GM-SCF,IL-21和Rae-1基因联合治疗对肝癌小鼠模型的影响
2015-07-07吕一峰程明荣吴衍
吕一峰,程明荣,吴衍
(上海市浦东新区周浦医院 普通外科,上海 201318)
GM-SCF,IL-21和Rae-1基因联合治疗对肝癌小鼠模型的影响
吕一峰,程明荣Δ,吴衍
(上海市浦东新区周浦医院 普通外科,上海 201318)
目的 将基因巨噬细胞集落刺激因子(GM-SCF),白介素-21(IL-21)和视黄酸早期转录因子-1(Rae-1)构建成重组质粒,观察重组质粒对小鼠肝癌皮下模型的疗效。方法 利用RT-PCR的方法将GM-SCF,IL-21和Rae-1构建成重组质粒,将小鼠皮下植模型分6组,分别为control组,IRES/GFP,IRES/IL21,IRES/GM-SCF,IRES/GM-SCF-IL21和IRES/combination组,每组15只,分别予以相应的基因治疗,观察各组60 d的生存率,并观察小鼠机体干扰素-γ(IFN-γ)和白介素-2(IL-2)水平的变化。结果 pGM-CSF-GFP-IRES-Rae-1-IL-21已经成功构建,control组到第14天小鼠全部死亡;IRES/GFP组到第16天小鼠全部死亡;治疗60 d后,IRES/GM-SCF组剩2只模型小鼠存活,其存活率为13.33%;IRES/IL21组的模型存活仅剩1只,其存活率为6.67%;IRES/GM-SCF-IL21模型存活11只模型小鼠,其存活率最高为73.33%。IRES/GM-SCF-IL21组的存活率明显高于其他各组(P<0.05或<0.01)。治疗后1~6 d IRES/combination,IRES/GM-SCF-IL21,IRES/GM-SCF和IRES/IL21组的IL-2和INF-γ水平出现逐渐升高,以IRES/combination水平最高,IRES/GM-SCF-IL21次之,IRES/GM-SCF和IRES/IL21组水平相对较低(P<0.01),6~10 d,IRES/combination组的IL-2和INF-γ水平仍为稳步增长,而IRES/GM-SCF-IL21,IRES/GM-SCF和IRES/IL21组出现逐渐下降。治疗结束时,IRES/GM-SCF-IL21组的IL-2和INF-γ水平较IRES/GM-SCF和IRES/IL21组明显增高(P<0.01),而IRES/GM-SCF和IRES/IL21组的IL-2和INF-γ水平较IRES/GFP和control组明显增高(P<0.01),以IRES/combination在的IL-2和INF-γ水平最高(P<0.01)。结论 联合GM-SCF,IL-21和Rae-1基因具有明显抑制小鼠肝癌的作用,其机理与机体的免疫激活有关。
巨噬细胞集落刺激因子;白介素-21;视黄酸早期转录因子-1;肝癌
肿瘤细胞能够通过多种机制逃避机体免疫系统识别和攻击,从而得以在体内生存和增殖。提高机体免疫细胞活性,或者促使肿瘤细胞表达能被免疫细胞识别的抗体或者配体,就能够促进机体自身识别和杀灭肿瘤细胞,从而达到肿瘤治疗的目的。前期实验及文献均发现巨噬细胞集落刺激因子(GM-SCF)和白介素-21(IL-21)基因融合质粒能明显抑制肿瘤的生长,并能促进NK细胞及CTL细胞的免疫活性[1-4]。体外实验发现高表达视黄酸早期转录因子-1(Rae-1)的肿瘤细胞更易被免疫细胞所识别[5]。本研究在前期实验的基础上,将Rae-1基因和绿色荧光蛋白(GFP)基因与融合质粒GM-CSF-IL-21通过基因拼接技术拼接成多功能融合质粒,其中GM-CSF和IL-21基因的功能是促进机体免疫系统的激活,尤其是NK细胞和CTL细胞的激活。Rae-1基因转染到肿瘤细胞后,主要促进肿瘤细胞表达Rae-1,后者促进肿瘤细胞被NK细胞和CTL细胞的识别。GFP基因主要用于监测基因体内外的转染情况。采用GM-SCF、IL-21和Rae-1三基因联合治疗小鼠原位肝癌模型,现报道如下。
1 材料与方法
1.1 材料及仪器 Balb/c小鼠购自上海复旦大学动物中心[SCXK(沪)2007-0002],雌性,7周龄,体质量约20 g左右。所有小鼠饲养条件为SPFB级,小鼠肝癌细胞(H22)购自 the China Center for Type Culture Collection (CCTCC,武汉, 中国)。RPMI 1640培养基(四季青生物工程材料有限公司产品,杭州)。实验得到上海市周浦医院和复旦大学医学院伦理委员会审查批准。四甲基偶氮唑蓝(MTT,Sigma有限公司, 上海);大剂量质粒抽提试剂盒购自Promega公司;干扰素(IFN-γ)和白细胞介素2(IL-2)、酶联免疫吸附(ELISA)测定试剂盒(圣克鲁斯生物工学, 美国),酶标仪(美国Bio-rad公司)。
1.2 方法
1.2.1 重组质粒pGM-CSF-GFP -IRES-IL-21-Rae-1的构建:从小鼠脾脏中调取GM-CSF、IL-21基因,化学合成Rae-1,GFP基因。针对GM-CSF和IL-21基因的CDS区序列设计并合成PCR 引物(见表1),在GM-CSF基因的5’端添加XhoI酶切位点,3’端添加EcoRI酶切位点。在GFP基因的5’端添加EcoRI酶切位点,3’端添加MluI酶切位点。在Rae-1基因的5’端添加Xba1酶切位点,3’端添加SalI酶切位点。在IL-21基因的5’端添加SalI酶切位点,3’端添加Not1酶切位点。GFP片段与目的载体pIRES的连接,获得pGFP-IRES;GM-CSF基因与目的载体pGFP-IRES的连接,获得pGM-CSF-GFP-IRES载体;将Rae-1基因PCR产物插入pGM-CSF-GFP-IRES载体,获得pGM-CSF-GFP-IRES-Rae-1载体;将IL21片段插入pGM-CSF-GFP-IRES-Rae-1载体,获得pGM-CSF-GFP-IRES-Rae-1-IL-21真核表达载体。MCS A处是GM-CSF-GFP,MCS B处是Rae-1-IL-21。

表1 扩增GM-CSF、GFP、Rae-1和 IL-21基因全长的引物序列Tab.1 Amplification of GM-CSF, GFP, Rae-1, and IL-21 gene sequences
加粗字体分别为酶切位点XhoI和EcoRI;EcoRI和MluI;XbaI和SalI;SalI和NotI
1.3 动物模型建立 用小鼠肝癌细胞株(H22)建立小鼠肝癌皮下模型[3]。将小鼠处死后解剖出肿瘤组织,挑选生长旺盛新鲜的肿瘤组织,制成6×107个/mL 的肿瘤细胞悬液。用1 mL注射器注射肿瘤细胞悬液50 μL注入右前肢腋窝皮下。
1.3.1 重组质粒治疗小鼠皮下肝癌模型:待小鼠肝癌皮下模型成模后第5天,肝癌组织约4~6 mm,按随机数字法,将小鼠分为6组,分别为control组,IRES/GFP,IRES/GM-SCF,IRES/IL21,IRES/GM-SCF-IL21和IRES/combination组,每组15只。control组:肿瘤内注射PBS 200 μL;IRES/GFP,IRES/GM-SCF,IRES/IL21,IRES/GM-SCF-IL21和IRES/combination组分为在肿瘤内注射200 μL(分别含100 μg相应的质粒),每天1次,连续5天,到治疗第10天,60天后,观察并记录各组小鼠的生存率。
1.4 IL-2和INF-γ的ELISA检测 各组分别于治疗0、2、4、6、8、10 d检测模型IL-2和INF-γ的水平,其具体方法为:小鼠中分离各组小鼠的血清标本,-20 ℃保存,取血清标本于37 ℃恒温箱解冻,双蒸馏水稀释至500 mL,倍比稀释标准品至8000 μg/L,每孔加入测定稀释液150 μL,继加入标准品或测定样品50 μL,15 min内完成,充分振荡摇匀后室温孵育2 h;取尽液体后,加入400 μL洗涤液重复。洗涤4次,加入辣根过氧化物酶标记IL-2和INF-γ多抗200 μL用上述方法振荡摇匀,室温孵育2 h;再取尽液体后,加入400 μL洗涤液重复洗涤4次;将显色剂A,B等量混匀后加入200 μL酶标抗体,室温避光孵育30 min;加50 μL终止液终止酶反应;立即置酶标仪中测定,以450 nm波长读光密度值,根据标准曲线计算样品的含量。

2 结果
2.1 pGM-CSF-GFP-IRES-Rae-1-IL21重组载体酶切鉴定 从图1可知,采用EcoRI和MluI双酶切pGM-CSF-GFP-IRES-Rae-1-IL21得目的条带(红色箭头)与目的基因GFP大小一致的片段;采用XhoI和EcoRI双酶切pGM-CSF-GFP-IRES-Rae-1-IL21得目的条带(红色箭头)与目的基因GM-CSF大小一致的片段;采用Xba1和Sal1双酶切pGM-CSF-GFP-IRES-Rae-1-IL21得目的条带(红色箭头)与目的Rae-1一致的片段;采用Sal1和Not1双酶切pGM-CSF-GFP-IRES-Rae-1-IL21得目的条带(红色箭头)与目的基因IL21一致的片段,得到的基因GFP,GM-CSF,Rae-1和IL21与Genbank的原序列完全一致,证明载体构建成功。

图1 pGM-CSF-GFP-IRES-Rae-1-IL21重组载体酶切鉴定泳道M:Marker 5000(从上到下分别为5000,3000,2000,1500,1000,750,500,250,100bp);泳道1:EcoRI和MluI酶切pGM-CSF-GFP-IRES-Rae-1-IL21(目的条带GFP:720bp);泳道2:XhoI和EcoRI酶切pGM-CSF-GFP-IRES-Rae-1-IL21(目的条带GM-CSF:425bp);泳道3:Xba1和Sal1酶切pGM-CSF-GFP-IRES-Rae-1-IL21(目的条带Rae-1:1106bp);泳道4:Sal1和Not1酶切pGM-CSF-GFP-IRES-Rae-1-IL21(目的条带IL21:441bp);泳道5:XhoI和Not1酶切pGM-CSF-GFP-IRES-Rae-1-IL21(目的条带GM-CSF-GFP-IRES-Rae-1-IL21:3.3kb);泳道6:XhoI和Not1酶切pIRES空载(目的条带:5.5kb +673bp(IRES));泳道7:pGM-CSF-GFP-IRES-Rae-1-IL21质粒注:因IL21的381位有EcoRI酶切位点所以用EcoRI和MluI酶切时有条2160bp条带(泳道1),用XhoI和EcoRI酶切时有条2880的条带(泳道2),见上图中蓝色箭头指示。Fig.1 Design and construction of the new expression vector pGM-CSF-GFP- IRES-Rae-1-IL21 and their enzyme cleavage products.Lane M: Marker 5000 (5000, 3000, 2000, 1500, 1000, 750, 500, 250, and 100 bp ordered reading top to bottom).Lane 1, EcoRI and MluI cleavage of pGM-CSF-GFP-IRES- Rae-1-IL21 (target band GFP, 720 bp).Lane 2: XhoI and EcoRI cleavage of pGM-CSF-GFP- IRES-Rae-1-IL21 (target band GM-CSF, 425 bp).Lane 3: Xba1 and Sal1 cleavage of pGM-CSF-GFP-IRES-Rae-1-IL21 (target band Rae-1, 1106 bp).Lane 4: Sal1 and Not1 cleavage of pGM-CSF-GFP-IRES-Rae-1-IL21 (target band IL21, 441 bp).Lane 5: XhoI and Not1 cleavage of pGM-CSF-GFP-IRES-Rae-1-IL21 (target band GM-CSF-GFP-IRES- Rae-1-IL21, 3.3 kb).Lane 6: XhoI and Not1 cleavage of pIRES (target band IRES, 5.5kb +673bp);Lane 7: pGM-CSF-GFP-IRES-Rae-1-IL21 plasmidNote: EcoRI cleavage site was the 381 of IL21 sequence.So, a band of 2160 bp is present in EcoRI and MluI cleavage band (Lane 1) and a band of 2880 bp is present in EcoRI and XhoI cleavage band (Lane 2), indicated by blue arrow.
2.2 pGM-CSF-GFP-IRES-Rae-1-IL21重组质粒对小鼠肝癌模型的抑制作用 将原位肝癌移植模型,随机分为6组,每组15只,按照前述的方法予以治疗,并用Kaplan-Meier生存分析进行统计分析。control组从第6天开始有小鼠死亡,到第14天小鼠全部死亡,其中位生存时间为11 d;IRES/GFP组从第5天开始死亡,到第16 d小鼠全部死亡,中位生存时间为12 d;IRES/GM-SCF组小鼠第17天开始死亡,到观察结束第60天剩2只模型小鼠存活,中位生存时间为35 d,其存活率为13.33%;IRES/IL21组的模型小鼠从第15天开始出现小鼠死亡,到第60天小鼠存活仅剩1只,中位生存时间为37 d,其存活率为6.67%;IRES/GM-CSF-IL-21模型小鼠从20 d开始死亡,到第60天存活4只小鼠模型,中位生存时间39 d,其存活率为26.67%;IRES/combination模型小鼠从30 d开始死亡,到第60天存活11只模型小鼠,未超过半数小鼠死亡,其存活率最高为73.33%。IRES/GM-SCF-IL21组的存活率明显高于其他各组(P<0.05,P<0.01)。

图2 重组质粒治疗后对小鼠荷肝癌模型的抑制作用(n=15)Fig.2 Inhibitory effects of different plasmids on liver cancer of tumor-bearing mice (n=15)
2.3 pGM-CSF-GFP-IRES-Rae-1-IL21重组质粒对小鼠IL-2和INF-γ水平的影响 从图3,表2~3可知,治疗后1~6 d IRES/combination,IRES/GM-SCF-IL21,IRES/GM-SCF和IRES/IL21组的IL-2和INF-γ水平出现逐渐升高,以IRES/combination水平最高,IRES/GM-SCF-IL21次之,IRES/GM-SCF和IRES/IL21组水平相对较低(P<0.01),6~10 d,IRES/combination在的IL-2和INF-γ水平仍为稳步增长,而IRES/GM-SCF-IL21,IRES/GM-SCF和IRES/IL21组出现逐渐下降。治疗结束时,IRES/GM-SCF-IL21组的IL-2和INF-γ水平较IRES/GM-SCF和IRES/IL21组明显增高(P<0.01),而IRES/GM-SCF和IRES/IL21组的IL-2和INF-γ水平较IRES/GFP和control组明显增高(P<0.01),以IRES/combination在的IL-2和INF-γ水平最高(P<0.01),而IRES/GM-SCF和IRES/IL21组,IRES/GFP和control组之间的IL-2和INF-γ水平差异无统计学意义。在1~10 d,IRES/GFP和control组小鼠的IL-2和INF-γ水平变化不明显。

图3 各组对模型小鼠血清IL-2和INF-γ水平的影响(n=3)**P<0.01,与control组比较,##P<0.01,与IRES/GM-SCF或IRES/IL-21组比较,△△P<0.01,与IRES/GM-SCF-IL21组比较Fig.3 IL-2 and INF-γ levels in mouse serum and expression of Rae-1 in liver cancer tissue in each treatment group(n=3)*P<0.05 , ** P<0.01, compared with control group; #P<0.05 ,##P<0.01, compared with IRES/GM-SCF and IRES/IL-21 group;△P<0.05, △△P<0.01, compared with IRES/GM-SCF-IL21
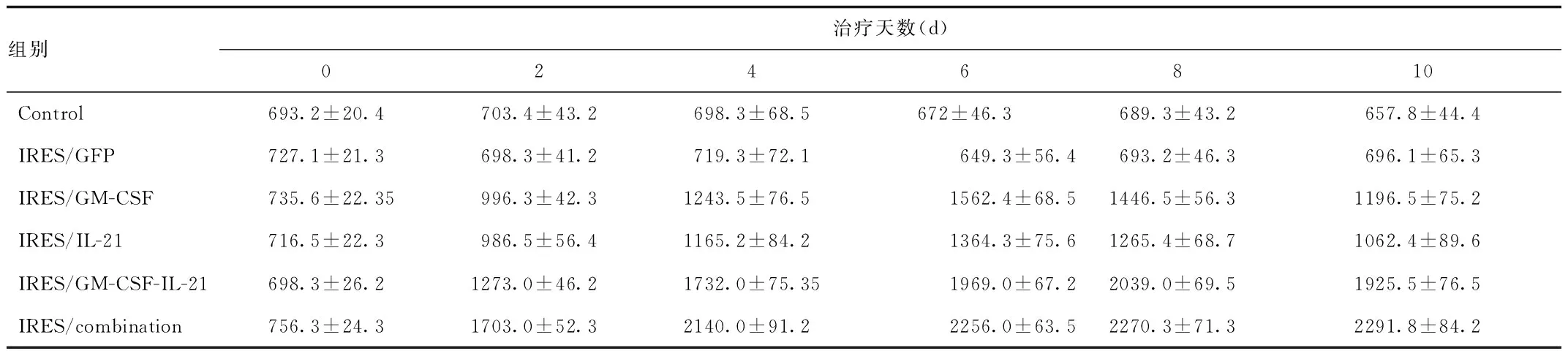
组别治疗天数(d)0246810Control693.2±20.4703.4±43.2698.3±68.5672±46.3689.3±43.2657.8±44.4IRES/GFP727.1±21.3698.3±41.2719.3±72.1649.3±56.4693.2±46.3696.1±65.3IRES/GM-CSF735.6±22.35996.3±42.31243.5±76.51562.4±68.51446.5±56.31196.5±75.2IRES/IL-21716.5±22.3986.5±56.41165.2±84.21364.3±75.61265.4±68.71062.4±89.6IRES/GM-CSF-IL-21698.3±26.21273.0±46.21732.0±75.351969.0±67.22039.0±69.51925.5±76.5IRES/combination756.3±24.31703.0±52.32140.0±91.22256.0±63.52270.3±71.32291.8±84.2

表3 各组治疗后对模型小鼠机体INF-γ水平的动态变化(pg/mL)Tab.3 Dynamic change of INF-γlevels in model mice after different treatment(pg / mL)
3 讨论
肿瘤的发生发展与机体的免疫具有明显的相关性[6],肝癌与其他肿瘤一样出现了机体的免疫功能的抑制,由于肝癌细胞表面表达的分子的改变,可以使肝癌细胞逃避免疫监视的识别[7],导致肝癌细胞不能被NK细胞和CTL细胞识别并清除,同时肿瘤细胞表达被CTL和NK细胞识别的分子(如配体MHC-Ⅰ类相关分子,小鼠中为Rae-1)明显减少或者消失,导致肝癌细胞能在机体内长期存活[8-9]。现在研究较多的是用GM-SCF和(或)IL21基因治疗肿瘤,以提高CTL和NK细胞活性[10-11],没有同时提高肿瘤组织表达又能被NK和CTL细胞识别的基因,或者仅有提高肿瘤组织表达但不能被NK和CTL细胞识别的基因[12],而没有同时提高能促进机体NK和CTL细胞活性的基因治疗。为了提高机体的免疫功能,同时又增强肿瘤组织表达被免疫细胞识别的蛋白,本实验成功构建了“免疫逃逸抑制系统”即重组质粒pGM-CSF-GFP-IRES-Rae-1-IL21,且通过RT-PCR和基因测序证实。前期实验[3]已经证实GM-SCF和IL-21能够明显抑制小鼠肝癌模型,通过激活机体的NK和CTL细胞活性达到抑制小鼠模型的作用。本组实验在前期基础上构建了重组质粒,对小鼠肝癌皮下模型治疗发现,通过60d的观察,IRES/combination的生存期最长,其存活率高达73.33%,明显优于IRES/GM-SCF-IL21,IRES/GM-SCF和IRES/IL21组。而双基因的IRES/GM-SCF-IL21组的疗效明显优于单基因治疗组(IRES/GM-SCF和IRES/IL21组),说明IRES/combination的联合治疗抑制模型小鼠生长作用最强,联合GM-SCF,IL-21和Rae-1基因治疗小鼠肝癌模型的疗效确切。
细胞因子是免疫细胞产生的一类小分子活性物质,具有明显的免疫调节作用,并在肿瘤的免疫中具有重要作用[13-14]。现已知GM-SCF和IL-21可以促进机体的INF-γ和IL-2水平的表达,提高机体CTL细胞和NK细胞的活性,从而达到抑制肿瘤生长的作用[15-16]。本组研究表明GM-CSF,IL-21和Rae-1联合治疗后,小鼠体内的INF-γ和IL-2水平呈持续升高,并且呈高水平的表达,较其他基因治疗明显增强。同时发现IRES/combination组的CTL和NK细胞的数量和活性均明显增强,较IRES/GM-SCF-IL-21,IRES/GM-SCF和IRES/IL-21组明显增强,说明GM-SCF,IL-21和Rea-1三种基因具有协同促进INF-γ和IL-2水平,及CTL和NK细胞的数量及活性。INF-γ是一族糖蛋白,具有明显的抗病毒、免疫调节和抗增殖等生物特性,其主要由CTL和NK细胞分泌[17-18],对肿瘤细胞具有明显的抑制作用,同时具有一定的免疫调节活性,能激活和促进单核巨噬细胞和NK细胞的吞噬作用,促进B细胞和T细胞的分化成熟,增强CTL细胞的细胞毒作用,并可以诱导肿瘤细胞表达MHC-I类抗原的表达,增强免疫细胞如CTL和NK细胞的识别[19]。IL-2作为T淋巴细胞的生长因子,对T淋巴细胞的激活、增殖,对B细胞和巨噬细胞的活化过程具有重要作用,同时活化的T细胞可以分泌IL-2[20]。故说明GM-SCF,IL-21和Rea-1 3种基因促进肝癌小鼠皮下模型机体的免疫系统的激活,促进机体NK细胞和CTL数量和细胞毒性增强,激活的CTL细胞和NK 细胞分泌INF-γ水平明显增加,而高水平INF-γ又促进NK细胞和CTL细胞的激活和细胞毒性;同样激活的CTL细胞分泌的IL-2水平明显增加,而IL-2又促进机体CTL细胞的激活,形成良性循环,从而达到抑制肿瘤生长作用。
综上所述,联合GM-SCF,IL-21和Rae-1基因治疗明显抑制小鼠肝癌生长的作用,其机理与机体的免疫激活有关。
[1] Dou J, Hong X, Zhao F, et al.Investigation of GM-CSF immune accessory effects in tumor-bearing mice by direct gene immunization[J].Immunol Invest,2006,35(2):227-237.
[2] Zhao F, Dou J, Wang J, et al.Investigation on the anti-tumor efficacy by expression of GPI-anchored mIL-21 on the surface of B16F10 cells in C57BL/6 mice[J].Immunobiology,2010,215(2):89-100.
[3] Cheng M, Li Q, Wan T, et al.Synthesis and efficient hepatocyte targeting of galactosylated chitosan as a gene carrier in vitro and in vivo[J].J Biomed Mater Res B Appl Biomater,2011,99(1):70-80.
[4] Pan X C, Li L, Mao J J, et al.Synergistic effects of soluble PD-1 and IL-21 on antitumor immunity against H22 murine hepatocellular carcinoma[J].Oncol Lett,2013,5(1):90-96.
[5] Routes J M,Ryan S, Morris K, et al.Adenovirus serotype 5 E1A sensitizes tumor cells to NKG2D-dependent NK cell lysis and tumor rejection[J].J Exp Med,2005,202(11):1477-1482.
[6] Yigit R, Massuger L F, Figdor C G, et al.Ovarian cancer creates a suppressive microenvironment to escape immune elimination[J].Gynecol Oncol,2010,117(2):366-372.
[7] Nagao M, Nakajima Y, Hisanaga M, et al.The alteration of Fas receptor and ligand system in hepatocellular carcinomas: how do hepatoma cells escape from the host immune surveillance in vivo?[J].Hepatology,1999,30(2):413-421.
[8] Siddle H V, Kreiss A, Tovar C, et al.Reversible epigenetic down-regulation of MHC molecules by devil facial tumour disease illustrates immune escape by a contagious cancer[J].Proc Natl Acad Sci U S A,2013,110(13):5103-5108.
[9] Li M S, Ma Q L, Chen Q, et al.Alpha-fetoprotein triggers hepatoma cells escaping from immune surveillance through altering the expression of Fas/FasL and tumor necrosis factor related apoptosis-inducing ligand and its receptor of lymphocytes and liver cancer cells[J].World J Gastroenterol,2005,11(17):2564-2569.
[10] Zhang P, Wang J, Wang D, et al.Dendritic cell vaccine modified by Ag85A gene enhances anti-tumor immunity against bladder cancer[J].Int Immunopharmacol,2012,14(3):252-260.
[11] Onishi H, Koya N, Kiyota A, et al.A new method for rapid cytotoxic T-lymphocyte induction using a multiple cytokine cocktail[J].Anticancer Res,2012,32(6):2385-2390.
[12] Baba T,Iwasaki S, Maruoka T, et al.Rat CD4+CD8+ macrophages kill tumor cells through an NKG2D- and granzyme/perforin-dependent mechanism[J].J Immunol,2008,180(5):2999-3006.
[13] Barber A, Sentman C L.Chimeric NKG2D T cells require both T cell- and host-derived cytokine secretion and perforin expression to increase tumor antigen presentation and systemic immunity[J].J Immunol,2009,183(4):2365-2372.
[14] Pellegrini M, Mak T W, Ohashi P S.Fighting cancers from within: augmenting tumor immunity with cytokine therapy[J].Trends Pharmacol Sci,2010,31(8):356-363.
[15] Maeda M, Yanagawa Y, Iwabuchi K, et al.IL-21 enhances dendritic cell ability to induce interferon-gamma production by natural killer T cells[J].Immunobiology,2007,212(7):537-547.
[16] Penafuerte C, Bautista-Lopez N, Boulassel M R, et al.The human ortholog of granulocyte macrophage colony-stimulating factor and interleukin-2 fusion protein induces potent ex vivo natural killer cell activation and maturation[J].Cancer Res,2009,69(23):9020-9028.
[17] Ramstead A G, Schepetkin I A, Quinn M T, et al.Oenothein B, a cyclic dimeric ellagitannin isolated from Epilobium angustifolium, enhances IFNgamma production by lymphocytes[J].PLoS One,2012,7(11):e50546.
[18] Ramstead A G, Jutila M A.Complex role of gammadelta T-cell-derived cytokines and growth factors in cancer[J].J Interferon Cytokine Res,2012,32(12):563-569.
[19] Zanon R G, Cartarozzi L P, Victorio S C, et al.Interferon (IFN) beta treatment induces major histocompatibility complex (MHC) class I expression in the spinal cord and enhances axonal growth and motor function recovery following sciatic nerve crush in mice[J].Neuropathol Appl Neurobiol,2010,36(6):515-534.
[20] Zhao M F, Qu X J, Qu J L, et al.The role of E3 ubiquitin ligase Cbl proteins in interleukin-2-induced Jurkat T-cell activation[J].Biomed Res Int,2013,2013:430861.
(编校:谭玲)
Inhibitory effect of joint gene expression of GM-SCF, IL-21, and Rae-1 in treatment of liver cancer model mouse
LV Yi-feng, CHENG Ming-rongΔ, WU Yan
(Department of General Surgery, Pudong New Area Zhoupu Hospital, Shanghai 201318,China)
ObjectiveTo observe recombinant plasmids were constructed with the macrophage colony-stimulating factor (GM-SCF), interleukin -21 (IL-21) and retinoic acid early transcription factor -1 (Rae-1), and observe the inhibitory effects in subcutaneous liver cancer model in mice with the recombinant plasmids.MethodsThe recombinant plasmids of GM-SCF, IL-21 and Rae-1 were constructed with RT-PCR method, mouse model was constructed, the model mice were randomly divided into six groups including control, IRES/GFP, IRES/IL21, IRES/GM-SCF, IRES/GM-SCF-IL21 and IRES/combination with 10 mice included in each group, each groups (15 mice) were treated with the corresponding gene therapy.The survival rate were observed after 60 days.The blood levels of interferon -γ (IFN- γ) and interleukin -2 (IL-2) were detected in each group.ResultsThe pGM-CSF-GFP-IRES-Rae-1-IL-21 has been successfully constructed.All mice had demised 14 and 16 days after treatment in the control and IRES/GFP groups, respectively.There were 2, 1, 11 mice remaining after 60 days of treatment in the IRES/GM-SCF, IRES/IL21 and IRES/GM-SCF-IL21 groups respectively.The survival rate of mice at 60 days of treatment was 73.33%, 13.33%, and 6.67% for groups IRES/GM-SCF-IL21, IRES/GM-SCF and IRES/IL21, respectively.The survival rate of the mice was significantly higher in IRES/GM-SCF-IL21 than the other groups.The levels of IL-2 and INF-γ of mice 1-6 days after treatment gradually increased in the IRES/combination groups, including IRES/GM-SCF-IL21, IRES/GM-SCF and IRES/IL21.They were highest in the IRES/combination group and lowest (P< 0.01) in the IRES/GM-SCF and IRES/IL21 groups, with the IRES/GM-SCF-IL21 group showing intermediate levels.By 6-10 days after treatment, IL-2 and INF-γ levels had stably increased in the IRES/combination groups, but had gradually decreased in the IRES/GM-SCF-IL21, IRES/GM-SCF and IRES/IL21 groups.At the end of treatment, IL-2 and INF-γ levels were significantly (P<0.01) higher in the IRES/GM-SCF-IL21 than were found in either the IRES/GM-SCF group or IRES/IL21 group, which were also significantly (P<0.01) higher than either the IRES/GFP or control groups.The levels of IL-2 and INF-γ were highest in the IRES/combination group (P<0.01) and not significantly different among the IRES/GM-SCF, IRES/IL21, IRES/GFP, and control groups.ConclusionThe inhibitory effects in subcutaneous liver cancer model in mice were obvious significantly, and its mechanism maybe be related to the activation of the body’s immune.
macrophage colony-stimulating factor; interleukin-21; retinoic acid early transcription factor-1; liver cancer
上海市自然基金生物引导类基因(114119a4700);上海市科委纳米专项(12nm0502202)
吕一峰,男,硕士,主治医师,研究方向:肝癌的靶向治疗,E-mail:ssmu_lyf@163.com;程明荣,通讯作者, 男,硕士,副主任医师,研究方向:肝癌的靶向治疗,E-mail:15921300758@126.com。
R574.62;R574.63
A
1005-1678(2015)06-0017-05
