四种深共融溶剂的性质:密度、电导率、动力粘度及折光率
2015-09-03宿洪祯尹静梅刘青山李长平
宿洪祯 尹静梅 刘青山 李长平,*
(1大连大学环境与化学工程学院,辽宁 大连 116622; 2沈阳农业大学理学院,沈阳 110866)
四种深共融溶剂的性质:密度、电导率、动力粘度及折光率
宿洪祯1尹静梅1刘青山2,*李长平1,*
(1大连大学环境与化学工程学院,辽宁 大连 116622;2沈阳农业大学理学院,沈阳 110866)
制备了四种四丁基氯化铵类深共融溶剂,包括四丁基氯化铵∶丙酸[TBAC∶2PA]、四丁基氯化铵∶乙二醇[TBAC∶2EG]、四丁基氯化铵∶聚乙二醇[TBAC∶2PEG]、四丁基氯化铵∶苯乙酸[TBAC∶2PAA].在288.15–338.15 K温度范围内,测定了它们的密度、电导率、动力粘度及折光率.讨论了温度对密度、电导率、动力粘度及折光率等性质的影响.通过经验方程估算了深共融溶剂的热膨胀系数、分子体积、标准摩尔熵及晶格能等热力学性质参数.利用Vogel-Fulcher-Tamman(VFT)方程和Arrhenius方程,将测量的电导率和动力粘度对温度拟合,得到了动力粘度和电导率随温度变化方程式.有关研究对深共融溶剂的工业化应用具有重要意义.
深共融溶剂; 密度; 电导率; 动力粘度; 折光率
1 Introduction
In the recent years,deep eutectic solvents have been attracting widely scientific and technological attention because of their low cost as green solvents similar to ionic liquids(ILs).The deep eutectic solvents(DESs) are composed of two or three cheap and green components through hydrogen bond formation.1Compared to ILs,the DESs have many advantages,such as the simple synthesis and purification process,low cost of raw materials,and renewable,good biocompatibility,etc.2,3
DESs have been widely used for replacing the organic solvents in industrial and scientific communities,especially for bioactive natural products dissolution,4green media,5catalysis,6–8organic synthesis,9electrochemistry,10,11reaction medium,12separation process,13,14etc.In our group,series of IL-type DESs were designed,synthesized,and applied for the extraction desulfurization of fuels successfully.15Moreover,the extraction process was optimized and extraction mechanism was probed systematically and the hydrogen bond formed accounting for the higher desulfurization efficiencies.
Although the DESs have exhibited so many excellent properties,the research on their physicochemical properties is still scarce.The limited existing study of the properties is mainly on the melting temperature and dynamic viscosity at several points,which will hinder its industrial and engineering applications to a great extent.16–19
In this work,four IL-type tetrabutylammonium chloride based DESs,namely tetrabutylammonium chloride:propionic acid [TBAC:2PA],tetrabutylammonium chloride:ethylene glycol [TBAC:2EG],tetrabutylammonium chloride:polyethylene glycol [TBAC:2PEG],and tetrabutylammonium chloride:phenylacetic acid [TBAC:2PAA],were synthesized.As the basic material,the TBAC has been used for the phase transfer catalysis in some reactions.The raw materials are cheap and easily obtained for the syntheses of DESs.The samples have also exhibited highly extraction desulfurization efficiencies,simple and environmentally friendly synthesis process.Their main physicochemical properties,including the density,dynamic viscosity,electrical conductivity,and refractive index were characterized at 288.15–338.15 K.
2 Experimental
2.1 Materials
The deep eutectic solvents were synthesized and characterized according to our previous method.15Tetrabutylammonium chloride(TBAC),propionic acid(PA),thylene glycol(EG),polyethylene glycol(PEG),and phenylacetic acid(PAA) were obtained from Tianjin Kermel,Aladdin,Tianjin Kermel,and Tianjin Kermel,respectively.All the materials were purified before use according to crystallization,distillation,and vacuum drying.The purified compounds of TBAC and PA(or EG,PEG,PAA) were put into a round-bottomed flask with the molar ratio of 1:2.The system was stirred vigorously with a magnetic stirrer at 353 K for 8 h.The purity and the structure of the DESs have been characterized with1H NMR,which were shown in literature.15The1H NMR spectra are also listed in Table S1(Supporting Information).The purity of the DESs and the mass fraction contribution of the impurities are estimated and given in Table 1.The impurities are the H-bond acceptor for TBAC and H-bond donor for PA,EG,PEG,and PAA,respectively.The properties of the raw materials are listed in Table 2.
2.2 Measurements of the density,electrical conductivity,dynamic viscosity,and refractive index
The water contents were determined before determination of the properties by a Cou-Lo Aquamax Karl Fischer moisture meter v.10.06(Mettler Toledo,Switzerland).The water contents were 190 × 10–6,160 × 10–6,180 × 10–6,and 200 × 10–6(mass fraction) for [TBAC:2PA],[TBAC:2EG],[TBAC:2PEG],and [TBAC:2PAA],respectively.
The density,electrical conductivity,dynamic viscosity,and refractive index were determined by a previous method at atmosphere.20,21The uncertainties of the experimental values are also estimated according to literarue.22,23
The density was determined by a density meter system DDM2911(Rudolph Research Analytical Co.,USA) at((288.15·–338.15) ± 0.01) K within an experimental error of 0.05 kgm–3.The electrical conductivity was carried out on a MP522 conductivity instrument(Shanghai San-Xin Instrumentation,P.R.China) with the cell constants of 1 cm–1(the cell was calibrated with the aqueous KCl solution) at((288.15–338.15) ± 0.05) K and the uncertainties were estimated to be 1%.The measurement frequency and voltage of the electrical conductivity are 50 Hz and 9 V,respectively.The dynamic viscosity was determined using an Ostwald viscometer(Dalian Instruments an Meters Co.,P.R.China) at((288.15–338.15) ± 0.1) K and the uncertainties were estimated to be 1%.The refractive index was carried out according to the literature.24The data were carried on an Abbe refractometer(Shanghai Shen Guang Instrument Co.,P.R.China) and the frequency was 50 Hz.The degassed water refractive index was determined by the instrument at(298.15 ± 0.05) K and the value is in good agreement with the literature.25
The above experimental values are listed in Tables S2 to S5(Supporting Information).
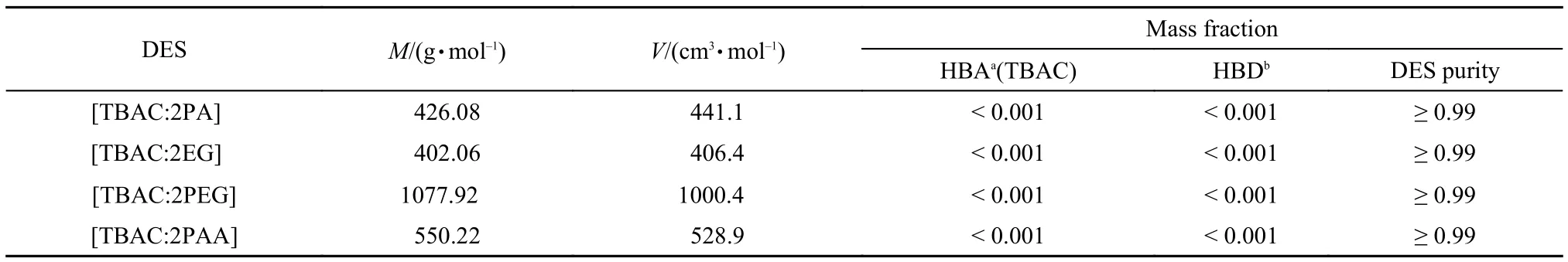
Table 1 Purity of DESs and mass fraction contribution of the impurities
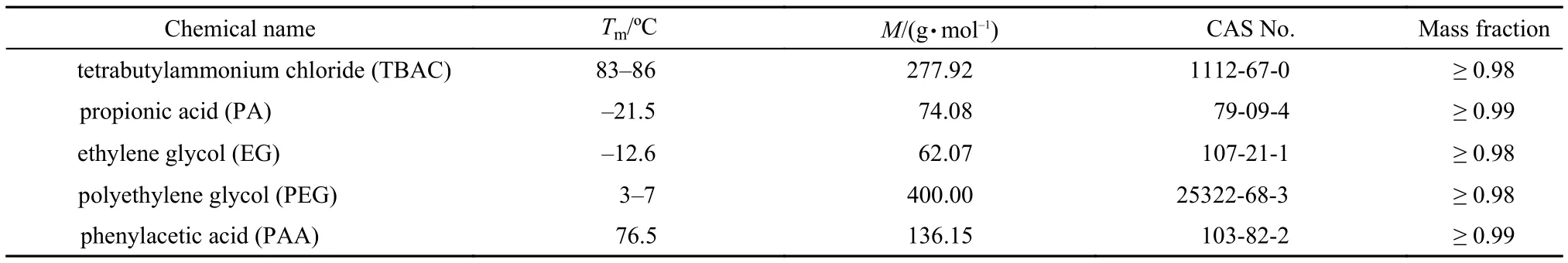
Table 2 Properties of the raw materials
3 Results and discussion
The temperature dependence on density(ρ),electrical conductivity(σ),dynamic viscosity(η),and refractive index(nD) of the four DESs are plotted in Figs.1–4.
It can be seen that with the increase of the temperature,the lines of density and refractive are linear one.While for the electrical conductivity and dynamic viscosity,the lines are nonlinear ones with temperature increasing.From the Tables S2–S5(Supporting Information) and Figs.1–4,the density,dynamic viscosity,and refractive index decrease with the temperature increasing.And the electrical conductivity increases with the temperature increasing.
For density,the sequence of the four DESs is [TBAC:2PA] <[TBAC:2EG] < [TBAC:2PAA] < [TBAC:2PEG].The values of the DESs of [TBAC:2PA] are lower than those of [TBAC:2PAA] and the values of the DESs of [TBAC:2EG] are lower than those of [TBAC:2PEG],the tendency is different from the traditional ILs.Usually,the traditional ILs exhibit the high density which has the relative small molecule for the series ILs,for example,the series of pyridinium ILs and imidazolium ILs.21,24
For electrical conductivity,the tendency of the four DESs is [TBAC:2PEG] ≈ [TBAC:2PAA] < [TBAC:2PA] < [TBAC:2EG].The samples exhibited the same tendency with the traditional ILs.The small molecule has the low electrical conductivity.The glycol type DESs exhibit the higher electrical conductivity than acid type EDSs.
For dynamic viscosity,the tendency of the four DESs is [TBAC:2EG] < [TBAC:2PA] ≈ [TBAC:2PEG] < [TBAC:2PAA].The small molecule exhibits the better liquidity than the relative big molecule.And the glycol type DESs have the better liquidity than acid type EDSs.

Fig.1 Density vs temperature plots for four DESs■ [TBAC:2PA];● [TBAC:2EG];▲ [TBAC:2PEG];▼ [TBAC:2PAA]

Fig.2 Electrical conductivity vs temperature plots for the four DESs■ [TBAC:2PA];● [TBAC:2EG];▲ [TBAC:2PEG];▼ [TBAC:2PAA]

Fig.3 Dynamic viscosity vs temperature plots for the four DESs■ [TBAC:2PA];● [TBAC:2EG];▲ [TBAC:2PEG];▼ [TBAC:2PAA]

Fig.4 Plot of nDvs temperature for the four DESs■ [TBAC:2PA];● [TBAC:2EG];▲ [TBAC:2PEG];▼ [TBAC:2PAA]
For refractive index,the sequence of the four DESs is [TBAC:2PA] < [TBAC:2EG] < [TBAC:2PEG] < [TBAC:2PAA].The big molecule exhibits higher refractive index than the relative small molecule.
From the above results,it can be seen that,with same hydrogen bond acceptor(HBA) tetrabutylammonium chloride,the changing of hydrogen bond donor(HBD) influences the properties of the DESs greatly.
3.1 Density(ρ)
The lnρ vs T can be fitted according to the following straight line.The thermal expansion coefficient,α,can be obtained.

where ρ is the density values,b is an empirical constant,α is the thermal expansion coefficient,T is the experimeantal temperature.The thermal expansion coefficients are listed in Table 3.The values in the range of 5 × 10–4and 7 × 10–4K–1were obtained by Jacquemin.26
The molecular volume,Vm,standard molar entropy,S0,and lattice energy,UPOT,were calculated from density values according to empirical equations.27

where M is molar mass,N is Avogadro's constant,ρ is density,Vmis molecular volume,S0is standard molar entropy,and UPOTis lattice energy.The calculated values of the above are listed in Table 3.From Table 3,the DESs have exhibited low lattice energy which is an underlying reason for the l ·iquid state of the DESs at room temperature(UPOT,CsI=613 kJmol–1).25
3.2 Molar electrical conductivity(Λ)
The molar electrical conductivities can be calculated from density and electrical conductivity by the following equation:

where Λ is the molar electrical conductivity,σ is the electrical conductivity,M is the molar mass,and ρ is the density.The molar electrical conductivity values are listed in Table 4.From Table 4,at 298.15 K,the tendency of the four DESs is [TBAC:2PAA] < [TBAC:2PA] < [TBAC:2PEG] < [TBAC:2EG].
3.3 Electrical conductivity(σ)
Usually,the Vogel-Fulcher-Tamman(VFT) and Arrhenius equations22are used for the fitting of the electrical conductivity values,σ,vs temperature,T:

where σ is the electrical conductivity;σ0,B are fitting parameters;Eσis the activation energy,which indicates the energy needed for an ion to hop to a free hole;σ∞is the maximum electrical conductivity,and kBis the Boltzmann constant.

Table 3 Estimated physicochemical property values for the four DESs at 298.15 K at atmospheric pressure

Table 4 Calculated values of molar electrical conductivity for the four DESs at 288.15–338.15 K under atmospheric pressure
The fitted parameters are listed in Table 5 by the equation(6)(see Fig.2).From Table 5,the correlation coefficient,R,is more than 0.9999,which indicates that the VFT equation can be well used for the electrical conductivity fitting.By the Arrhenius equation,the lnσ vs 1/T was plotted for the four DESs(see Fig.5).
In Fig.5,the dashed lines are straight lines for fitting of lnσ vs 1/T,the solid lines are smooth curves for lnσ vs 1/T.From the equation,the experimental points should follow a straight line,however,it is obvious that the experimental points are not on the straight line in Fig.5.So,the electrical conductivity does not follow the Arrhenius behavior well.
The electrical conductivity activation energies for the four DESs can be calculated by the modified VFT equation that Vila et al.28have built,recently.

here,σ0=σ∞and B=Eσ/kB.σ is the electrical conductivity;σ0,B are fitting parameters;Eσis the activation energy;σ∞is the maximum electrical conductivity,and kBis the Boltzmann constant.The calculated values of electrical conductivity activation energies are listed in Table 5.
3.4 Dynamic viscosity(η)
Like the electrical conductivity,the VFT and Arrhenius equations were also used for the fitting of the dynamic viscosity,η,vs temperature,T.

Table 5 Fitted values of electrical conductivity vs temperature for the four DESs by VFT and modified VFT equations

Fig.5 Plots of lnσ vs 1/T for the four DESs■ [TBAC:2PA];● [TBAC:2EG];▲ [TBAC:2PEG];▼ [TBAC:2PAA].The dashed lines are straight lines.
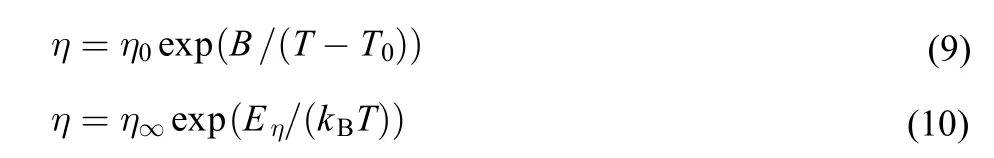
where η is the dynamic viscosity;η0,B are fitting parameters;Eηis the activation energy for dynamic viscosity;η∞is the maximum dynamic viscosity,and kBis the Boltzmann constant.The best fitted parameters are listed in Table 6 by the equation(9)(see Fig.3).From Table 6,the correlation coefficient,R,is more than 0.999,which indicates that the VFT equation can also be used for the dynamic viscosity fitting.
According to equation(10),the lnη vs 1/T was described in Fig.6 for the four DESs.
In Fig.6,the dashed lines are straight lines for fitting of lnη vs 1/T,the solid lines are smooth curves for lnη vs 1/T.From the Fig.6,similar to the electrical conductivity,the same result was obtained that the experimental dynamic viscosity does not follow the Arrhenius behavior well.
The same for dynamic viscosity,the modified VFT equation was obtained by Vila et al:28

here,η0=σ∞and B=Ea/kB.η is the dynamic viscosity;η0,B are fitting parameters;Eais the activation energy for dynamic viscosity;η∞is the maximum dynamic viscosity,and kBis the Boltzmann constant.The dynamic viscosity activation energies for DESs are listed in Table 6.
3.5 Walden product
The relationship of molar conductivity and dynamic viscosity can be built by the following Walden equation:

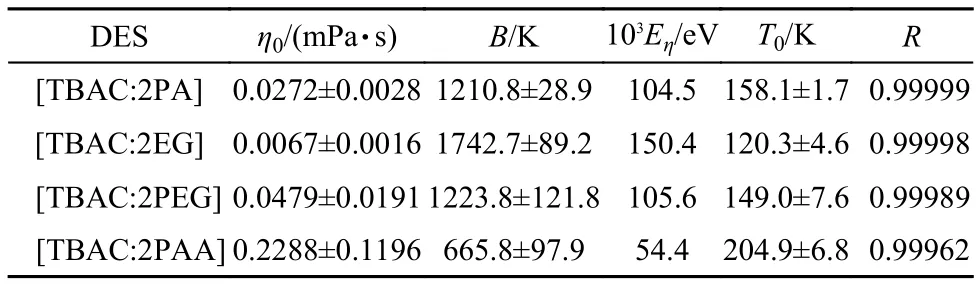
Table 6 Fitted values of dynamic viscosity vs temperature for the four DESs by VFT and modified VFT equations

Fig.6 Plots of lnη vs 1/T for the four DESs■ [TBAC:2PA];● [TBAC:2EG];▲ [TBAC:2PEG];▼ [TBAC:2PAA].The dashed lines are straight lines.

Fig.7 Plots of lgΛ vs lgη–1for the four DESs from 288.15 to 338.15 K■ [TBAC:2PA];● [TBAC:2EG];▲ [TBAC:2PEG];▼ [TBAC:2PAA].The solid straight line is the ideal line for aqueous KCl solutions.

Table 7 Fitted values of refractive index vs temperature for the four DESs according to equation(14)
where Λ is the molar conductivity,η is the dynamic viscosity,k is a temperature dependent constant,α is the fitting parameter.The description of lgΛ vs lgη–1is shown in Fig.7 for four DESs from 288.15 to 338.15 K.
The slopes,α,can be obtained and the values are 0.653 for [TBAC:2PA],0.564 for [TBAC:2EG],0.621 for [TBAC:2PEG],and 0.710 for [TBAC:2PAA],respectively.From Fig.7,all of the Walden plots are lower than the ideal line,the position ofthe ideal line is obtained using aqueous KCl solutions at high dilution.29The lines of the four DESs lie below the ideal KCl line.So,the four DESs are called "sub-ionic".30According to the fitted values of the slopes,α,the values are lower than the other traditional ILs.21,30
3.6 Refractive index(nD)
The relationship of refractive index and temperature can be described by a linear equation:

where nDis the refractive index;A,B are fitting parameters;T is experimental temperature.The fitted values of A and B are listed in the Table 7.From Table 7,the correlation coefficients for the four DESs are higher than 0.999.
4 Conclusions
The density,electrical conductivity,dynamic viscosity,and refractive index of the four DESs were determined at atmospheric pressure at 288.15–338.15 K.The DESs exhibited lower lattice energies than the traditional melt salts,it is the reason that the DESs are liquids in room temperature.The VFT equation can be used for fitting of the electrical conductivity and dynamic viscosity to temperature very well and Arrhenius equation can not.The relative big molecule DESs exhibited the higher density,electrical conductivity,and refractive index than the relative small molecule,but,the poorer liquidity.According to the Walden rule,the slopes were obtained and the values are 0.653 for [TBAC:2PA],0.564 for [TBAC:2EG],0.621 for [TBAC:2PEG],and 0.710 for [TBAC:2PAA],respectively.The temperature dependence on refractive index was fitted by the linear equation.This study will be significant for DESs' industrial and engineering applications.
Supporting Information:The1H NMR spectra and the experimental values of density,dynamic viscosity,electrical conductivity,and refractive index have been included.This information is available free of charge via the internet at http://www.whxb.pku.edu.cn.
(1)Zhang,Q.H.;Vigier,K.D.O.;Royer,S.;Jérôme,F.Chem.Soc.Rev.2012,41,7108.doi:10.1039/c2cs35178a
(2)Jhong,H.R.;Wong,D.S.H.;Wan,C.C.;Wang,Y.Y.;Wei,T.C.Electrochem.Commun.2009,11,209.doi:10.1016/j.elecom.2008.11.001
(3)Singh,B.S.;Lobo,H.R.;Shankarling,G.S.Catal.Commun.2012,24,70.doi:10.1016/j.catcom.2012.03.021
(4)Dai,Y.;Sprosen,J.V.;Witkamp,G.J.;Verpoorte,R.;Choi,Y.H.Anal.Chim.Acta 2013,766,61.doi:10.1016/j.aca.2012.12.019
(5)Francisco,M.;Bruinhorst,A.V.D.;Kroon,M.C.Angew.Chem.Int.Edit.2013,52,3074.doi:10.1002/anie.201207548
(6)Krystof,M.;Pérez-Sánchez,M.;María,P.D.D.ChemSusChem 2013,6,630.doi:10.1002/cssc.201200931
(7)Maugeri,Z.;María,P.D.D.ChemCatChem 2014,6,1535.
(8)Serrano,M.C.;Gutiérrez,M.C.;Jiménez,R.;Ferrer,M.L.;Monte,F.D.Chem.Commun.2012,48,579.doi:10.1039/C1CC15284J
(9)Zhang,Z.H.;Zhang,X.N.;Mo,L.P.;Li,Y.X.;Ma,F.P.Green Chem.2012,14,1502.doi:10.1039/c2gc35258c
(10)Carriazo,D.;Gutirrez,M.C.;Pic,F.;Rojo,J.M.;Fierro,J.L.G.;Ferrer,M.L.;Monte,F.D.ChemSusChem 2012,5,1405.doi:10.1002/cssc.v5.8
(11)Abbott,A.P.;Ttaib,K.E.;Frisch,G.;Ryder,K.S.;Weston,D.Phys.Chem.Chem.Phys.2012,14,2443.doi:10.1039/c2cp23712a
(12)Monte,F.D.;Carriazo,D.;Serrano,M.C.;Gutirrez,M.C.;Ferrer,M.L.ChemSusChem 2014,7,999.doi:10.1002/cssc.201300864
(13)Abbott,A.P.;Cullis,P.M.;Gibson,M.J.;Harris,R.C.;Raven,E.Green Chem.2007,9,868.doi:10.1039/b702833d
(14)Shahbaz,K.;Mjalli,F.S.;Hashim,M.A.;AlNashef,I.M.Energy Fuels 2011,25,2671.doi:10.1021/ef2004943
(15)Li,C.P.;Li,D.;Zou,S.S.;Li,Z.;Yin,J.M.;Wang,A.L.;Cui,Y.N.;Yao,Z.L.;Zhao,Q.Green Chem.2013,15,2793.doi:10.1039/c3gc41067f
(16)Abbott,A.P.;Capper,G.;Davias,D.L.;Rasheed,R.K.;Tambyrajah,V.Chem.Commun.2003,70,70.
(17)Maugeri,Z.;María,P.D.D.RSC Adv.2012,2,421.doi:10.1039/C1RA00630D
(18)Ruß,C.;König,B.Green Chem.2012,14,2969.doi:10.1039/c2gc36005e
(19)Bahadori,L.;Manan,N.S.;Chakrabarti,M.H.;Hashim,M.A.;Mjalli,F.S.;AlNashef,I.M.;Hussain,M.A.;Low,C.T.J.Phys.Chem.Chem.Phys.2013,15,1707.doi:10.1039/C2CP43077K
(20)Liu,Q.S.;Yang,M.;Yan,P.F.;Liu,X.M.;Tan,Z.C.;Welz-Biermann,U.J.Chem.Eng.Data 2010,55,4928.doi:10.1021/je100507n
(21)Liu,Q.S.;Li,P.P.;Welz-Biermann,U.;Chen,J.;Liu,X.X.J.Chem.Thermodyn.2013,66,88.doi:10.1016/j.jct.2013.06.008
(22)Chen,Y.;Zhuo,K.;Chen,J.;Bai,G.J.Chem.Thermodyn.2015,86,13 doi:10.1016/j.jct.2015.02.017
(23)Chen,Y.;Zhang,H.;Li,A.;Zhuo,K.Fluid Phase Equilibr.2015,388,78.doi:10.1016/j.fluid.2014.12.038
(24)Tong,J.;Hong,M.;Chen,Y.;Wang,H.;Guan,W.;Yang,J.Z.J.Chem.Thermodyn.2012,54,352.doi:10.1016/j.jct.2012.05.012
(25)Lide,D.R.Handbook of Chemistry and Physics,82nd ed.;CRC Press:Boca Raton,FL,2001–2002.
(26)Jacquemin,J.;Husson,P.;Padua,A.A.H.;Majer,V.Green Chem.2006,8,172.doi:10.1039/B513231B
(27)Glasser,L.Thermochim.Acta 2004,421,87.doi:10.1016/j.tca.2004.03.015
(28)Vila,J.;Ginés,P.;Pico,J.M.;Franjo,C.;Jiménez,E.;Varela,L.M.;Cabeza,O.Fluid Phase Equilibr.2006,242,141.doi:10.1016/j.fluid.2006.01.022
(29)Schreiner,C.;Zugmann,S.;Hartl,R.;Gores,H.J.J.Chem.Eng.Data 2010,55,1784.doi:10.1021/je900878j
(30)Belieres,J.P.;Angell,C.A.J.Phys.Chem.B 2007,111,4926.doi:10.1021/jp067589u
Properties of Four Deep Eutectic Solvents:Density,Electrical Conductivity,Dynamic Viscosity and Refractive Index
SU Hong-Zhen1YIN Jing-Mei1LIU Qing-Shan2,*LI Chang-Ping1,*
(1College of Chemical Engineering and the Environment,Dalian University,Dalian 116622,Liaoning Province,P.R.China;2School of Science,Shenyang Agricultural University,Shenyang 110866,P.R.China)
Four deep eutectic solvents(DESs) were prepared from tetrabutylammonium chloride∶tetrabutylammonium chloride∶propionic acid [TBAC∶2PA],tetrabutylammonium chloride∶ethylene glycol [TBAC∶2EG],tetrabutylammonium chloride∶polyethylene glycol [TBAC∶2PEG],and tetrabutylammonium chloride∶phenylacetic acid [TBAC∶2PAA].The density,electrical conductivity,dynamic viscosity,and refractive index of the samples were measured at 288.15–338.15 K under atmospheric pressure.The influence of the temperature on the density,electrical conductivity,dynamic viscosity,and refractive index are discussed.The thermal expansion coefficient,molecular volume,standard molar entropy,and lattice energy were determined from the measured values using empirical equations.The temperature dependences on the electrical conductivity and dynamic viscosity of the DESs were fitted by the Vogel-Fulcher-Tamman(VFT) equation.The Arrhenius equation is also discussed for the electrical conductivity and dynamic viscosity.The above study will be of great significance for the industrial and engineering applications of DESs.
Deep eutectic solvent; Density; Electrical conductivity; Dynamic viscosity;Refractive index
March 13,2015;Revised:June 10,2015;Published on Web:June 11,2015.
O642
icle]
10.3866/PKU.WHXB201506111 www.whxb.pku.edu.cn
*Corresponding authors.LIU Qing-Shan,Email:liuqingshan@dicp.ac.cn;Tel:86-13478787524.LI Chang-Ping,Email:changpingli@dicp.ac.cn;Tel:+86-13795164535.
The project was supported by the National Natural Science Foundation of China(21203193,21306016,21176033).
国家自然科学基金(21203193,21306016,21176033)资助项目© Editorial office of Acta Physico-Chimica Sinica
