi:10.1016/j.apsusc.2012.09.164
(12)Wang,W.W.;Zhu,Y.J.Inorg.Chem.Commun.2004,7,1003.doi:10.1016/j.inoche.2004.06.014
(13)Hou,X.M.;Zhou,F.;Sun,Y.B.;Liu,W.M.Mater.Lett.2007,61,1789.doi:10.1016/j.matlet.2006.07.133
(14)Movahedi,M.;Kowsari,E.;Mahjoub,A.R.;Yavari,I.Mater.Lett.2008,62,3856.doi:10.1016/j.matlet.2008.05.002
(15)Goharshadi,E.K.;Ding,Y.L.;Nancarrow,P.J.Phys.Chem.Solids 2008,69,2057.doi:10.1016/j.jpcs.2008.03.002
(16)Wang,L.;Xu,S.Z.;Li,H.J.;Chang,L.X.;Su,Z.;Zeng,M.H.;Wang,L.N.;Huang,Y.N.J.Solid State Chem.2011,184,720.doi:10.1016/j.jssc.2011.01.032
(17)Chen,C.Y.;Li,Q.;Nie,M.;Lin,H.;Li,Y.;Wu,H.J.;Wang,Y.Y.Mater.Res.Bull.2011,46,888.doi:10.1016/j.materresbull.2011.02.017
(18)Sanes,J.;Carrion,F.J.;Bermudez,M.D.Appl.Surf.Sci.2009,255,4859.doi:10.1016/j.apsusc.2008.12.023
(19)Lee,K.M.;Chiu,W.H.;Hsu,C.Y.;Cheng,H.M.;Lee,C.H.;Wu,C.G.J.Power Sources 2012,216,330.doi:10.1016/ j.jpowsour.2012.05.079
(20)Min,Y.L.;Zhang,K.;Chen,L.H.;Chen,Y.C.;Zhang,Y.G.Diamond &Related Materials 2012,26,32.doi:10.1016/ j.diamond.2012.04.003
(21)Sabbaghan,M.;Shahvelayati,A.S.;Bashtani,S.E.Solid State Sci.2012,14,1191.doi:10.1016/j.solidstatesciences.2012.05.034
(22)Tong,L.;Liu,Y.;Rong,H.;Gong,L.Mater Lett.2013,112,5.doi:10.1016/j.matlet.2013.08.119
(23)Rong,H.;Li,W.;Chen,Z.Y.;Wu,X.M.J.Phys.Chem.B 2008,112,1451.doi:10.1021/jp0774591
(24)Lu,F.;Chen,Y.N.;Liu,N.;Cao,Y.Z.;Feng,L.Chem.J.Chin.Univ.2014,35(2),368. [卢 飞,陈雨宁,刘 娜,曹莹泽,冯 琳.高等学校化学学报,2014,35(2),368.]
(25)Yu,D.Z.;Cai,R.X.;Liu,Z.H.Spectrochim.Acta Part A 2004,60,1617.doi:10.1016/j.saa.2003.09.003
(26)Daneshvar,N.;Salari,D.;Khatee,A.R.J.Photochem.Photobiol.A 2004,162(2/3),317.
(27)Su,B.T.;Hu,C.L.;Zuo,X.W.;Lei,Z.Q.Chin.J.Inorg.Chem.2010,26(1),96. [苏碧桃,胡常林,左显维,雷自强.无机化学学报,2010,26(1),96.]
(28)Razali,R.;Zak,A.K.;Majid,W.H.A.;Darroudi,M.Ceram.Int.2011,37,3657.doi:10.1016/j.ceramint.2011.06.026
Preparation of Micro/Nano ZnO Pompons and Their Catalytic Activity for the Solar Degradation of Organic Dyes
TONG La-Ga LIU Jin-Yan WANG Cen-Chen RONG Hua LI Wei*
(College of Chemical Engineering,Beijing Institute of Petro-chemical Technology,Beijing 102617,P.R.China)
A novel,high-yielding synthesis of micro/nano ZnO pompons using glutamic acid fluo·borate(GluBF4) ionic liquid is reported.The precursor was prepared with zinc acetatedihydrate [Zn(Ac)22H2O] and sodium hydroxide(molar ratio=1∶6) as starting materials in an aqueous solution of the GluBF4ionic liquid at room temperature,which was then heated by microwave to form nano-ZnO powder.The ZnO pompons were characterized using field emission scanning electron microscopy(FESEM),X-ray diffraction(XRD),specific Brunauer-Emmett-Teller(BET) surface area method,and energy dispersive spectrometry(EDS).The product displayed a hexagonal wurtzite structu·re.The pompon diameter was determined to be 20.4 nm,with a pompon specific surface area of 28.3 m2g–1.A possible mechanism for the formation of the nano-ZnO pompons is discussed.The ZnO pompons displayed high photocatalytic reactivity and photostability under sunlight.Aqueous solutions of methyl orange(MO) and methyl violet(MV) containing the ZnO pompons were exposed to sunlight and the decolorization rates were determined by monitoring the drop in color intensity.After 5 h,the solutions reached 74.3% and 96.9% degradation,respectively.The total organic carbon(TOC) content decreased as the photodegradation process occurred.The morphology,color,and weight of the ZnO pompons remained unchanged even after being reused five times.
Amino acid ionic liquid; Microwave heating; Micro/nano ZnO; Organic contaminant;Photocatalytic degradation
February 3,2015;Revised:May 11,2015;Published on Web:May 14,2015.
O644
icle]
10.3866/PKU.WHXB201505141 www.whxb.pku.edu.cn
*Corresponding author.Email:liwei77@bipt.edu.cn;Tel:+86-10-81292127.
The project was supported by the National Undergraduates Research Training Program,China(URT:201300078) and Breeding Project of Outstanding Academic Leaders of Beijing Institute of Petro-chemical Technology,China(BIPT-BPOAL-2014).
国家级大学生创新创业训练计划(URT:201300078)及北京石油化工学院优秀学科带头人培育计划(BIPT-BPOAL-2014)资助项目
© Editorial office of Acta Physico-Chimica Sinica
