高倍率性能纳微结构锂离子电池正极材料0.6Li2MnO3-0.4LiNi0.5Mn0.5O2的简易制备
2015-09-03史侠星廖世宣钟艳君钟本和刘郭孝东
史侠星 廖世宣 袁 炳 钟艳君 钟本和刘 恒 郭孝东,*
(1四川大学化学工程学院,成都 610065; 2四川大学材料科学与工程学院,成都 610065)
高倍率性能纳微结构锂离子电池正极材料0.6Li2MnO3-0.4LiNi0.5Mn0.5O2的简易制备
史侠星1廖世宣1袁 炳1钟艳君1钟本和1刘 恒2郭孝东1,*
(1四川大学化学工程学院,成都 610065;2四川大学材料科学与工程学院,成都 610065)
采用快速共沉淀法合成了立方体的层状无钴富锂固溶体正极材料0.6Li2MnO3-0.4LiNi0.5Mn0.5O2.通过X射线衍射(XRD),X射线光电子能谱(XPS),电感耦合等离子体(ICP),扫描电子显微镜(SEM),透射电子显微镜(TEM)及电性能测试等手段对材料进行了表征.结果表明,材料具有典型的α-NaFeO2六方层状晶体结构且具有与目标材料相似的化学组成.SEM和TEM结果表明,材料由粒径为40–200 nm的纳米颗粒组装成立方体结构.在文中给出了一个立方团聚体可能的形成机理.电化学性能测试(2.0–4.8 V电压范围内(vs Li/Li+))显示该材料具有优异的倍率性能,0.1C和10C倍率下的放电比容量分别是243和143 mAh·g–1.此外,该材料具有良好的循环稳定性,即使在大倍率测试后,0.5C倍率下循环72次仍显示出90.7%的高容量保持率.这种具有简易操作步骤和优异结果的共沉淀方法是一种经济的能够促进锂离子电池正极材料大规模应用的技术手段.
正极材料; 简易快速共沉淀; 立方体结构; 电化学性能; 锂离子电池
1 Introduction
Among the cathode materials used at present,Li-rich layered oxide materials zLi2MnO3-(1–z)LiMO2(0 < z < 1,M=Mn0.5Ni0.5,MnxNiyCo(1–x–y),0 < x,y < 0.5) are extremely attracti·ve because of the high specific capacity over 250 mAhg–1,1–3though they suffer from some problems such as large irreversible capacity loss in the first cycle,unsatisfactory rate capacity performance,inferior cyclic stability and so on.In particular,xLi2MnO3-(1–x)Li(Mn1/3Ni1/3Co1/3)O2materials have been proved to be glamorous in terms of superior electrochemical performance.4–7Nevertheless,in large-scale applications,those Co-doped Li-rich layered manganese oxide materials(xLi2MnO3-(1–x)Li(Mn1/3Ni1/3Co1/3)O2) are less competitive than the Co-free materials(xLi2MnO3-(1–x)LiMn0.5Ni0.5O2) due to the expensiveness and toxicity of cobalt resource.Generally,the physical and chemical properties(i.e.,crystal structure,particle size,morphology,and tap density,etc.),the determining factors of the electrochemical performance of cathode powders,are related directly to the synthetic routes.8,9Therefore,to optimize the physical and chemical properties,numerous research articles have been focused on the synthetic aspects of these materials so far.Shojan et al.10prepared 0.3Li·2MnO3-0.7LiMn0.5Ni0.5O2with capacity less than 200 mAhg–1(0.1C) and poor cycle performance by sol-gel method.Wei et al.4synthesized nanoplate Li(Li0.17Ni0.25Mn0.58)O2material with excellent electrochemical performance,but there are still plenty of limiting factors for the industrialization of the hydrothermal method.Nowadays,co-precipitation method in combination with solid state has become a primary preparation technique for these materials.However,these traditional coprecipitation methods in combination with solid state10–12are complex and time-consuming for the slow precipitation reaction,pH control,filtering,and mixing of Li-source.In some cases,preventing Mn(II) oxidation with inert gas in the synthesis process induces operation complexity.11,13
In this work,we proposed a facile quick co-precipitation approach to synthesize the layered composites 0.6Li2MnO30.4 LiMn0.5Ni0.5O2with cuboid hierarchical micro/nanostructure.(1) This approach adopts quick waterfall-addition with subsequent evaporation to replace complicated processes including slow dropwise-addition,aged procedure,filtering and drying step,and mixing of Li-source process of the traditional co-precipitation method.(2) Oxalate is chosen as precipitant to avoid using of inert gas protection(The inert gas is used to control the valence state of Mn(II)).13,14With the implementation of two changes mentioned above,the co-precipitation method has been greatly simplified.In the previous reports,12,15–17the primary nanoparticles prepared by oxalic precipitation can’t self-assemble to secondary particles with special morphology,unless polyethylene glycol-assisted,hydrothermal method or solvothermal route are used.Herein,we obtained a cuboid hierarchical micro/nanostructured 0.6Li2MnO3-0.4LiMn0.5Ni0.5O2material composed of the accumulation of small nanoparticles by masterly utilizing the properties of oxalate.Encouragingly,the assynthetized novel cuboid aggregates deliver an excellent electrochemical performance,especially rate capability.
2 Experimental
2.1 Material synthesis
The reagents 0.16 mol LiOH·H2O(Chengd ·uKelong Chemical Co.,AR,99%),0.02 mol Ni(CH3COO)24H2O(Chengdu Kelong Chemical Co.,AR,98%) and 0.08 mol Mn(CH3COO)2·4H2O(Tianjing Damao Chemical Co.,AR,99%) were respectively dissolved in distilled water in term of the stoichiometric ratio(Li excess 5%).After neutralizing by excess acetic acid(Chengdu Kelong Chemical Co.,AR,99.5%),the LiOH solution was added into the mixture of Ni2+/Mn2+solution(nickel acetate/manganese acetate),resulting in a weakly acidic Li+/Ni2+/Mn2+mixed solution.At the same time,mix 37 g oxalate(Chengdu Kelong Chemical Co.,AR,99.5%) used as both precipitant and structure-directing agent with 100 mL distilled water and heat the mixture until oxalate was dissolved completely(about 60°C).Light blue sediment which contains nickel oxalate and manganese oxalate precipitation was obtained after pouring aqueous solution of oxalate into the weakly acidic Li+/Ni2+/Mn2+mixed solution mentioned above.Then the water in the resulting solution was evaporated at 95°C with continuously stirring until a powder precursor was obtained.Finally,the dried precursor was preheated at 400°C for 3 h,then calcinated orderly at 500°C for 5 h,and 900°C for 10 h in air.
2.2 Materials characterization
The X-ray diffraction pattern wa·s conducted with Cu Kαradiation at a scanning rate of 0.06(°)s–1in the 2θ range from 10° to 70°.The general morphology and size of the sample were determined by field emission scanning electron microscopy(SEM)(JEOL JSM-5900 LV).The Li,Mn,and Ni molar ratio in the synthesized sample was analyzed with a Spectro AR-COS FHS12 inductively coupled plasma-atomic emission spectrometer(ICP-AES).The structure of the sample was characterized by a transmission electron microscopy(TEM)(JEM-2100).The valence states of the transition metal ion in the sample were determined by X-ray photoelectron spectroscopy(XPS)(PHI QUANTUM 2000) with monochromatic Al-Kαanode source with pass energy of 1486.6 eV,the binding energy(EB) of XPS was referenced to C1s spectrum of carbon support at 284.6 eV.
2.3 Electrochemical measurements
The electrochemical properties of the sample were measured using a CR-2032-type coin cell.The cathode material was prepared as follows:80%(w) active material,13%(w) acetylene black,and 7%(w) polyvinylidene fluoride(PVDF) were mixed with N-methyl pyrrolidinone(NMP) which is selected as solvent.The slurry was spread on Al foil and dried at 100°C for 12 h to obtain positive electrode.A lithium foil and a Celgard 2400 membrane were used as the negative electrode and separator,respectively.The dried positive electrode sheet was cut into disks with diameter of 14 mm and the loading of the active material in the electrode was ~2.5 mg·cm–2.The galvanostatic charge-discharge tests were performed on a battery measurement system(Neware BTS-610,5 V,10 mA) with a cut-off voltage of 2.0–4.8 V(vs Li/Li+) at room temperature.After the initial charge-discharge cycle,the cell was cycled at increasing rates:7 cycles at 0.1C,6 cycles at 0.2C,11 cycles each at 0.5C,1C,2C,5C and 10C,72 cycles at 0.5C,then 51 cycles at 1C,finally 10 cycles at 0.1C in succession.The current value for 1C rate was 200 mA·g–1in our definition.Cyclic voltammetry(CV) curves were recorded from 2.0 to 4.8 V at a scan rate of 0.1 mV·s–1on a LK9805 electrochemical workstation.
3 Results and discussion
The crystallinity and crystal phase of the sample are investigated by XRD.The XRD pattern of the as-prepared solid solution sample is shown in Fig.1.This pattern is extremely similar to the pattern of lithium-rich layered oxides reported in the previous literature.18No impurity and overlapping peaks are detected in the XRD pattern.The majority of the diffraction peaks in the pattern can be indexed as a layered α-NaFeO2-type structure(space group Rm,hexagonal),while a few weak super-lattice reflections peaks presented between 21° to 25° are indexed based on a layered Li2MnO3-type structure(space group C2/m),3,7,19,20which are known to be caused by the super-lattice ordering of Li+and Mn4+in the transition metal layers.21In general,the intensity ratio of the I(003)/I(004)can be used to estimate degree of cation mixing between Ni2+and Li+in the Li-layers.22The I(003)/I(004)intensity ratio of the as-prepared cathode material is 1.45(greater than 1.20),which is an indication that the extent of cation disorder between Ni2+and Li+in the crystal structure is low.23,24The clear separation of the pair reflection peaks(006)/(012) and(108)/(110),24and the high c/a ratio(c/a=4.995) reflect the successful formation of a well-ordered layer-structure.
The Li/Mn/Ni atomic ratio measured by ICP is 1.223:0.615:0.154,which is closely to the theoretical metal atomic ratio of raw Li1.231Mn0.615Ni0.154O2material.To further confirm the chemical valence state of the transition metals in the component,XPS characterization was employed.The Mn2p,Ni2p and O1s XPS spectra of as-prepared sample are shown in Fig.2.The Mn2p spectra(Fig.2(a)) exhibits two major peaks at 642.1 and 653.6 eV without any satellite peak,suggesting a predominantly valence of Mn4+.25,26Four signals are observed in the Ni2p XPS spectra(Fig.2(b)),two strong signals around 872.1 and 854.4 eV correspond to Ni2p1/2and Ni2p3/2,while their shakeup satellite peaks are respectively at 879.0 and 861.2 eV.Such satellite peaks of Ni2p3/2originating from the multiple splitting in the energy level of the Ni-oxides have been observed in other Ni2+containing oxides such as NiO,Li(Mn1.5Ni0.5)O4,and Li(Mn1/3Ni1/3Co1/3)O2.25Fig.2(c) shows O1s peak of the sample.The narrow peak located at 529.35 eV is characteristic of O2–anions belonging to the crystalline network.27The XPS results show that the predominant oxidation states of Ni and Mn in this compound are 2+ and 4+,respectively.According to the electroneutrality principle,the as-prepared composite can be given the formula of Li1.231Mn0.615(Ⅳ)Ni0.154(Ⅱ)O2.28

Fig.1 XRD pattern for the sample
As is well-known,the morphology and structure of lithium cathode materials have great influence on the electrochemical performances.Fig.3(a–c) present SEM images of the precipitation,the precursor calcined at 400°C for 3 h,and final product powder of the sample synthesized with oxalic acid,respectively.As shown in Fig.3(a),the cuboid precipitation is assembled with acicular particles.After low temperature treatment,the primary particle size of the precursor is about 50 nm and these particles pile closely to form the secondary particles(10–20 μm) with irregular cuboid(Fig.3(b)).Subsequently,as shown in Fig.3(c),the main cuboid shape of the secondary particles can be maintained after high temperature.Here,in this formation process of the cuboid precipitation,oxalate serves a vital function.But from the insert of Fig.3(c),a particular section of a high-resolution image,a slight differences in morphology compared to the precursor can be observed.Obviously,the primary particles liking rock-shaped grains(40–200 nm) of the as-prepared sample are larger and denser than the precursor’s.Ingeneral,nanoparticles with proper particle size can enhance the lithium-ion diffusion by shortening the intergranular and the interior diffusion pathways,29which can improve the rate performance of the materials remarkably.Besides,the number of the pore in the as-prepared sample is increased in comparison to the precursor.These pores deriving from the decomposition of the oxalic acid root provide sufficient contact area to the electrolyte,which is beneficial for the penetration of electrolyte into the materials and the Li-ion insertion/extraction at high current rate.On the other hand,the secondary microstructure yields good structural stability by suppressing the dissolution of the primary nanoparticles into electrolyte.16Thus,this sample with cuboid hierarchical micro/nanostructure may have a superior electrochemical performance.

Fig.2 XPS spectra of the sample(a) Mn2p;(b) Ni2p;(c) O1s

Fig.3 SEM images of(a) precipitation,(b) precursor calcined at 400°C,(c) as-prepared sample synthesized with oxalate,and(d) the sample synthesized without adding oxalate
For the sake of investigating the role of oxalate in the formation of cuboid aggregation,a sample is synthesized via the same way but without adding oxalate.The SEM images of the sample are shown in Fig.3(d).It can be seen clearly that there is no any nearly cuboid aggregation but only unordered nanoparticles in the compound synthesized without adding oxalic acid.The different morphology of the two samples indicates that oxalate plays a vital role in the formation of the cuboid precipitation.Based on these,we propose a formation mechanism of the cuboid aggregation as shown in Scheme 1.In step 1,the saturated oxalate solution of 60°C is poured into the weakly acidic Li+/Ni2+/Mn2+metal ion solution of 30°C.When the saturated oxalate solution at 60°C touches the metal ion solution at apparently lower temperature(30°C),the acicular oxalate crystals begin to crystallize out in the interface of these two kinds of solution.In the following reaction,the tiny acicular oxalate crystals serve as precipitant and structure-directing agent that assists the formation of cuboid micro-sized matrix.With crystallization and precipitation happening in succession,the acicular precipitations self-assemble into the cuboid shape(shown inFig.3(a)).The further growth of the cuboids is achieved under the high calcined temperature.In step 2,the oxalate in precursors is decomposed into gas and oxide,leaving behind nanoparticles in the bulk matrix.This process utilizes thermal variation to realize the dissolution and recrystallization of oxalate,further guiding the formation of cuboid aggregation.
The TEM characterization of as-synthesized materials is displayed in Fig.4.Homogeneous particles with a size below 200 nm can be seen in Fig.4(a) and Fig.4(b),which agree with the SEM observations above.A high resolution transmission electron micrograph of as-synthesized material is shown in Fig.4(c),from which clear lattice fringes with d-spacing of 0.47 nm can be observed.This value coincides with the interplanar distance of(001) plane of Li2MnO3and/or the(003) plane of LiMO2,which agrees with the XRD analysis results.
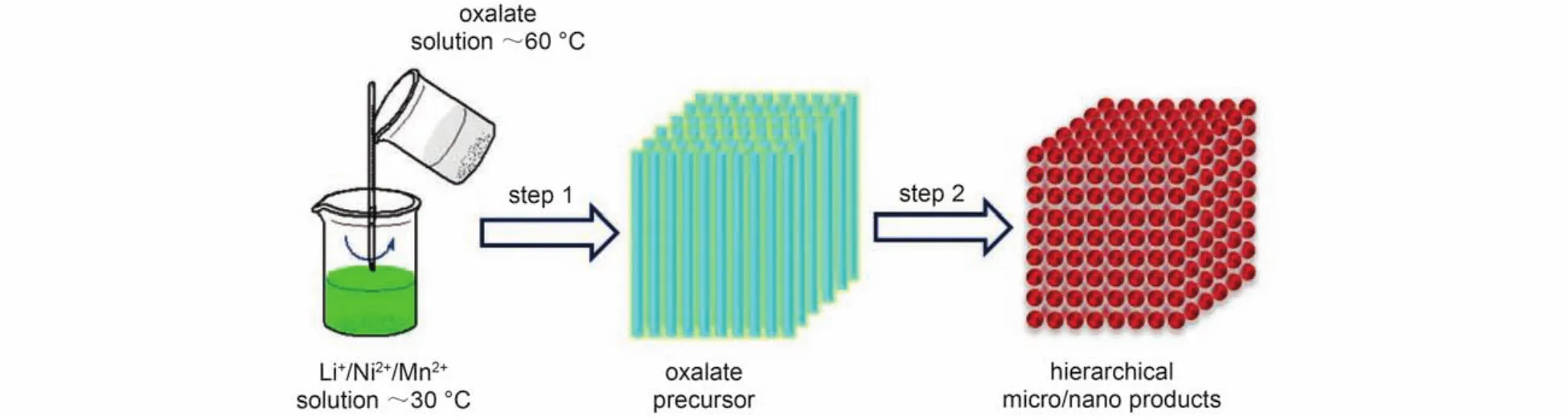
Scheme 1 Schematic illustration of the material preparation process

Fig.4 (a) TEM image of the sample,(b) a magnified image of a single nanoparticles and(c) HR-TEM image

Fig.5 Cyclic performance of the samples synthesized with oxalate and without oxalate at different rates
To investigate the effect of oxalate on the electrochemical properties,the electrochemical performances in terms of rate property and cycling capability for the samples synthesized with and without addition of oxalate are illustrated in Fig.5.It can be clearly observed that material synthesized with oxalate displays better specific capacity at all rates than the material synthesized without oxalate.The cuboid material synthesized with oxalate in this work delivers very excellent electr·ochemical properties with discharge capacity over 160 mAhg–1even under the rate of 5C(in Fig.5(a)).As shown in Fig.5(a) and Fig.6(c),the initial charge and discharge capacities ·at a rate of 0.1C are respectively measured as 319 and 243 mAhg–1corresponding to the coulombic efficiency(CE) of 76.3% which is larger than that of sample synthesized without oxalate(72.0%).When the rate is increased to 0.2C,0.5C,1C,2C,5C and 10C,the discharge capacity· are measured at about 214,188,177,168,163,and 143 mAhg–1,respectively(Fig.5(a)).In contrast,the corresponding capacities for the ·sample without oxalate are 195,165,142,125,90,and 65 mAhg–1,respectively.No obvious fading of discharge capacity is observed at each current rate.Fig.5(b) and Fig.5(c) show the cycle stability under 0.5C and 1C rate of the materials after high rate performance tests.It turns out to be that the discharge capacity can be recovered when the rate turns back to 0.5C after rate capability tests,implying the retentive and stable of sample synthesized with oxalate.In the cycling performance tests,the in·itial discharge capacity at the current rate of 0.5C ·is 193 mAhg–1and the capacity maintains a value of 175 mAhg–1after 72 cycles,yielding the high capacity retention of 90.7%.All above indicate that the assynthesized material has a stable structure.This excellent electrochemical performance,especially superior rate performance is closely related to the nanosized primary particles which enhances the lithium-ion diffusion by shortening the pathway for Li+diffusion.Simultaneously,the porous structure derived from decomposition of oxalate precursors can accelerate electrolyte penetration into the nanoparticles,ensuring the efficient contact area between the electrode and electrolyte.Furthermore,the second cuboid micro-sized matrix structure of the as-prepared material can inhibit the dissolution of the primary particles into electrolyte during the charge-discharge process,revealing a relatively stable structure and better electrochemical performance.In the fur·ther cycles,the ·discharge capacity fades slowly from 160 mAhg–1to 136 mAhg–1at 1C.When the rat·e goes back to 0.1C,the average special capacity is 210 mAhg–1,which is 87.5%· of the discharge capacity in the first seven cycles(~240 mAhg–1).This capacity retention(87.5%) after about 200 cycles including cycles with high current rate is attractive compared to the capacity retention(82.8%) in Gu’s work.30

Fig.6 Discharge curves(a) and the corresponding dQ/dV curves(b) under differentC-rate for 0.6Li2MnO3-0.4LiNi0.5Mn0.5O2electrode;(c) charge-discharge curves of 1st,2nd,3rd,and 198th;(d) cyclic voltammograms profiles
Fig.6 gives further electrochemical analysis for 0.6Li2MnO3-0.4LiNi0.5Mn0.5O2prepared with oxalate addition.In the discharge voltage curves(Fig.6(a)) under different currents,voltage decay is observed with the increase of current rate and cycle number.This voltage decay signals the layered to spinel transformation,27,30which causes the fading of discharge capacity.The corresponding dQ/dV curves are illustrated in Fig.6(b).A shape peak around 4.5 V in the initial charge process is attributed to the irreversible Li2O removal from the Li2MnO3.31The reduction peak at ~ 4.3 V noted as R1and the reduction peak lower than 3.5 V noted as R2in the first discharge process are associated with the reduction reaction of Ni4+/Ni2+and the reduction of Mn4+to Mn3+which happens in the charge-discharge process after the electrochemical activation of Li2MnO3component,32respectively.Clearly,R1becomes more and more smoothly with the increase of C-rate and the cycle number which indicates the contribution of Ni to capacity is on the decrease.It is also obvious that the R2shifts to the low voltage region clearly with the increase of C-rate and cycle number.At last,this peak is below 3.0 V completely at the rate of 10C.It is considered that the reduction peak below 3.0 V can be attributed to the spinel-like phase.22The weaken of the reduction peak around 4.3 V corresponding to Ni4+/Ni2+and the shift of the reduction peak around 3.0 V demonstrate that parts of the layered structure has been gradually transformed into spinel structure in crystallography(which is corresponding to the result of Fig.6(a)).The charge and discharge curves of 1st,2nd,3rd,and 198th are shown in Fig.6(c).The initial charge profile is composed of a sloping region below 4.5 V and a plateau region ab·ove 4.5 V.The former region provides a capacity of 111 mAhg–1which is attributed to the Li+extraction from the layered LiNi0.5Mn0.5O2by oxidation of Ni2+to Ni4+.Then the lo·ng flat plateau above 4.5 V provides a capacity of 208 mAhg–1.This is a process that Li+extraction from the transition metal layer with the loss of Li2O from the layered Li2MnO3component leads to a large initial irreversible capacity loss(23.7%) and is consistent with the sharp peak at 4.5 V in the first charge process in Fig.6(b).The subsequent cycles(seen the charge and discharge curves of 2nd,3rd,and 198th in Fig.6(c)) indicate that the cycles are almost reversible after the initial irreversible cycle.The voltage decay phenomenon which is contributed to the cycling-driven structural evolution(layered to spinel transformation) can also be observed in Fig.6(c).The plateau between 3.0 and 3.5 V in the 198th charge curve is more noticeable than in the 2nd and 3rd,which indicates more redox reaction of Mn4+/Mn3+.Cyclic voltammogram(CVs) of the prepared material is recorded to gather information about the individual redox process during the charging and discharging,which is shown in Fig.6(d).The first anodic peak around 4.0 V in the initial charging is predominantly associated with Ni oxidation from Ni2+to Ni4+.The second anodic peak at higher potential(> 4.5 V) is corresponding to the removal of Li2O which is consistent with the result of Fig.6(b).The two cathodic peaks at ~ 3.25 V and ~ 3.75 V are associated with the reduction of Mn and Ni,respectively.33The overlap between 2nd,3rd,and 4th cycle profiles indicates the good reversibility of the as-prepared material.These changes mentioned above and the polarization caused by the current rate increasing during charge and discharge result in a gradual capacity fading.Although alterations have occurred to the electrode,the electrode is still a stable one as depicted in 192nd–201st cycles.Furthermore,the electrode shows good reversibility in all cycles(2nd–201st cycles) with high coulombic efficiency nearly 100%.
4 Conclusions
In summary,a cuboid nanostructured 0.6Li2MnO3-0.4LiNi0.5Mn0.5O material was successfully synthesized through a facile quick co-precipitation method.The XRD,SEM,and TEM results show that the layered as-synthesized material with pure phase possesses a cuboid morphology consisted of nanoparticles of 40 to 200 nm in size.The charge-discharge experiments demonstrate that the sample exhibits excellent electrochemical properties especially rate performance.At the high rate of 10C,the discharge capacity is measured as high as 143 mAh·g–1which is 58.8% of the average capacity at the rate of 0.1C.Moreover,the as-prepared material has a good cycling stability,even after the high rate measurement,delivering high capacity retention of 90.7% after 72 cycles at 0.5C.This study has thrown a new light upon facile preparation of hierarchical micro/nanostructure which is very promising for large-scale commercialization of the Co-free and Li-rich cathode material xLi2MnO3-(1–x)LiMn0.5Ni0.5O2to synthesize Lirich layered cathode with excellent performance.
(1)Cheng,F.;Xin,Y.;Chen,J.;Lu,L.;Zhang,X.;Zhou,H.J.Mater.Chem.A 2013,1,5301.doi:10.1039/c3ta00153a
(2)Tabuchi,M.;Nabeshima,Y.;Takeuchi,T.;Kageyama,H.;Imaizumi,J.;Shibuya,H.;Akimoto,J.J.Power Sources 2013,221,427.doi:10.1016/j.jpowsour.2012.08.055
(3)Xue,Q.R.;Li,J.L.;Xu,G.F.;Hou,P.F.;Yan,G.;Dai,Y.;Wang,X.D.;Gao,F.Acta Phys.-Chim.Sin.2014,30,1667.[薛庆瑞,李建玲,徐国峰,侯朋飞,晏 刚,代 宇,王新东,高 飞.物理化学学报,2014,30,1667.] doi:10.3866/PKU.WHXB201406251
(4)Wei,G.Z.;Lu,X.;Ke,F.S.;Huang,L.;Li,J.T.;Wang,Z.X.;Zhou,Z.Y.;Sun,S.G.Adv.Mater.2010,22,4364.doi:10.1002/adma.v22:39
(5)Cho,T.H.;Shiosaki,Y.;Noguchi,H.J.Power Sources 2006,159,1322.doi:10.1016/j.jpowsour.2005.11.080
(6)Lei,C.H.;Bareño,J.;Wen,J.G.;Petrov,I.;Kang,S.H.;Abraham,D.P.J.Power Sources 2008,178,422.doi:10.1016/j.jpowsour.2007.11.077
(7)Johnson,C.S.;Li,N.;Lefief,C.;Thackeray,M.M.Electrochem.Commun.2007,9,787.doi:10.1016/j.elecom.2006.11.006
(8)Kim,S.;Johnson,C.S.;Vaughey,J.T.;Thackeray,M.M.;Hackney,S.A.;Yoon,W.;Grey,C.P.Chem.Mater.2004,16,1996.doi:10.1021/cm0306461
(9)Lin,J.;Mu,D.;Jin,Y.;Wu,B.;Ma,Y.;Wu,F.J.Power Sources 2013,230,76.doi:10.1016/j.jpowsour.2012.12.042
(10)Shojan,J.;Chitturi,V.R.;Torres,L.;Singh,G.;Katiyar,R.S.Mater.Lett.2013,104,57.doi:10.1016/j.matlet.2013.04.001
(11)Liu,G.B.;Liu,H.;Shi,Y.F.Electrochim.Acta 2013,88,112.doi:10.1016/j.electacta.2012.10.054
(12)Wu,F.;Lu,H.;Su,Y.;Li,N.;Bao,L.;Chen,S.J.Appl.Electrochem.2010,40,783.doi:10.1007/s10800-008-0057-2
(13)Zhu,Z.;Zhu,L.J.Power Sources 2014,256,178.doi:10.1016/j.jpowsour.2014.01.068
(14)Xue,Q.R.;Li,J.L.;Xu,G.F.;Zhou,H.W.;Wang,X.D.;Kang,F.Y.J.Mater.Chem.A 2014,2,18613.doi:10.1039/C4TA04024D
(15)Zhang,X.;Cheng,F.;Zhang,K.;Liang,Y.;Yang,S.;Liang,J.;Chen,J.RSC Adv.2012,2,5669.doi:10.1039/c2ra20669b
(16)Fu,F.;Deng,Y.P.;Shen,C.H.;Xu,G.L.;Peng,X.X.;Wang,Q.;Xu,Y.F.;Fang,J.C.;Huang,L.;Sun,S.G.;Electrochem.Commun.2014,44,54.doi:10.1016/j.elecom.2014.04.013
(17)Zhang,L.;Borong,W.;Ning,L.;Feng,W.Electrochim.Acta 2014,118,67.doi:10.1016/j.electacta.2013.11.186
(18)Jiang,Y.;Yang,Z.;Luo,W.;Hu,X.;Huang,Y.Phys.Chem.Chem.Phys.2013,15,2954.doi:10.1039/c2cp44394e
(19)Kim,D.;Gim,J.;Lim,J.;Park,S.;Kim,J.Mater.Res.Bull.2010,45,252.doi:10.1016/j.materresbull.2009.12.027
(20)Kim,J.H.;Choi,S.H.;Son,M.Y.;Kim,M.H.;Lee,J.K.;Kang,Y.C.Ceram.Int.2013,39,331.doi:10.1016/j.ceramint.2012.06.029
(21)Wang,Z.Y.;Li,B.;Ma,J.;Xia,D.G.RSC Adv.2014,4,15825.doi:10.1039/c3ra47044j
(22)Wu,F.;Wang,Z.;Su,Y.;Guan,Y.;Jin,Y.;Yan,N.;Tian,J.;Bao,L.;Chen,S.J.Power Sources 2014,267,337.doi:10.1016/j.jpowsour.2014.05.097
(23)Ryu,J.H.;Park,B.G.;Kim,S.B.;Park,Y.J.J.Appl.Electrochem.2009,39,1059.doi:10.1007/s1008-008-9757-2
(24)Kim,G.Y.;Yi,S.B.;Park,Y.J.;Kim,H.G.Mater.Res.Bull.2008,43,3543.doi:10.1016/j.materresbull.2008.01.011
(25)Yu,C.;Li,G.;Guan,X.;Zheng,J.;Li,L.;Chen,T.Electrochim.Acta 2012,81,283.doi:10.1016/j.electacta.2012.06.084
(26)Li,L.;Zhang,X.;Chen,R.;Zhao,T.;Lu,J.;Wu,F.;Amine,K.J.Power Sources 2014,249,28.doi:10.1016/j.jpowsour.2013.10.092
(27)Sathiya,M.;Rousse,G.;Ramesha,K.;Laisa,C.;Vezin,H.;Sougrati,M.T.;Doublet,M.L.;Foix,D.;Gonbeau,D.;Walker,W.Nat.Mater.2013,12,827.doi:10.1038/nmat3699
(28)Liao,S.X.;Zhong,B.H.;Guo,X.;Shi,X.X.;Hua,W.B.Eur.J.Inorg.Chem.2013,2013,5436.doi:10.1002/ejic.v2013.31
(29)Zhang,L.;Wu,B.;Li,N.;Mu,D.;Zhang,C.;Wu,F.J.Power Sources 2013,240,644.doi:10.1016/j.jpowsour.2013.05.019
(30)Gu,M.;Belharouak,I.;Zheng,J.;Wu,H.;Xiao,J.;Genc,A.;Amine,K.;Thevuthasan,S.;Baer,D.R.;Zhang,J.G.ACS Nano 2012,7,760.
(31)Chen,L.;Chen,S.;Hu,D.Z.;Su,Y.F.;Li,W.K.;Wang,Z.;Bao,L.Y.;Wu,F.Acta Phys.-Chim.Sin.2014,30,467.[陈 来,陈 实,胡道中,苏岳峰,李维康,王 昭,包丽颖,吴 峰.物理化学学报,2014,30,467.] doi:10.3866/PKU.WHXB 201312252
(32)Li,Q.;Li,G.;Fu,C.;Luo,D.;Fan,J.;Li,L.ACS Appl.Mater.Interfaces 2014,6,10330.doi:10.1021/am5017649
(33)Wang,Y.;Yan,X.;Bie,X.;Fu,Q.;Du,F.;Chen,G.;Wang,C.;Wei,Y.Electrochim.Acta 2014,116,250.doi:10.1016/j.electacta.2013.10.215
Facile Synthesis of 0.6Li2MnO3-0.4LiNi0.5Mn0.5O2with Hierarchical Micro/Nanostructure and High Rate Capability as Cathode Material for Li-Ion Battery
SHI Xia-Xing1LIAO Shi-Xuan1YUAN Bing1ZHONG Yan-Jun1ZHONG Ben-He1LIU Heng2GUO Xiao-Dong1,*
(1College of Chemical Engineering,Sichuan University,Chengdu 610065,P.R.China;
2College of Materials Science and Engineering,Sichuan University,Chengdu 610065,P.R.China)
The cuboid layered 0.6Li2MnO3-0.4LiNi0.5Mn0.5O2cobalt-free lithium-rich solid-solution cathode material was synthesized by a facile quick co-precipitation method.The prepared material was characterized by X-ray powder diffraction(XRD),X-ray photoelectron spectroscopy(XPS),inductively coupled plasma(ICP) spectroscopy,field-emission scanning electron microscopy(SEM),transmission electron microscopy(TEM),and electrochemical measurements.It was found that the as-prepared material has a typical hexagonal α-NaFeO2layered structure with Rm space group,and the chemical composition of this material is similar to the corresponding target material.SEM and TEM images reveal that the cuboid structures are assembled from nanoparticles with particle sizes of 40–200 nm.A possible formation mechanism of this cuboid aggregation is proposed.The electrochemical tests(in the voltage range 2.0–4.8 V vs Li/Li+) indicate that the as-prepared material exhibits excellent rate capability.It deliversapproximately 243 and 143 mAh·g–1corresponding to 0.1C and 10C,respectively.Moreover,the asprepared material has good cycling stability even after high rate measurement,delivering a high capacity retention of 90.7% after 72 cycles at 0.5C.This co-precipitation approach,which has facile operation processes and good results,is a economic technique that could facilitate the application of Li-rich cathode on a large scale.
Cathode material; Facile quick co-precipitation; Cuboid structure; Electrochemical performance; Li-ion battery
April 22,2015;Revised:June 15,2015;Published on Web:June 15,2015.
O646
icle]
10.3866/PKU.WHXB201506151 www.whxb.pku.edu.cn
*Corresponding author.Email:xiaodong2009@scu.edu.cn;Tel:+86-28-85406702;Fax:+86-28-85405517.
The project was supported by the Science and Technology Pillar Program of Sichuan University,China(2014GZ0077) and Research Fund for the Doctoral Program of Higher Education,China(20120181120103).
四川大学科技支撑计划(2014GZ0077)和高等学校博士学科点专项科研基金(20120181120103)资助项目
© Editorial office of Acta Physico-Chimica Sinica
