大鼠脑创伤后MKP-1表达上调、NO的生成减少
2015-07-31刘丽君王弘凯冉建华
张 雪,王 琦,刘丽君,王弘凯,冉建华*
研究论文
大鼠脑创伤后MKP-1表达上调、NO的生成减少
张 雪1,2,王 琦1,2,刘丽君1,2,王弘凯1,2,冉建华1,2*
(重庆医科大学 1.神经科学研究中心; 2.解剖学教研室, 重庆 400016)
目的研究创伤性脑损伤(TBI)周围皮质中丝裂原活化蛋白激酶磷酸酶-1(MKP-1)的表达变化,探讨其对一氧化氮(NO)含量的影响及机制。方法用自由落体法制作大鼠TBI模型;酶化学法检测周围皮质中NO的含量;免疫组织化学和免疫印迹等方法检测脑皮质MKP-1、eNOS及MAPKs的表达。结果对照组脑皮质NO水平为34.4±3.2 μmol/L,TBI损伤后3、6、24及72 h均显著下降,分别为20.8±2.5、23.9±3.8、24.0±1.6及26.8±2.6 μmol/L (均P<0.05)。脑损伤周围皮质中胞质和突起呈棕黄色的MKP-1免疫阳性细胞数量增加。脑损伤后3和6 h MKP-1蛋白表达水平明显增加(P<0.05),随后降低至正常水平;eNOS蛋白表达水平在脑损伤后3和6h则明显降低(均P<0.05),至24 h接近正常水平;pERK和p-P38 MAPK蛋白表达水平在脑损伤后3和6 h也分别下降(均P<0.05)。结论大鼠TBI后,皮质中 MKP-1表达上调可能负向调控MAPK信号而降低eNOS水平,引起NO生成减少造成脑血流灌注不足。
大鼠;创伤性脑损伤;丝裂原活化蛋白激酶磷酸酶-1;一氧化氮
创伤性脑损伤(traumatic brain injury,TBI)是意外伤害导致青壮年死亡或残疾的首要因素,包括机械性原发损伤和继发性损伤两个病理过程[1]。继发性损伤以创伤周围脑血流下降为早期病理改变,是引发氧自由基损伤、钙超载等后续病理过程的关键因素;脑创伤早期脑血流量低于每分钟18 mL/100 g(不可逆缺血性脑损伤的临界值)提示患者预后不良甚至死亡[2-6]。内皮型一氧化氮合酶(eNOS)催化精氨酸(L-arginine)产生的NO是引起血管扩张、调节脑血流的重要分子[3]。创伤性脑损伤后,患者血浆和脑组织中NO水平持续下降,并与创伤周围eNOS表达下调和脑血流量的下降密切相关;吸入NO可提高患者脑血流灌注量,减轻继发性损伤的严重程度[2],但其调控机制尚不清楚。丝裂原活化蛋白激酶磷酸酶-1(mitogen-activated protein kinases phosphatase-1,MKP-1)是MAPK信号通路的特异性抑制剂,通过抑制NOS的表达在缺血再灌注脑损伤、神经炎性反应中发挥病理作用[7-9]。然而,TBI后损伤脑组织中eNOS表达、NO含量的改变是否受到MKP-1的调节尚未见报道。为此,本实验拟通过制作大鼠创伤性脑损伤模型,检测脑损伤周围组织中NO含量以及MKP-1、eNOS和MAPKs相关信号分子的表达变化,探讨TBI后MKP-1的表达规律,其对NO生成的影响及分子机制。
1 材料与方法
1.1 实验动物
清洁级雄性SD大鼠90只,体质量200~250 g[重庆医科大学动物中心提供,合格证号SCXK(渝)2012-0001]。
1.2 主要试剂
兔抗多克隆抗MKP-1抗体(Santa Cruz公司);兔抗多克隆抗eNOS抗体(Abcam公司);免疫组化试剂盒(北京中杉公司);一氧化氮含量检测试剂盒(南京建成公司);细胞裂解液(碧云天公司)。
1.3 动物模型制作
参考Feeney’s自由落体模型,设计、改装打击装置后制作重度创伤性脑损伤模型[10]。严格遵循动物模型入选标准,将入选的实验动物随机分为假手术组和创伤性脑损伤组,假手术组作为对照组。TBI组分别于术后3、6、24和72 h取材。
1.4 一氧化氮含量测定
将取好的新鲜大鼠脑组织称重并匀浆,离心后取上清液。NO含量检测参见南京建成相关试剂盒的说明书。采用酶标仪检测550 nm处每孔的吸光度,根据说明书上所给公式换算得到NO浓度值。
1.5 免疫组化检测MKP-1蛋白的表达变化
对照组和模型组的脑组织石蜡切片经脱水及修复后,用山羊血清工作液进行封闭37 ℃ 30 min;滴加MKP-1抗体(1∶500)4 ℃过夜;复温漂洗后,滴加SP工作液,37 ℃孵育30 min,DAB显色后苏木素复染细胞核,脱水、透明,中性树胶封片,镜下观察结果。阴性对照取相同量的正常羊血清代替MKP-1抗体,其余步骤同上。采用Image-Pro Plus 6.0图像分析软件,测定单位面积MKP-1蛋白平均吸光度值。
1.6 免疫印迹MKP-1、eNOS、MAPKs信号分子的表达水平

1.7 统计学分析
2 结果
2.1 大鼠脑损伤区周围皮质一氧化氮含量测定
脑损伤周围皮质NO含量较对照组显著下降 (P<0.05)(图1)。
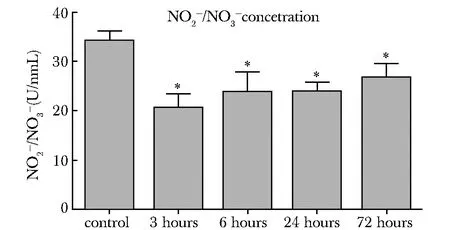
NO concentration in cortex between control group and TBI group was determined by a commercial NO assay kit; *P<0.05 compared with control group图1 对照组和TBI后不同时间点脑皮质中NO含量Fig 1 NO concentration in cortex between control group and TBI group with different time points
2.2 脑损伤周围皮质MKP-1的表达改变
阴性对照未见MKP-1蛋白表达。对照组的大脑皮质可见散在分布,胞质和突起呈棕黄色的阳性表达细胞。脑损伤周围皮质中MKP-1免疫阳性细胞数量和染色强度在6 h明显增加,同时在6、24和72 h部分血管周围可见染色较强的免疫阳性细胞。TBI后脑损伤周围皮质中MKP-1的平均吸光度值均高于对照组(P<0.01)(图2,表1)。

表1 免疫组化各组中MKP-1的平均吸光度值Table 1 Average optical density of MKP-1 in immunohistology(±s, n=10)
*P<0.01,**P<0.001 compared with control group.
2.3 脑损伤周围皮质中MKP-1、eNOS及MAPKs的表达改变
与对照组相比,TBI组脑损伤周围皮质中MKP-1蛋白表达水平从3 h开始明显上调,至6 h达到峰值(P<0.05),随后从24 h明显降低至72 h达到正常水平。与此同时,eNOS的表达水平从3 h开始明显下调(P<0.05),至6 h达到最低值(P<0.05),缓慢回升至72 h接近对照组水平(图3)。与对照组相比,TBI组损伤周围脑组织中pERK和p-P38 MAPK的表达水平在3和6 h降低 (P<0.05)(图4)。
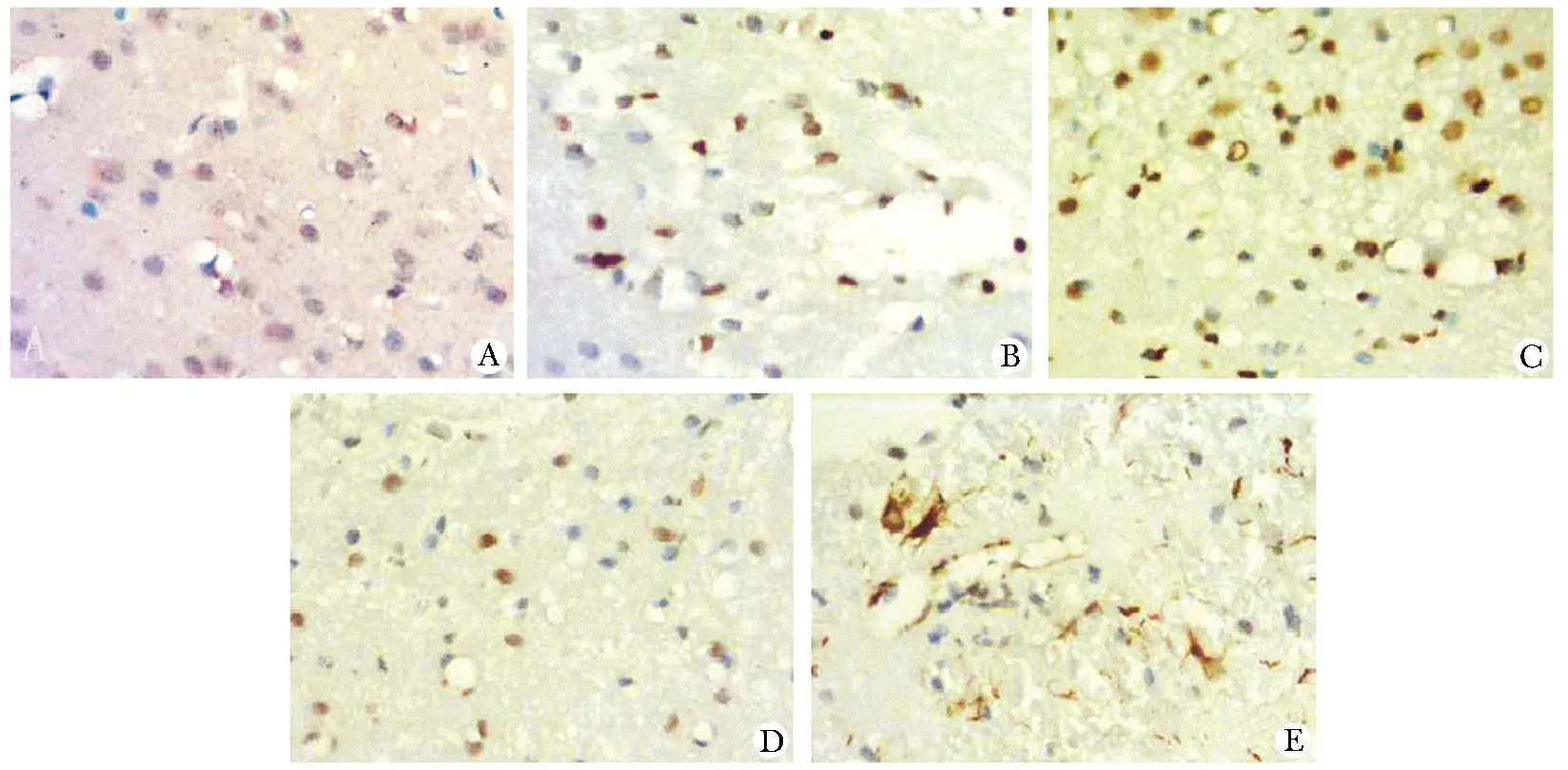
The changes expression of MKP-1 in rat cortex by DAB staining (A~E); A.expression of MKP-1 in the cerebral cortex of rats as control group; B~E.expression of MKP-1 in the cerebral cortex at 3, 6, 24 and 72 hours after brain injury TBI
图2 对照组和TBI后不同时间点脑皮质中MKP-1的表达
Fig 2 Expression of MKP-1 in cortex between control group and TBI group with different time points(×400)
3 讨论
脑内NO作为一种重要的气体信号分子,由精氨酸(L-arginine)经内皮型一氧化氮合酶(endothelial nitric oxide synthase,eNOS)、神经型一氧化氮合酶(neural nitric oxide synthase,nNOS)和诱导型一氧化氮合酶(induced nitric oxide synthase,iNOS)催化产生,其中eNOS催化产生的NO是调节血管扩张的主要来源;而脑外伤后eNOS的表达下调是引起内皮型NO减少、脑血灌注量下降的主要原因[11]。本研究发现,TBI 3 h后损伤周围脑皮质NO水平降低明显,从6 h开始NO水平缓慢回升,至72 h仍显著低于对照组,这与国外的报道结果一致[12]。与之相应的是eNOS的表达水平从3 h明显下调,至6 h到达最低值,然后开始上调至72 h接近正常水平。NO含量上升早于eNOS水平上调时间,可能与eNOS的酶活性或iNOS表达改变有关。然而,创伤性脑损伤后eNOS改变的机制尚不明确。

Western blot analysis for MKP-1 and eNOS protein expression was performed; left graph shows the representative films; Bar graph (right) shows the density ratio between MKP-1 and eNOS;*P<0.05,#P<0.05 compared with control group
图3 对照组和TBI后不同时间点脑皮质中MKP-1、eNOS的表达水平
Fig 3 Level of MKP-1 and eNOS in cortex between control group and TBI group with different time points(n=3)
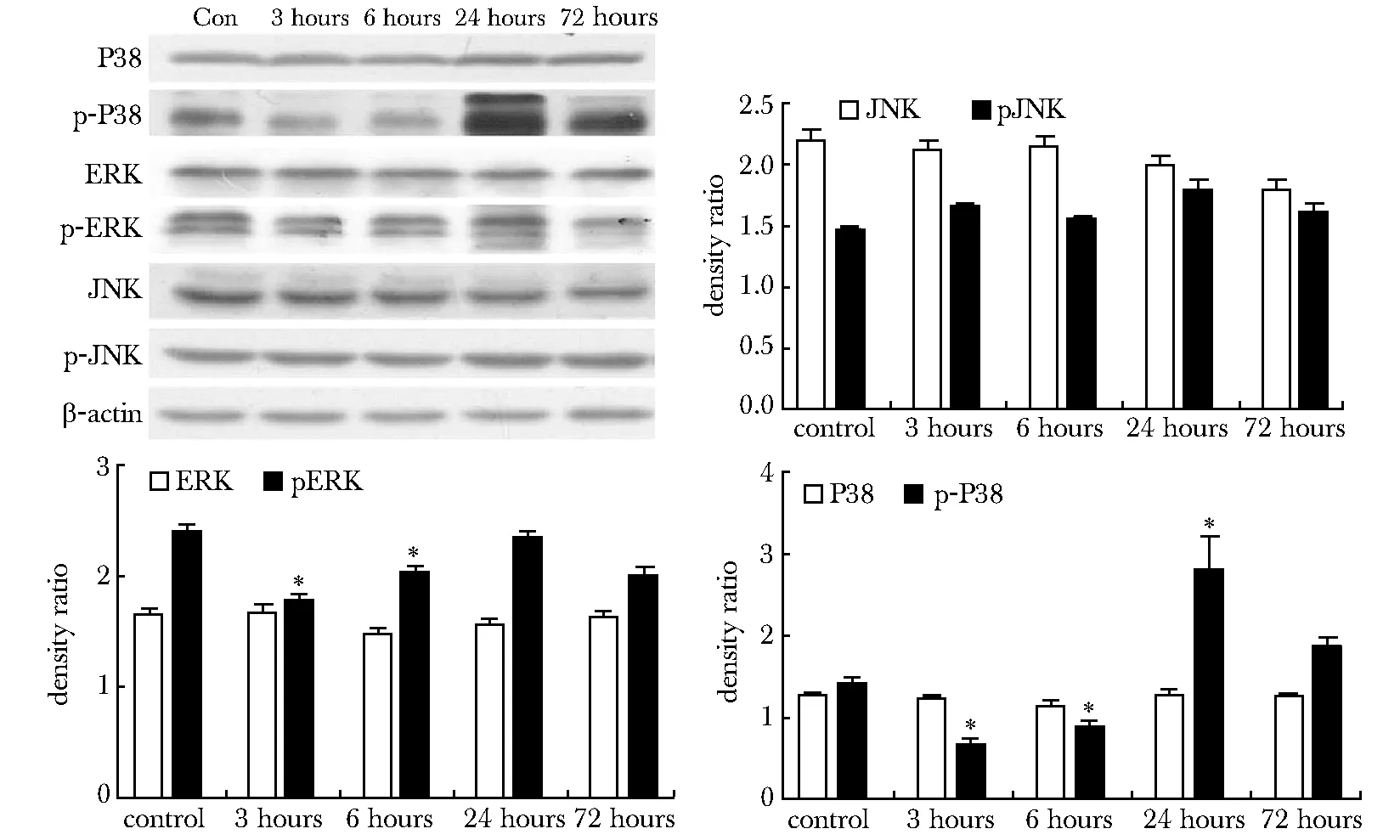
Western blot analysis for P38,p-P38,ERK,p-ERK,JNK and p-JNK protein expression was performed;*P<0.05 compared with control group
图4 不同时间点大鼠损伤周围脑组织MAPKs信号分子的表达变化
Fig 4 MAPKs signaling levels were detected in pericontusional region at different time points after TBI(n=3)
MKP是双特异性磷酸酶(dual specificity phosphatase,DUSP)家族成员,通过去磷酸化作用抑制MAPK(P38、ERK1/2、JNK)活性,参与了细胞分化、凋亡和突触可塑性等多种病理生理过程的调节[13]。近年来,MKP对NOS的表达调控引起关注。MKP-1可抑制MAPKs信号分子的磷酸化水平,缓解脂多糖(lipopolysaccharide,LPS)诱导鼠肺泡巨噬细胞中NOS过度活化所致的炎性反应[7]。脑冷冻伤3~6 h后MKP-1的表达显著上调,并与脑血流量的减少密切相关[14]。脑缺血再灌注损伤后,MKP-3表达可抑制ERK1/2信号通路,下调eNOS表达及NO合成[8]。本研究发现,TBI后损伤周围皮质中MKP-1阳性神经元数量和染色强度显著增加,并呈现出围绕血管周围分布的特征;MKP-1表达水平在3 h开始明显上调,至6 h达到峰值后开始下降,至72 h恢复至正常水平。随着MKP-1表达上调,pERK和p-P38 MAPK的表达水平在3和6 h明显降低,随后恢复至正常水平,而JNK的表达水平无明显改变。上述分子的时间变化趋势与eNOS及NO的改变一致,提示MKP-1及相关MAPK信号参与了TBI后损伤周围皮质中eNOS的表达调节过程。
综上所述,TBI后 MKP-1主要通过负向调控pERK和pp38 MAPK的表达水平,继而引起eNOS的表达下调和NO产量的降低,加重局部脑血流量的下降,引起继发性损伤的发生发展,MKP-1可能成为创伤性脑损伤新的治疗靶点。
[1] Walker KR, Tesco G. Molecular mechanisms of cognitive dysfunction following traumatic brain injury [J].Front Aging Neurosci,2013, 5:29.
[2] Terpolilli NA, Kim SW, Thal SC,etal. Inhaled nitric oxide reduces secondary brain damage after traumatic brain injury in mice [J].Cereb Blood Flow Metab,2013,33:311-318.
[3] Hlatky R, Lui H, Cherian L,etal. The role of endothelial nitric oxide synthase in the cerebral hemodynamics after controlled cortical impact injury in mice [J]. Neurotrauma, 2003, 20:995-1006.
[4] Zhou YF, Li WT, Han HC,etal. Allicin protects rat cortical neurons against mechanical trauma injury by regulating nitric oxide synthase pathways [J].Brain Res Bull, 2014,100:14-21.
[5] Rangel-Castilla L, Ahmed O, Goodman JC,etal. L-arginine reactivity in cerebral vessels after severe traumatic brain injury [J]. Neurol Res. 2010,32:1033-1040.
[6] Robertson CS, Gopinath SP, Valadka AB,etal. Variants of the endothelial nitric oxide gene and cerebral blood flow after severe traumatic brain injury [J]. Neurotrauma, 2011,28:727-737.
[7] Nelin LD, Wang X, Zhao Q,etal. MKP-1 switches arginine metabolism from nitric oxide synthase to arginase following endotoxin challenge [J].Am J Physiol Cell Physiol, 2007, 293:632-640.
[8] Yang D, Xie P, Liu ZH. Ischemia/reperfusion-induced MKP-3 impairs endothelial NO formation via inactivation of ERK1/2 pathway [J]. PLoS one, 2012,7:1-14.
[9] Stefania M, Stefania G, Katia V,etal. Cannabinoid CB2 receptors modulate ERK-1/2 kinase signalling and NO release in microglial cells stimulated with bacterial lipopolysaccharide [J].Br J Pharmacol, 2012,165: 1773-1788.
[10] Feeney DM, Boyeson MG, Linn RT,etal. Responses to cortical injury: Methodology and local effects of contusions in the rat [J]. Brain Res,1981,211:67-77.
[11] Garry PS, Ezra M, Rowland MJ,etal. The role of the nitric oxide pathway in brain injury and its treatment-From bench to beside [J]. Exp Neurol, 2015,263:235-243.
[12] Cherian L, Hlatky R, Robertson CS. Nitric oxide in traumatic brain injury [J]. Brain Pathol, 2004,14:195-201.
[13] Boutros T, Chevet E, Metrakos P. Mitogen-activated protein (MAP) kinase/MAP kinase phosphatase regulation: roles in cell growth, death and cancer [J]. Pharmacol Rev, 2008,60:261-310.
[14] Hermann DM, Mies G, Hossmann KA. Expression of c-fos, junB, c-jun, MKP-1 and hsp72 following traumatic neocortical lesions in rats—relation to spreading depression [J]. Neuroscience, 1999,88:599-608.
Increased expression of MKP-1 and reduced production of NO in brain tissue of rat after traumatic injury
ZHANG Xue1,2, WANG Qi1,2, LIU Li-jun1,2, WANG Hong-kai1,2, RAN Jian-hua1,2*
(1.Neuroscience Research Center; 2.Dept. of Anatomy, College of Medicine, Chongqing Medical University, Chongqing 400016, China)
Objective To detect expressive changes of MKP-1 and measure NO content in pericontusional cerebral cortex of rat after traumatic brain injury (TBI), to explore regulatory role and molecular mechanism of MKP-1 on NO generation after TBI. Methods We constructed TBI model by Feeney’s method. Nitric oxide detection kits were used to measure NO content in pericontusional cerebral cortex between control group and TBI group. Distribution of MKP-1 was detected by immunohistochemistry. Western blot analysis was performed to detect expression level of MKP-1, endothelial NOS and MAPKs in the rats of pericontusional cerebral cortex after TBI. Results NO level significantly decreased at 3, 6, 24 and 72 h with concentration as (20.8±2.5)μmol/L,(23.9±3.8)μmol/L, (24.0±1.6)μmol/L,(26.8±2.6)μmol/L(P<0.05) respectively, compared with the control as (34.4±3.2)μmol/L after TBI. The positive staining MKP-1 cells were scattered and distributed in pericontusional cerebral cortex with brown cytoplasma and processes and increased significantly at 3 h and 6 h after TBI. Compared to the control group, the protein expressions of MKP-1 were increased at 3 h and 6 h respectively with normal level at 24 h after TBI(P<0.05). While the protein level of eNOS decreased at 3 h and 6 h respectively after TBI (P<0.05). Both protein expression of pERK and p-P38 decreased at 3 h and 6 h respectively after TBI compared with control group (P<0.05).Conclusions Increased MKP-1 expression in pericontusional cerebral cortex after TBI specifically inhibits the expression level of pERK and p-P38 MAPK with the pJNK intact to down-regulated the expression of endothelial NOS, which decrease NO content and attribute to the defect of cerebral blood flow.
rats; traumatic brain injury; MKP-1; NO
2014-12-09
2015-03-25
国家自然科学基金(30500171);重庆市教委项目(KJ120330);重庆市渝中区科委项目(20120202)
*通信作者(corresponding author):1315038024@qq.com
1001-6325(2015)08-1015-05
R749.16
A
