均相Co(Ⅱ)/PMS体系对二氯喹啉酸的降解特性研究
2015-08-25钟美娥龚道新姚倩钰丁春霞杨丽华湖南农业大学理学院湖南长沙408湖南农业大学资源环境学院湖南长沙408湖南农业大学东方科技学院湖南长沙408
钟美娥,李 季,龚道新,姚倩钰,丁春霞,杨丽华(.湖南农业大学理学院,湖南 长沙 408;.湖南农业大学资源环境学院,湖南 长沙 408;3.湖南农业大学东方科技学院,湖南 长沙 408)
均相Co(Ⅱ)/PMS体系对二氯喹啉酸的降解特性研究
钟美娥1,2,3,李 季1,龚道新2*,姚倩钰2,丁春霞1,杨丽华2(1.湖南农业大学理学院,湖南 长沙 410128;2.湖南农业大学资源环境学院,湖南 长沙 410128;3.湖南农业大学东方科技学院,湖南 长沙 410128)
采用高级氧化技术,以Co2+为催化剂分解单过氧硫酸氢钾(PMS)所产生的强氧化性硫酸根自由基(SO4·-)降解水中的二氯喹啉酸(QC).考察了PMS用量、Co(Ⅱ)/PMS比值和Cl-浓度以及QC初始浓度对该均相Co(Ⅱ)/PMS体系降解QC的影响.结果表明,QC的降解遵循准一级动力学过程.当QC初始浓度在0.02~0.2mmol/L时,QC的降解速率随着QC/PMS比值的降低而增大,但当QC/PMS比值小于1/100时,则相反.QC的降解速率随着PMS浓度升高而线性增大,当PMS浓度为32mmol/L时,4h内QC的降解率可达94%.增大Co(Ⅱ)/PMS的摩尔比能够促进QC的降解,而Cl-对QC的降解有一定的抑制作用.LC/MS分析结果表明,3,7-二氯-8-羟基喹啉和7-氯-8-喹啉甲醛为QC降解过程中两种主要的中间产物.
高级氧化技术;单过氧硫酸氢钾;硫酸根自由基;降解;二氯喹啉酸
二氯喹啉酸是一种生长激素类除草剂,主要用于防除稻田单子叶杂草,尤其对稗草有极高活性,是我国稻田芽前苗后的主要除草剂品种之一[1-4].但是,由于前茬稻田使用除草剂二氯喹啉酸,其在土壤中的残留会导致后茬作物(如烤烟、黄瓜、蚕豆等)出现植株畸形生长,严重影响农作物的产量和质量.目前,针对水田二氯喹啉酸污染问题的治理措施主要有物理方法治理、化学药剂补救和微生物降解等措施.物理防治措施主要利用活性炭、蒙脱石等具有吸附特性的物质吸附土壤中残留的二氯喹啉酸[5-6],但不能从根本上去除二氯喹啉酸的影响;化学药剂补救主要是针对已经出现二氯喹啉酸药害的植株进行修复,如赤霉素、植保素、施嘉乐等化学药剂均体现出不同的修复效果[7],但是喷施化学药剂只能一定时间内缓解植株畸形,因为随着植株的生长,不断吸入土壤中存在的二氯喹啉酸,使体内二氯喹啉酸含量不断增加,植株畸形逐渐加重;微生物降解主要利用对二氯喹啉酸具有高效降解功能的菌株进行修复[8-9],但存在菌株筛选困难、降解周期长等缺点.可见,这些治理措施均存在一定的弊端,因此,开发一种高效、安全去除水田环境中二氯喹啉酸的方法成为环境科学领域亟待解决的问题.
1 材料与方法
1.1试剂与仪器
试剂:二氯喹啉酸(QC,江苏天容,96%),单过氧硫酸氢钾复合盐(PMS),七水合硫酸钴.实验用水均为去离子水.
仪器:Agilent1260高效液相色谱仪(色谱柱:250mm×4.6mm,5μm,反相C18柱),恒温震荡器,超高效液相色谱联合飞行时间串联质谱仪(Agilent, UHPLC(1290)-Q-TOF(6530), USA)等.
1.2试验方法
向50mL的敞口反应容器中依次加入一定浓度的二氯喹啉酸溶液和PMS溶液,最后加入一定量的Co2+溶液开始反应并计时,实验过程均未调节pH值.反应过程在150r/min恒温振荡器中进行,于 25℃振荡一定时间,按照一定的时间间隔进行取样,每次取样 1mL于样品瓶中,然后加入等体积的甲醇进行淬灭,摇匀后用液相色谱仪对溶液中剩余的二氯喹啉酸浓度进行检测,流动相为甲醇和水(1%冰乙酸)(V:V=60:40),检测波长240nm,流动相速度1.0mL/min,进样量20μL.
2 结果与讨论
2.1二氯喹啉酸初始浓度对其降解的影响
保持反应体系中 Co2+浓度为 0.04mmol/L,PMS浓度为4mmol/L,改变二氯喹啉酸的初始浓度,研究QC初始浓度对其降解速率的影响,所得结果如图1所示.
均相Co(Ⅱ)/PMS体系催化降解有机污染物的反应动力学一般用准一级动力学方程来描述[22-24].本研究按照准一级动力学方程(1)式求出不同QC初始浓度时的反应动力学常数:

式中:C0和C分别为t=0及t时反应体系中QC的浓度;k为反应速率常数;t为反应时间.不同QC初始浓度下反应速率常数变化如表1所示.
图1和表1结果均表明,在维持Co2+浓度和PMS浓度不变的情况下,随着二氯喹啉酸初始浓度的降低,QC的降解速率逐渐加快,但当其初始浓度小于0.04mmol/L,即QC与PMS的摩尔比小于1/100时,继续降低二氯喹啉酸的初始浓度,对其降解速率产生了一定程度的抑制.这与文献报道的随污染物初始浓度的降低其体系的降解速率总是逐渐加快的现象不一致[15,25].原因可能是由于:如式(2)所示,当Co2+浓度和PMS浓度不变时,体系产生的硫酸根自由基量是一定的,当二氯喹啉酸浓度越低,相对所获得的硫酸根自由基的进攻越多,越有利于QC的降解.另一方面,由式(3)可以看出,当产生的硫酸根自由基来不及消耗时,过多的硫酸根自由基之间相互反应生成过硫酸盐,从而影响了二氯喹啉酸的反应速率[26].
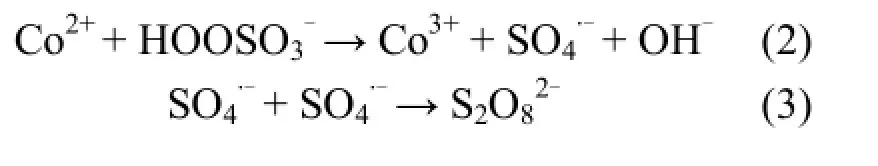
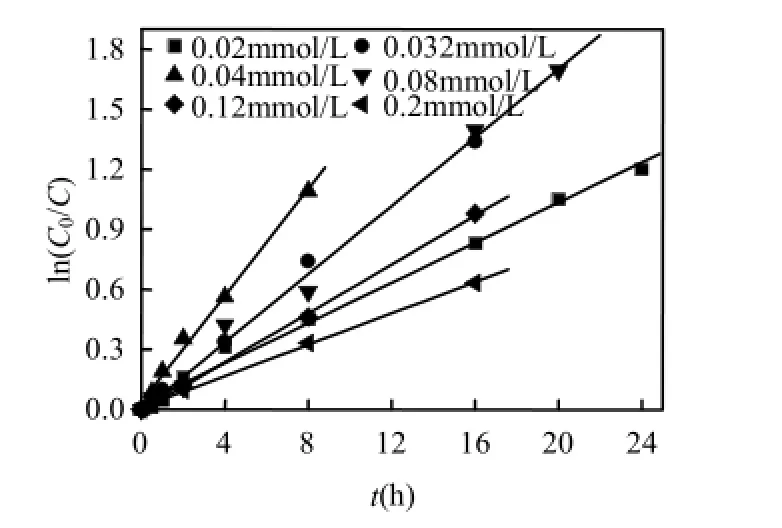
图1 不同二氯喹啉酸初始浓度下ln(C0/C)与反应时间的拟合曲线Fig.1 Plots of ln(C0/C) versus reaction time at various [QC]
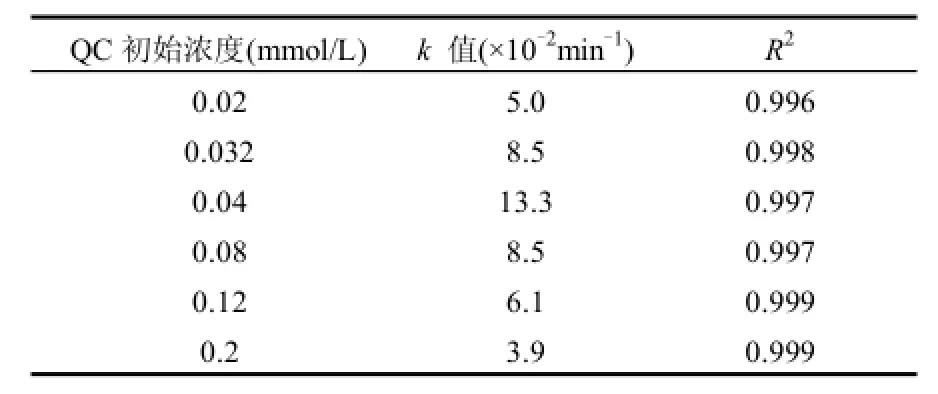
表1 不同二氯喹啉酸初始浓度下降解速率常数的变化Table 1 Effect of QC initial concentration on its degradation rate constant
2.2PMS用量对二氯喹啉酸降解的影响
保持反应体系中二氯喹啉酸初始浓度不变,为0.04mmol/L,Co2+与PMS摩尔浓度之比始终保持在Co(Ⅱ)/PMS=1/100,改变PMS的投加量,使其在0~32mmol/L范围内变化,研究PMS用量对QC降解速率的影响,所得结果如图 2(a)所示.由图2(a)可以看出,随着PMS用量的增大,QC的降解速率逐渐加快,当PMS浓度为20mmol/L时,4h 内QC的降解率可达80%左右;当继续增加PMS浓度至32mmol/L时,4h内QC的降解率为94%. 图2(b)描述了PMS浓度在0~32mmol/L范围内变化时对反应速率常数 k的影响.由图 2(b)可知,QC的降解速率随着PMS浓度的升高而线性增大.表明Co(Ⅱ)/PMS体系降解QC的一级动力学常数与PMS的浓度成正比[27].
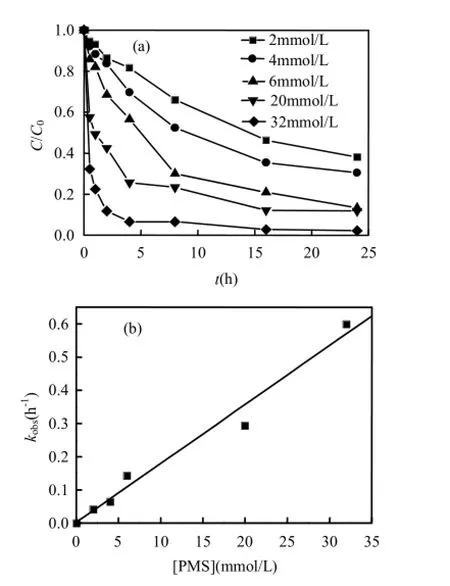
图2 PMS浓度对Co(Ⅱ)/PMS体系中二氯喹啉酸降解的影响Fig.2 Effect of PMS concentration on the degradation of quinclorac in the Co(Ⅱ)/PMS system
2.3Co2+用量对二氯喹啉酸降解的影响
保持溶液中二氯喹啉酸和PMS初始浓度不变,分别为0.02mmol/L和4mmol/L,改变钴盐的加入量,使Co2+浓度和PMS浓度之比分别为不添加Co2+和Co(Ⅱ)/PMS=1/10000、1/1000与l/100,研究Co2+用量的变化对Co(Ⅱ)/PMS体系降解二氯喹啉酸的影响.实验结果如图3所示.由图3可知,随着Co2+用量的增加,二氯喹啉酸的降解率逐渐增大,当体系中只有PMS时,二氯喹啉酸的降解率仅为 8%,当 Co(Ⅱ)/PMS=1/10000、1/1000 与l/100时其降解率分别为 24%、30%和 70%.虽然 Co2+用量的增加能够促进二氯喹啉酸的降解,但是Co2+不是环境友好物质,在使用过程中不宜过多投加,因此,实验选定 Co(Ⅱ)/PMS= l/100为适宜添加量.

图3 Co(Ⅱ)/PMS摩尔比对Co(Ⅱ)/PMS体系中二氯喹啉酸降解的影响Fig.3 Effect of molar ratio of Co(Ⅱ)/PMS on the degradation of quinclorac in the Co(Ⅱ)/PMS system
2.4Cl-浓度对二氯喹啉酸降解的影响
目前,在诸多阴离子中,研究较多的是 Cl-,因此实验选择添加NaCl来考察无机阴离子Cl-对Co(Ⅱ)/PMS体系降解二氯喹啉酸的影响,所得结果如图4所示.

图4 Cl-浓度对Co(Ⅱ)/PMS体系中二氯喹啉酸降解的影响Fig.4 Effect of Cl-concentration on the degradation of quinclorac in the Co(Ⅱ)/PMS system
由图 4可以看出,当氯离子的添加量为5mmol/L时,其对Co(Ⅱ)/PMS体系催化降解二氯喹啉酸的影响较小,QC的降解率从 70%下降到64%,仅降低了6%;继续增大Cl-用量时,QC的降解率继续降低,但总体下降幅度不大,当氯离子的添加量高达50mmol/L时,QC降解率仍有56%.可见,氯离子对 Co(Ⅱ)/PMS体系降解二氯喹啉酸有一定的抑制作用.这可能是因为能够氧化 Cl-生成氯自由基,如式(4)所示,而氯自由基的氧化活性比低,从而成为的淬灭剂[28-29].

2.5二氯喹啉酸在均相 Co(Ⅱ)/PMS体系中的降解机理
二氯喹啉酸在均相Co(Ⅱ)/PMS体系中的降解产物经过LC/MS分析,发现产物主要为图5中的a[m/z=214,179,150,122]和b[m/z=188,173,130].根据质谱图,当降解产物的m/z=214时,推测该物质可能是二氯喹啉酸脱羰基后的产物,为 3,7-二氯-8-羟基喹啉(242-CO=214),特征碎片有 7-氯-8-羟基喹啉(214-Cl+H=179)、1,2,3,4-四氢-8-羟基喹啉(214-2Cl+6H=150)、2-氨基-3-甲基-苯酚(214-2Cl-CH-N+6H=122).当降解产物的m/z =188时,推断二氯喹啉酸的另一降解产物为7-氯-8-喹啉甲醛(242-Cl-OH-H=188);特征碎片有 7-氯-8-甲基喹啉(188-O+H=173)、喹啉(188-Cl-CO+5H=130).另外,向二氯喹啉酸降解产物中添加AgNO3溶液时,观察到有白色沉淀形成,进一步证实二氯喹啉酸的降解产物中形成了脱氯物质.
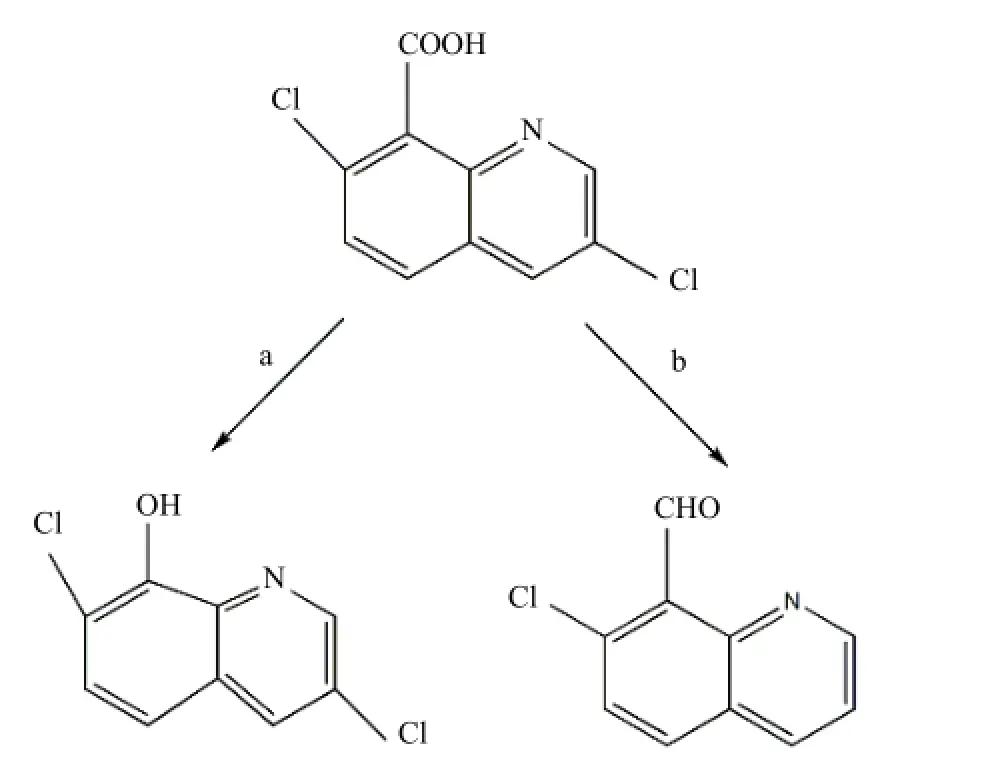
图5 二氯喹啉酸在Co(Ⅱ)/PMS体系中可能的降解途径Fig.5 Possible degradation pathways of quinclorac in the Co(Ⅱ)/PMS system
因此,根据这一分析结果,提出了二氯喹啉酸在均相 Co(Ⅱ)/PMS体系中的降解途径,所得结果如图5所示.由图5可推测,在均相Co(Ⅱ)/PMS体系中,Co2+催化PMS分解生成.然后硫酸自由基进攻二氯喹啉酸分子两个不同的位置:第一,进攻羧酸基团上的羰基生成相应的脱羰基化合物 a;第二,进攻喹啉环上氯原子和羟基生成相应的醛类化合物 b.这一研究结果与 Li等[8]和Lucía等[30]采用生物技术和光催化技术降解二氯喹啉酸生成的降解产物相类似.
3 结论
3.1采用 Co2+活化单过氧硫酸氢钾(PMS)的方法可以有效降解二氯喹啉酸,降解过程符合准一级反应动力学.
3.2在 Co(Ⅱ)/PMS体系中随着二氯喹啉酸初始浓度的降低,QC降解速率先升高后降低,QC的降解速率随着PMS浓度的升高而线性增大,Co2+促进二氯喹啉酸的降解,Cl-对QC降解有抑制作用.
3.3LC/MS鉴定结果表明二氯喹啉酸的降解是一个脱羰基和脱氯的过程,降解的主要产物为3,7-二氯-8-羟基喹啉和7-氯-8-喹啉甲醛.
[1] Yukari Sunohara, Hiroshi Matsumoto. Quinclorac-induced cell death is accompanied by generation of reactive oxygen species in maize root tissue [J]. Phytochemistry, 2008,69(12):2312-2319.
[2] Maria Vittoria Pinna, Alba Pusino. Direct and indirect photolysis of two quinolinecarboxylic herbicides in aqueous systems [J]. Chemosphere, 2012,86(6):655-658.
[3] Lü Zhenmei, Min Hang, Wu Shuwen, et al. Phylogenetic and degradation characterization of burkholderia cepacia wz1 degrading herbicide quinclorac [J]. Journal of Environmental Science and Health Part B-pesticides, food contaminants, and agricultural wastes, 2003,B38(6):771-782.
[4] Yukari Sunohara, Shinjiro Shirai, Hiroki Yamazaki, et al. Involvement of antioxidant capacity in quinclorac tolerance in Eleusine indica [J]. Environmental and Experimental Botany,2011,74:74-81.
[5] 丁春霞,何紫君,郑 琛,等.HDTMAB改性蒙脱石对二氯喹啉酸的吸附研究 [J]. 农业环境科学学报, 2014,33(9):1755-1761.
[6] 杨丽华,龚道新,袁雅洁,等.低分子量有机酸对粘土矿物吸附二氯喹啉酸的影响 [J]. 农药学学报, 2013,15(3):323-330.
[7] 郑雄志,李宏光,龙世平,等.几种解毒剂对烟田二氯喹啉酸次生药害的修复效果 [J]. 南方农业学报, 2013,44(3):426-430.
[8] Li Zimu, Shao Tiejuan, Min Hang, et al. Stress response of Burkholderia cepacia WZ1 exposed to quinclorac and the biodegradation of quinclorac [J]. Soil Biology and Biochemistry,2009,41(5):984-990.
[9] Lü Zhenmei, Li Zimu, Sang Liya, et al. Characterization of a strain capable of degrading a herbicide mixture of quinclorac and bensulfuronmethyl [J]. Pedosphere, 2008,18(5):554-563.
[10] 刘佳露,卢 伟,张凤君,等.活化过硫酸盐氧化地下水中苯酚的动力学研究 [J]. 中国环境科学, 2015,35(9):2677-2681.
[11] Li Huanxuan, Wan Jinquan, Ma Yongwen, et al. Influence of particle size of zero-valent iron and dissolved silica on the reactivity of activated persulfate for degradation of acid orange 7 [J]. Chemical Engineering Journal, 2014,237:487-496.
[12] Han Donghui, Wan Jinquan, Ma Yongwen, et al. New insights into the role of organic chelating agents in Fe(II) activated persulfate processes [J]. Chemical Engineering Journal, 2015,269:425-433.
[13] Shi Yafei, Yang Jiakuan, Yu Wenbo, et al. Synergetic conditioning of sewage sludge via Fe2+/persulfate and skeleton builder: Effect on sludge characteristics and dewaterability [J]. Chemical Engineering Journal, 2015,270:572-581.
[14] 何洋洋,唐素琴,康婷婷,等.响应面法优化硫酸根自由基高级氧化深度处理渗滤液生化尾水 [J]. 中国环境科学, 2015,35(6):1749-1755.
[15] Javier Fernandez, Pichai Maruthamuthu, Albert Renken, et al. Bleaching and photobleaching of Orange II within seconds by the oxone/Co2+ reagent in Fenton-like processes [J]. Applied Catalysis B: Environmental, 2004,49(3):207-215.
[16] Do Si-Hyun, Jo Jeong-Hwan, Jo Young-Hoon, et al. Application of a peroxymonosulfate/cobalt (PMS/Co(II)) system to treat diesel-contaminated soil [J]. Chemosphere, 2009,77(8):1127-1131.
[17] Wang Zhaohui, Yuan Ruixia, Guo Yaoguang, et al. Effects of chloride ions on bleaching of azo dyes by Co2+/oxone regent:kinetic analysis [J]. Journal of Hazardous Materials, 2011, 190 (1-3):1083-1087.
[18] George P. Anipsitakis, Dionysios D. Dionysiou. Degradation of organic contaminants in water with sulfate radicals generated by the conjunction of peroxymonosulfate with cobalt [J]. Environ. Sci. Technol., 2003,37(20):4790-4797.
[19] Xu Lei, Yuan Ruixia, Guo Yaoguang, et al. Sulfate radicalinduced degradation of 2,4,6-trichlorophenol: A de novo formation of chlorinated compounds [J]. Chemical EngineeringJournal, 2013,217:169-173.
[20] Zhang Mi, Chen Xiaoqing, Zhou He, et al. Degradation of p-nitrophenol by heat and metal ions co-activated persulfate [J]. Chemical Engineering Journal, 2015,264:39-47.
[21] Li Shen-Xin, Wei Dong, Mak Nai-Ki, et al. Degradation of diphenylamine by persulfate: Performance optimization, kinetics and mechanism [J]. Journal of Hazardous Materials, 2009,164(1):26-31.
[22] Yang Shiying, Xiao Tuo, Zhang Jun, et al. Activated carbon fiber as heterogeneous catalyst of peroxymonosulfate activation for efficient degradation of Acid Orange 7 in aqueous solution [J]. Separation and Purification Technology, 2015,143:19-26.
[23] Sun Hongqi, Kwan ChungKeat, Suvorova Alexandra, et al. Catalytic oxidation of organic pollutants on pristine and surface nitrogen-modified carbon nanotubes with sulfate radicals [J]. Applied Catalysis B: Environmental, 2014,154-155:134-141.
[24] Zhou Lincheng, Ma Junjun, Zhang He, et al. Fabrication of magnetic carbon composites from peanut shells and its application as a heterogeneous Fenton catalyst in removal of methylene blue [J]. Applied Surface Science, 2015,324:490-498.
[25] Wang Xuxian, Sun Hongqi, Duan Xiaoguang, et al. A new magnetic nano zero-valent iron encapsulated in carbon sphere for oxidative degradation of phenol [J]. Applied Catalysis B:Environmental, 2015,172-173:73-81.
[26] 陈晓旸,王卫平,朱凤香,等.UV/K2S2O8降解偶氮染料AO7的研究:动力学及反应途径 [J]. 环境科学, 2010,31(7):1433-1537.
[27] Chen Xiaoyang, Qiao Xianliang, Wang Degao, et al. Kinetics of oxidative decolorization and mineralization of acid orange 7by dark and photoassisted Co2+-catalyzed peroxymonosulfate system [J]. Chemosphere, 2007,67(4):802-808.
[28] Chan K H, Chu W. Degradation of atrazine by cobalt-mediated activation of peroxymonosulfate: different cobalt counter anions in homogenous process and cobalt oxide catalysts in photolytic heterogeneous process [J]. Water Research, 2009,43(9):2513-2521.
[29] Lars R Bennedsen, Jens Muff, Erik G Søgaard. Influence of chloride and carbonates on the reactivity of activated persulfate [J]. Chemosphere, 2012,86(11):1092-1097.
[30] Lucía Pareja, Andrés Pérez-Parada, Ana Agüera, et al. Photolytic and photocatalytic degradation of quinclorac in ultrapure and paddy field water: Identification of transformation products and pathways [J]. Chemosphere, 2012,87(8):838-844.
致谢:本实验的液质(LC/MS)分析测试工作由湖南农业大学分析测试中心完成,在此表示感谢.
Degradation characteristics of quinclorac in homogeneous Co(Ⅱ)/PMS system.
ZHONG Mei-e1,2,3, LI Ji1, GONG Dao-xin2*, YAO Qian-yu2, DING Chun-xia1, YANG Li-hua2(1.College of Science, Hunan Agriculture University,Changsha 410128, China;2.College of Resource and Environment, Hunan Agricultural University, Changsha 410128,China;3.Orient Science and Technology College of Hunan Agricultural University, Changsha 410128, China).
China Environmental Science, 2015,35(11):3282~3287
An effective advanced oxidation process for the degradation of quinclorac (QC) in water is reported. This method is based on the oxidation of quinclorac by sulfate radicals generated from the decomposition of peroxymonosulfate (PMS) mediated by Co (Ⅱ) ion in the aqueous phase. The effects of the concentration of PMS and Cl-,molar ratio of Co (Ⅱ)/PMS as well as the initial concentration of QC on the degradation efficiency of QC were examined. The results showed that the degradation of QC in the homogeneous Co (Ⅱ)/PMS system fitted well to the pseudo-first-order kinetic model. The degradation rate of QC increased with the decreasing of molar ratio of QC/PMS, but declined as the ratio of QC/PMS lower than 1/100 when the initial concentration of QC was in the range of 0.02~0.2mmol/L. The reaction rates linearly increased with the increase of PMS concentration with a QC decomposition as high as 94% within 4 hours at an initial concentration of 32mmol/L PMS. The ratio of Co (Ⅱ)/PMS had positive effect on the degradation of QC, while Cl-had negative impact. The results of LC/MS analysis indicated that 3,7-dichloro-8-hydroxy quinoline and 7-chloro-8-quinoline carboxaldehyde were the two major intermediates of QC degradation.
advanced oxidation technologies;peroxymonosulfate;sulfate radical;degradation;quinclorac
X703.5
A
1000-6923(2015)11-3282-06
2015-04-15
湖南农业大学东方科技学院项目(14QNZ07);湖南省教育厅项目(15C0653);湖南省科技厅项目(2014SK3178)
* 责任作者, 教授, gdx4910@163.com
钟美娥(1979-),女,湖南邵阳人,讲师,博士,主要从事环境污染物的修复治理以及功能材料的制备与性能研究.发表论文 20余篇.
