Preparation and Surface Modification of High-Density Chitosan/Functionalized Multi-walled Carbon Nanotubes Hollow Fibers
2015-08-07SHAOMeiling邵梅玲DUPan杜盼YANGQing杨庆HSIAOBenjamin
SHAO Mei-ling(邵梅玲),DU Pan(杜盼),YANG Qing(杨庆),HSIAO Benjamin S
College of Material Science and Engineering,Donghua University,Shanghai201620,China
Preparation and Surface Modification of High-Density Chitosan/Functionalized Multi-walled Carbon Nanotubes Hollow Fibers
SHAO Mei-ling(邵梅玲),DU Pan(杜盼),YANG Qing(杨庆)*,HSIAO Benjamin S
College of Material Science and Engineering,Donghua University,Shanghai201620,China
Functionalized multi-walled carbon nanotubes(f-MWNTs)were prepared with chitosan via controlled surface deposition and crosslinking process and scanning electron m icroscopy (SEM),Fourier translation infrared spectroscopy(FT-IR)and X-ray diffraction(XRD)are used to character properties.A novel high-density chitosan(HCS)was dissolved in f-MWNTs dispersed dilute acetic acid with a maximal concentration of 5.8%.The hollow fibers can be made by extruding the solution into a dilute alkali solution through a wet-spinning process and the tensile properties of thematerialswere evaluated by universal tester.The surface property of fibers,pretreated by Helium(He)and the following grafted with gelatin was evaluated with X-ray photoelectron spectroscopy(XPS).As the hollow fibers were intended for neural tissue engineering,its suitability was evaluated in vitro using rat Schwann cells(RSC96)as model cells.The cells attachment,proliferation and morphology,were studied by variousm icroscopic techniques.Based on the results,the gelatin grafted HCS/f-MWNTs hollow fibers could be used as a potential cell carrier in neural tissue engineering.
chitosan;multi-walled carbon nanotubes(MWNTs); hollow fibers;wet-spinning;cold plasma treatment;biocompatibility
Introduction
In recent years,the treatment for injury-induced nerve defect typically relays on donor tissues from the patient,and it leads to the issue of function loss at the donor sites,formation of potential painful neuromas and structural differences between donor and recipientnerves[1-2].Consequently,the development of artificial matrices that can support cell attachment,proliferation and differentiation,as well as deliver bioactive factors and/or host cells has attracted a great deal of attention[3-4].At present,chitin and chitosan have become protagonist in the field of nerve regeneration thanks to the relevant biochem ical significance,and in particular itaccelerates macrophage m igration and nerve cells proliferation,and promotes granulation and vascularization[5].Besides,the solubility of chitosan with high density is quite high(nearly 3 times as ordinary chitosan)for the regular spatial structure,which will endow fiber production with good mechanical strength ow ing to the dense structure.
Carbon nanotubes(CNTs)have also attracted a great deal of attention for biological applications both at molecular and cellular levels due to their novel properties,such as superior strength,electrical conductivity and availability of chemical functionalization[6].Mattson et al.[7]reported the feasibility of using CNTs as a substrate for neuronal grow th and it had well elaborated neuritis and branching on multi-walled carbon nanotubes(MWNTs).In this work,chemically functionalized MWNTs(f-MWNTs)were dispersed in high-density chitosan (HCS)to obtain high strength hollow fibers.
Gelatin is a partial derivative of collagen,which is the major component of skin,bone,cartilage and connective tissues[8].It is commonly used in tissue engineering because of its biodegradability,biocompatibility and easy availability.In this paper,gelatin is used formodification of the hollow fibers' surface property to strengthen the interaction between fibers and cells.Among the various surface modification approaches available for chitosan,plasma treatment seems to be most prom ising.So cold plasma pretreatment is carried out to induce gelatin grafting and rat Schwann cells(RSC96)are used to assess its efficacy in promoting neuron attachment and proliferation in vitro.
1 Experimental
1.1 M aterials
HCS(bulk density≥0.8 g/mL)in our experiments was supplied by Golden-Shell Biochemical Co.,Ltd.,China with the deacetylation degree of 90%,and the viscosity-average molecular weight measured by Ubbelohde viscometer(NCY-4,China)is 6.81×105.MWNTs were obtained from Shenzhen Nanotech PortCo.Ltd.,China.Acetic acid,nitric acid,sulphuric acid,ethanol and sodium hydroxide(NaOH)are all purchased from National Pharmaceutical Group Chemical Reagent Co.,Ltd. China.Gelatin for surface grafting was obtained from Shanghai Fankelbio Science&Technology Co.,Ltd.,China.All of the cell-related reagentswere bought from Gibco(Invitrogen,USA).
1.2 Preparation of f-MWNTs
The purified and oxidized pretreatmentof raw MWNTswas performed in hot reflux condenser by heating concentrated sulphuric acid and nitrating acid mixtures(3∶1 by volume)at 110℃for12 h.Then themixturewas extracted through a0.22 μm polytetrafluoroethylene filter membrane.Follow ing that,the residue was washed by deionized water to neutrality after extraction and then dried in vacuum oven at 80℃.Then 100 mg HCS was dissolved in 100 m L dilute acetic acid solution (pH=2),and 100 mg pretreated MWNTs were added in the solution and ultrasonic treated for 2 h in a sonicating bath followed by magnetic stirring for 1 h.The weak aqua ammonia was dropped into the HCS/MWNTs dispersion to make pH of the system up to 10 and HCS would deposit on the surface of MWNTs.After adding 0.02 g glutaraldehyde(6%water solution)into themixture,itwas heated to 60℃for 1.5 h for deposited HCS cross linking reaction and unreacted HCS was removed by dilute acetic acid solution.Then f-MWNTs was obtained by collecting with centrifugation and drying to constant weight at50℃in a vacuum oven.
1.3 Preparation of HCS/f-MWNTs hollow fibers via wet spinning
The preparation of spinning solution was carried out as follows.A certain amount of f-MWNTs was ultrasonic welldispersed in 2%dilute acetic acid solution,and then dried HCS power was added and stirred rapidly.The concentration of HCS was unchanged(maintained at 5.8%)in the solution and the content of f-MWNTs were 0.1%,0.3%,0.5%and 0.7%(relative to the mass of HCS)respectively.The m ixture gradually turned into a homogeneous solution after stirring for 3 h.In this work,the wet spinning line used to produce the hollow fibers was our laboratory homemade instrument. Solutions were spun at room temperature with extrusion rate of 300m L/h and coagulated in coagulation baths(3%NaOH and 3%ethanol water mixture).The collected hollow fibers were washed in deionized water for about 24 h to remove residual solvent and dried at room temperature for 12 h.
1.4 Plasma pretreatment and surfacemodification
The hollow fibers were put into a vacuum chamber of the cold plasma processor;then evacuated the chamber until the atmospheric pressure dropped down to 2-3 Pa and the reactant He gas was charged into for 10 m in to remove residual air. Next,He was fed into constantly to maintain the working pressure at20 Pawith the discharge power of20-100W.After treated for 30-150 s,some of the pretreated samples were soaked into gelatin solution(10%)overnight to allow the graft reaction completed.Follow ing by washed with deionized water and cleaned ultrasonically for 3 times to remove absorbed gelatin,the products were immersed into deionized water overnight at 40℃to remove the unreacted gelatin thoroughly and then died at room temperature.
1.5 Cell culture on the hollow fibers
The samples were immersed in 75%(v/v)ethanol aqueous for 1 h followed by ultraviolet radiation for 1 h to sterilize and five parts with the same quality of every sample were selected to participate in the experiment to calculate the error bar.Then all the samples were pre-cultured in RCS96 culture medium that containing 90%Dulbecco's modified eagle'smedium(DMEM)solution,9%bovine serum albumin (BSA)and 1%Penicillin-Streptomycin for 24 h.Next,cell suspensionswith specific density were seeded on each sample in a 24-well plate and themedium was changed every third day.The density of cells for assessing attachmentand proliferation were2. 5×106cells/well and 1×104cells/well respectively.The cell/ sample constructswere incubated under sterile conditions at37℃in a humidified incubator of 5%CO2.
1.6 M easurements
The morphology of the samples and cell grow th on the fiberswere examined by scanning electron m icroscopy(SEM) (JSM-5600LV,Japan).For SEM analysis,the fibers were frozen in liquid nitrogen,fractured immediately,and vacuumdried.The cell/sample constructs immobilized with 4% paraformaldehyde and subjected to sequential dehydration for 15 min each with ethanol series(60%,70%,80%,90%and 100%),then dried at room temperature.Surface properties of the f-MWNTswere tested using the automatic target recognition (ATR)attachment of Fourier translation infrared spectroscopy (FT-IR,Nicolet8700,USA)under spectral range of650-4 000 cm-1at a resolution of 4 cm-1.The crystalline structure of the sampleswas examined using X-ray diffraction(XRD)(D/max- 2550 PC,Japan).The tensile properties of the fibers were measured by a universal testing machine(20 kN WDW3020,China).The sample was fixed in the clamp with a loading velocity of10mm/min and five parts of every samplewere tested to take the average.The surface elements and contents of fibers after modification were characterized with the X-ray photoelectron spectroscopy(XPS)(ESCALAB 200R,UK).
1.7 Cell attachment and proliferation on hollow fibers
Cell adhesion evaluation on the hollow fiberswasmeasured by the follow ing method in this paper:the cell/sample constructs after 2,8 and 24 h of incubation were washed with DMEM for 3 times to remove the cells thathad not adsorbed on the surface,then 0.5 m L Tryspin-ethylene diamine tetraacetic acid(EDTA)and 0.5 m L cell-culturemedium were added into every constructand fluttered lightly to help cells slough-off from the construct.The number of cells in the suspension was counted by using Cell Counter(Moxi Z,USA).The adhesion rate on the surface of cell-culture plate is regarded as100%,so the cell adhesion rate(R)was calculated as follows:R/%=where N is the cell number of attached on samples'1surface;N0is the number of blank plate.CCK-8(cell counting kit-8)is a kind of cell viability assay reagent with higher sensitivity and better reproducibility;and the cytotoxicity of fibers was measured by the common method:cell/sample constructs after1,3,5,7,9 and 11 d of culturewerewashed for 3 times with DMEM to remove dead cells,then incubated at 37℃for 2.5 h with the addition of 500μL CCK-8 reagent in medium solution(10%(v/v)).The cell activity can be obtained from the optical density(OD)of the solutionmeasured by Multifunctionalmicroplate reader(DG5031,USA).
2 Results and Discussion
Figure 1 is the representative SEM micrographs of MWNTs taken with large enough magnification before and after treatment.We can view directly that most of the tube bodies spread out with significant improvement of agglomeration that close to single dispersion and tangle also is eased after surface deposition and crosslinking process(from Fig.1(b)).In addition,during the process of pre-oxidation,amorphous carbon and some short tubes are removed by the role of concentrated acid,so the treatment also endows the tubes with uniform thickness,and it shows a little thicker than the raw tubes for the deposition of HCS coating around the tubes.
From photos of dispersivity(Fig.1(c)),we can clearly see that the dispersion of f-MWNTs ismuch better than the raw one and this superiority already appears after ultrasonication for half an hour.Even though the MWNTs dispersed as well as f-MWNTs after 2 h,it agglomerates together again after 12 h.


Fig.1 Themicromorphologies of MWNTs(a)before and(b)after surface functional treatment and(c)the dispersivity contrast in water
2.2 Chem ical and crystalline structures of f-MWNTs
Typical FT-IR spectra of MWNTs before and after being treated with acid mixture for 2 h and then deposited with HCS are shown in Fig.2(A),in which two new peaks around 1 425.1 and 1 027.0 cm-1appear.The peak at1 425.1 cm-1is normally assigned to the stretching vibration of C—O and bending vibration of O—H in COOH[9-10];the peak at 1027 cm-1that caused by stretching vibration of symmetrical C—O—C is also the characteristic peak of HCS.Meanwhile,after treatment peaks at around 3 327.1,2 923.1 and 2 363.0 cm-1are all weakened which ow ing to the attenuation of van der Waals forceswith strong acid.Moreover,the peak ataround 1 631.0 cm-1,which can be assigned to the stretching mode of—C=C—groups[11-12],shows a little shift to lower frequency with treatment,which also suggests some structural change in the tubes.

Fig.2 FT-IR spectra(A)and WAXD patterns(B)of raw and modified MWNTs
The crystallinity of MWNTs characterized by XRD is shown in Fig.2(B).It can be clearly seen there is a visible diffraction peak at around 18°which can represent the existence of HCS on the pattern of f-MWNTs.Based on the above conclusions,the result of this functional experiment agrees with our opinion that the HCS deposits on the MWNTs successfully.
2.3 Cross section morphologies of hollow fibers
Seen from the SEM cross section photos of hollow fibers in Fig.3,there are significant differences with increasing of f-MWNTs.The white highlights in these photos represent the existence of f-MWNTs,and we can get the follow ing conclusions.When the content of f-MWNTs increases to 0. 5%,it can be evenly distributed in the HCSmatrix thatendows the section with dense structures(from Fig.3(c))and the image also can further illustrate that the MWNTs after modification can disperse uniform ly in the solution;while if the content continues to increase to 0.7%,the excess f-MWNTs aggregates together that make the section rough and uneven (from Fig.3(d)).To sum up the above analysis,the adequate addition of f-MWNTs can offer perfect section morphology for hollow fibers.


Fig.3 SEM cross section photographs of hollow fibers with different contents of f-MWNTs:(a)0.0%,(b)0.3%,(c)0.5%,and(d)0.7%
2.4 M echanical properties of hollow fibers
从认知难度上看,循环小数、有限小数和分数形式的数的理解要比整数困难,但是RJ版教科书中以整数形式考查有理数的例题数目要多于分数和小数,对形式不易理解的数的举例少于易理解的数,甚至未涉及循环小数的例题,这易导致学生对难点掌握不牢固.CM教科书中以分数、有限小数形式考查有理数的例题数量要大于整数,相比RJ版教科书更遵循学生的认知难度.
Themechanical properties of hollow fibers with different contents of f-MWNTs are shown in Fig.4 and it shows that the addition of f-MWNTs can improve fibers'mechanical properties effectively.After comparative analysis,we can easily obtain that the hollow fiberswith 0.5%f-MWNTs exhibit the optimal tensile property with themaximum tensile strength and elasticity modulus of 9.33 MPa and 2.34 GPa,respectively(as shown in Fig.4(b)).It can be closely related to the dense structures as mentioned in the previous analysis.For one thing,in the solution flow field,f-MWNTs with excellent length/diameter ratio and flexibility orient along the fibers'axial direction,so the axial tensile force can be increased;for another,the addition of f-MWNTs with high crystallinity can enhance the intermolecular force thus affecting the movement of long segments,which leads to the reduced deformation of the macromolecules,and consequently there is the grow th of strength and modulus whereas elongation at break reduces slightly.When the amount of addition reaches 0.7%,the mechanical parameters reducemainly for the increase of internal defects in hollow fibers caused by excess particle agglomeration.To sum up,the results of mechanical analysis are nearly consistentwith themorphology analysiswhich follows thatmicrostructure is a direct factor to affect the mechanical properties.Thus,the hollow fibers with 0.5%f-MWNTs (HCS/f-MWNTs hollow fibers)will be chosen to carry out the follow ing experiments.
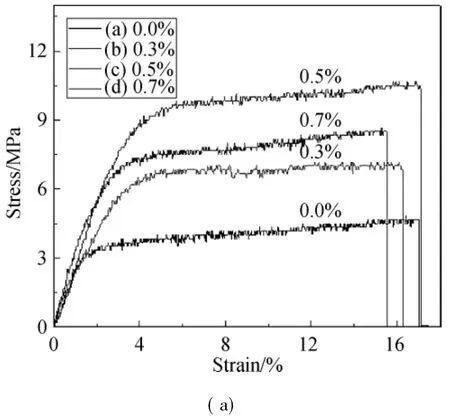

Fig.4 Stress-strain curves of the hollow fiberswith different contents of f-MWNTs(a)and the changes of tensile strength and elasticity moduluswith content(b)
2.5 Surface elemental analysis of the modified hollow fibers
Plasma treatment which is an efficient method with simplicity of operation has been w idely used in improving the surface properties of materials for a long time.In this paper,cold plasma technology was applied for the modification of hollow fibers to endure the surface with reactive property.In previous experiments,we found that the surface properties after plasma treatmentwas closely linked to the processing time and discharge power[13-14].From several exploratory experiments,we may draw a conclusion that,the HCS/f-MWNTs hollow fibers samples treated at 60 W maintained for 120 s displayed the optimal hydrophilicity which means the surface with active properties,so this value of the parameter will be chosen for surface grafting.
In this paper,XPS technology was carried out to characterize the changes of chem ical composition and combination of the surface before and after modification.The survey scan spectra that can be used to identify the elements existing on the surface of fibers are shown in Fig.5(A).The figure shows that there are C1s,N1s and O1s peaks at approximate 285,420 and 530 eV respectively on the surface of all fibers[15].The reinforcement of O1s peak on the fibers after He-plasma pretreated can testify that the increase in oxygen content of the surface and the obviousenhanced N1s peak on the gelatin-grafted fibers can also illustrate that gelatin has been introduced onto the surface successfully.In Fig.5,B.E. represents electron binding energy.
Figures 5(B)-(D)show the spectrogram of C1s after peak separation and fitting which can determ ine the elemental combination of themodified fibers.The peaks located at284.2,286.0 and 288.4 eV correspond to C—C,C= O and C= N,respectively[16-17].After carefully analyzing the research result,the integral peak areas of C= O and C= N bonds increased obviously after modification.This is a strong indication that there are more polar groups bonded on the surface aftermodification,such as carboxyl groups(—COOH)and amide bonds(—CO—NH—).
The percentage and ratio of elements of the samples based on XPSquantitative analysis are shown in Table1.The data can reflect the visual change of the carbon-containing function groups.After plasma pretreatment,the O/C ratio is significantly increased,from 42.01%to 65.71%.This can be associated with the formation of new oxygen-containing groups. During the pretreatment process,He-plasma acted on samples' surface and energy was transm itted by metastable chemical group with a relatively high energy,and then the samples were exposed to air to incorporate oxygen and resulted in the oxygen content significantly increasing(from 29.07%to 38.28%).In the follow ing grafting reaction,the active groups from gelatin reacted with the above-mentioned new groups to introduce a large amount of nitrogen(from 1.73%to 7.54%)to realize the grafting reaction.By data analysis,after modification the ratio of C—C drops from 61.03%down to 45.79%with the increase of both C= O(from 27.31%to 34.79)and C=N bonds(from 11.67%to 19.42%).The results indicate that during the treatment process,the C—C bond breaks down to form C= O and C= N bonds or other polar groups.


Fig.5 XPS survey scan spectra and C1s spectra of the hollow fibers before and after treatment:(A)survey scan spectra of three samples;(B)C1s of the untreated samples;(C)C1s of the He-plasma pretreated samples;(D)C1s of the gelatin-grafted samples

Table 1 Quantitative elementary analysis of the untreated,He-plasma pretreated,and gelatin-grafted samplesmeasured by XPS
2.6 Attachment and proliferation of RSC96 on hollow fibers
The results of cell attachmentand proliferation on fibers are shown in Fig.6.As indicated in Fig.6(a),RSC96 cells can be well attached on all the fibers after about 8 h and the rates of four fibers'change tendency with culture time are almost consistent.So we can come to the conclusions that a small amount of f-MWNTs in fibers with the rate of 77.44%that equal to HCS fibers(78.29%)after 24 h has no influence on cell adhesion;the rate of fibers after plasma pretreatment also accounts for 96.30%which mainly benefits from the improved hydrophilicity;as an important aspect,it shows that fibers after gelatin grafted with adhesion rate up to 75.10%have the best cell affinity than other fibers in the first2 h and this advantage has beenmaintained to the last(up to 101.86%).
The activity and proliferation of cellon different fibers after culturing for different days are shown in Fig.6(b)and the higher OD value representsmore live cells.As revealed in this figure,the tendency of cell proliferation on HCS/f-MWNTs fibers can nearly keep in step with the HCS fibers;after pretreated with plasma and especially grafted with gelatin,compared with others the viability of cell is improvedsignificantly,and obvious cells grow ths on the modified fibers are still observed after cultivation over one week.Reports by a few researchers demonstrated that gelatin enhanced the attachment and proliferation of cells[18-19].Gelatin has the same structure with extracellularmatrix.For the protein of cell wall,hydrophilic amino acids existed in outer region because of a repulsion caused by a protein and hydrophobic component at the inner region[20].So it can create amore favorable environment for cells and the cytocompatibility of the fibers is substantially improved by the bioactivity of the gelatin layer.

Fig.6 Grow th of RSC96 on different fibers:(a)adhesion rate in different time on hollow fibers before and aftermodification; (b)cell proliferation results obtained by the CCK-8 test
2.7 Cellmorphology on nano-fibersmats
The representative SEM micrographs of RSC96 cells cultured for5 d on the fibers are shown in Fig.7.It can be seen that the cell grow th on the composite hollow fibers with f-MWNTs is in the same way with HCS fibers(shown in Figs.7 (a)and(b)),meaning that the addition of f-MWNTs has no effect on cell grow th.In addition,after pretreatment the numbers of cells on fibers(Fig.7(c))are obviouslymore than the untreated samples and it provides an evidence for the stronger cell-fibers interaction with amore hydrophilic surface. In comparison,the gelatin grafted fibers culture has a more intense than other samples,even a part or portion that overlaps (Fig.7(d)).It justmeans that cells canmultiply rapidly on the fibers,so it indicates that gelatin grafting is beneficial for cell grow th with the provision of some factors by gelatin layerwhich can enhance cells cytoactivity and cytoproliferation throughout the culture period.

Fig.7 SEM micrographs of RSC96 cells cultured for 5 d on the different fibers:(a)HCS hollow fibers,(b)HCS/f-MWNTs fibers,(c)plasma pretreated HCS/f-MWNTs fibers,and(d)gelatin grafted HCS/f-MWNTs fibers
3 Conclusions
The dispersivity and compatibility with HCS of f-MWNTs thatobtained via a controlled surface deposition and crosslinking process had been improved a lot;the hollow fibers with the f-MWNTs content up to 0.5%relative to HCS exhibited the optimal tensile property with the maximum tensile strength and elasticity modulus of 9.33 MPa and 2.34 GPa,respectively. The fiber was then immersed into gelatin solution for grafting after pretreatment.XPS results showed that some oxygen elementwas introduced on the surface of the pretreated samples for subsequently exposing to air(atomic fraction rising from 29.07%to 38.28%);in the follow ing grafting reaction,the active groups from gelatin reacted with the new groups to introduced a large amountof nitrogen(from 1.73%to 7.54%) and the C—C bond broke down to form C= O and C= N bonds or other polar groups to realize the grafting reaction. From biological characterization such as cell attachment,cell morphology and proliferation,these results demonstrated the follow ing two aspects.First,the addition of a small amount of f-MWNTs in fiberswith the celladhesion rate of77.44%which equalled HCS fibers(78.29%)after 24 h had no effect on cell behavior;second,cells seeded in fibers grafted with gelatin had higher adhesion rate and cells could proliferate faster in the same timewhich proved that the superior ability of fibers after surface modification with gelatin could well support RSC96 grow th and proliferation.
[1]Richardson P M.Peripheral Nerve Regeneration:an Overview[M].New York:Academic Press,2009:557-560.
[2]Chen Y S,Liu C J,Cheng C Y,et al.Effect of Bilobalide on Peripheral Nerve Regeneration[J].Biomaterials,2004,25(3): 509-514.
[3]Nair L S,Laurencin C T.Biodegradable Polymers as Biomaterials[J].Progress in Polymer Science,2007,32(8/9): 762-798.
[4]Whang K,Goldstick T K,Healy K E.A Biodegradable Polymer Scaffold for Delivery of Osteotropic Factors[J].Biomaterials,2000,21(24):2545-2551.
[5]Jayakumar R,Prabaharan M,Sudheesh Kumar P T,et al.Biomaterials Based on Chitin and Chitosan in Wound Dressing Applications[J].Biotechnology Advances,2011,29(3):322-337.
[6]Hu H,Ni Y C,Montana V,et al.Chem ically Functionalized Carbon Nanotubes as Substrates for Neuronal Grow th[J].Nano Letters,2004,4(3):507-511.
[7]Mattson M P,Haddon R C,Rao A M.Molecular Functionalization of Carbon Nanotubes and Use as Substrates for Neuronal Grow th[J].Journal of Molecular Neuroscience,2000,14(3):175-182.
[8]Chen J,Su C L.Surface Modification of Electrospun PLLA Nanofibers by Plasma Treatment and Cationized Gelatin Immobilization for Cartilage Tissue Engineering[J].Acta Biomaterialia,2011,7(1):234-243.
[9]Mawhinney D B,Naumenko V,Kuznetsova A,et al.Infrared Spectral Evidence for the Etching of Carbon Nanotubes:Ozone Oxidation at 298 K[J].Journal of the American Chemical Society,2000,122(10):2383-2384.
[10]Bahr JL,Tour JM.Covalent Chem istry of Single-Wall Carbon Nanotubes[J].Journal of Materials Chemistry,2002,12(7): 1952-1958.
[11]Zhang JF,Zou H X,Qing Q,et al.Effect of Chemical Oxidation on the Structure of Single-Walled Carbon Nanotubes[J].Journal of Physical Chemistry B,2003,107(16):3712-3718.
[12]Chiang Y,Lin W H,Chang Y C.The Influence of Treatment Duration on Multi-walled Carbon Nanotubes Functionalized by H2SO4/HNO3Oxidation[J].Applied Surface Science,2011,257(6):2401-2410.
[13]Shi T N,Shao M L,Zhang H R,et al.Surface Modification of Porous Poly(tetrafluoro-ethylene)Film via Cold Plasma Treatment[J].Applied Surface Science,2011,258(4):1474-1479.
[14]Shao M L,Chen L,Yang Q.Preparation and Surface Modification of Electrospun Aligned Poly(butylene carbonate)Nanofibers[J].Journal of Applied Polymer Science,2013,130(1):411-418.
[15]Kull K R,Steen M L,Fisher E R.Surface Modification with Nitrogen-Containing Plasmas to Produce Hydrophilic,Low-Fouling Membranes[J].Journal of Membrane Science,2005,246(2):203-215.
[16]Kim E S,Yu Q S,Deng B L.Plasma Surface Modification of Nanofiltration(NF)Thin-Film Composite(TFC)Membranes to Improve Anti Organic Fouling[J].Applied Surface Science,2011,257(23):9863-9871.
[17]Ni H C,Lin Z Y,Hsu S H,et al.The Use of Air Plasma in Surface Modification of Peripheral Nerve Conduits[J].Acta Biomaterialia,2010,6(6):2066-2076.
[18]Ghasem iM L,Prabhakaran M P,Morshed M,et al.Electrospun Poly(ε-caprolactone)/Gelatin Nanofibrous Scaffolds for Nerve Tissue Engineering[J].Biomaterials,2008,29(34):4532-4539.
[19]Kwon O,M yung S,Lee C,et al.Comparison of the Surface Characteristics of Polypropylene Films Treated by Ar and M ixed Gas(Ar/O2)Atmospheric Pressure Plasma[J].Journal of Colloid and Interface Science,2006,295(2):409-416.
[20]Martino S,D'Angelo F,Armentano I,et al.Stem Cell-Biomaterial Interactions for Regenerative Medicine[J]. Biotechnology Advances,2012,30(1):338-351.
TQ342+.8
A
1672-5220(2015)04-0602-07
date:2014-03-26
s:State Key Laboratory for Modification of Chem ical Fibers and Polymer Materials,China(No.LZ0902);Shanghai Science and Technical Comm ittee,China(No.12DZ194030)
*Correspondence should be addressed to YANG Qing,E-mail:yangqing@dhu.edu.cn
猜你喜欢
杂志排行
Journal of Donghua University(English Edition)的其它文章
- Numerical Reality Method of the M icroburst Model
- Corporate Governance,Government Regulation and Bank Stability
- Cracking Patterns of Shear Walls in Reinforced Concrete Structure due to Strong Earthquake Based on Mohr-Coulomb Criterion
- Cooperative Compressive Spectrum Sensing in Cognitive Underwater Acoustic Communication Networks
- Numerical Simulation of Gas-Solid Two-Phase Flow in Reverse Blow ing Pickup Mouth
- Fuzzy Model Free Adaptive Control for Rotor Blade Full-Scale Static Testing
