冠状动脉内小剂量尿激酶溶栓联合支架植入对急性ST段抬高型心肌梗死患者心肌灌注及短期预后的影响
2015-06-28赵蓓刘利峰刘瑛琪彭佑华刘莉赵京涛王守力
赵蓓,刘利峰,刘瑛琪,彭佑华,刘莉,赵京涛,王守力
冠状动脉内小剂量尿激酶溶栓联合支架植入对急性ST段抬高型心肌梗死患者心肌灌注及短期预后的影响
赵蓓,刘利峰,刘瑛琪,彭佑华,刘莉,赵京涛,王守力
目的探讨急诊经皮冠状动脉介入术(PCI)中冠状动脉内小剂量尿激酶溶栓联合支架植入对急性ST段抬高型心肌梗死(STEMI)患者心肌灌注及短期预后的影响。方法选择2011年10月-2013年6月在解放军306医院因急性STEMI接受急诊PCI手术治疗且符合入组标准的患者共183例,随机分为尿激酶组(n=91)和对照组(n=92)。尿激酶组除常规介入治疗外,在支架植入术前经抽吸导管单次或继之在PCI术中反复多次给予5~10万U尿激酶,对照组不给予尿激酶。主要观察指标包括两组患者在PCI术后即刻的TIMI血流分级、校正TIMI帧数,心电图ST段回落,以及PCI术后7、30d左室功能情况。记录30d随访的主要不良心血管事件(MACEs,包括死亡、心绞痛、靶血管再次血运重建、心衰、脑卒中)。结果尿激酶组患者在PCI术后即刻的TIMI Ⅲ级血流及校正TIMI帧数明显优于对照组(83vs71,P=0.034;21.2±10.7vs29.6±15.3,P=0.012),术后90min ST段回落>70%患者百分比明显高于对照组(93.4%vs82.6%,P=0.025)。术后7d随访时心脏超声左室射血分数(LVEF)改善不明显(53.5%±9.4%vs51.6%±8.7%,P=0.158),30d时心脏超声显示,尿激酶组LVEF明显优于对照组(56.3%±9.8%vs53.5%±8.1%,P=0.036)。30d时尿激酶组MACEs发生率明显低于对照组(4.4%vs13.0%,P=0.038)。结论急诊PCI术中于支架植入前单次或反复多次冠脉内给予小剂量尿激酶可有效增加心肌再灌注及左室功能,改善急性心肌梗死患者的短期预后,且安全性较好。
心肌梗死;血管成形术,气囊,冠状动脉;尿激酶型纤溶酶原激活物;血栓溶解疗法
目前,经皮冠状动脉介入术(percutaneous coronary intervention,PCI)已成为急性ST段抬高型心肌梗死(ST-elevated myocardial infarction,STEMI)的主要治疗手段[1-4],但心外膜血管的开通并不意味着心肌灌注能彻底恢复,研究发现再灌注损伤以及微栓塞是影响缺血组织恢复灌注的主要因素[5]。急性心肌梗死患者的罪犯血管狭窄部位常有较严重的血栓负荷,血栓抽吸并不能清除所有血栓,介入操作尤其是球囊扩张及支架植入可导致局部形成微血栓,当前向血流恢复时,这些微血栓向前移动造成远端微血管栓塞,可进一步加重组织缺血损伤,造成慢血流,影响手术效果及患者预后,已成为临床上困扰心脏介入医生的重要问题[6],目前国内外已经开展了大量的相关研究[7-8]。本研究在PCI术中通过抽吸导管,在冠脉血栓内单次或多次反复给予小剂量尿激酶,观察其对PCI术后心肌灌注及短期预后的改善情况。
1 资料与方法
1.1 研究对象 选择2011年10月-2013年6月在解放军306医院因急性STEMI住院并接受急诊PCI手术治疗且术中出现慢血流的患者288例,最终纳入183例。纳入标准:①年龄18~74岁;②首次接受冠状动脉介入治疗;③持续性胸痛(缺血性胸痛发作<12h,或≥12h但仍有缺血性胸痛);④心电图显示相邻≥2个导联ST段抬高>1mV或新出现左束支传导阻滞伴心肌酶学证据;⑤冠脉造影显示罪犯血管TIMI血流分级为0或1级。排除标准:①伴心源性休克、急性肺水肿者等血流动力学不稳定的患者;②有严重出血倾向,近期有活动性出血;③严重血小板减少;④严重肝肾功能不全患者;⑤既往脑卒中史。无复流现象定义为:在球囊扩张或支架置入后,无撕裂或夹层的前提下,出现TIMI血流<3级者。本研究通过解放军306医院伦理委员会批准,所有纳入患者均签署知情同意书。研究遵循赫尔辛基宣言2007版[9]。患者具体入选流程见图1。

图1 入选患者流程图Fig.1 Flow chart of enrolled patients
1.2 方法
1.2.1 研究分组及给药方法 患者由电脑随机号码分成两组:尿激酶组(n=91),对照组(n=92)。所有患者的PCI均在解放军306医院心血管内科导管室进行,采用标准技术经股动脉或桡动脉穿刺径路,行冠状动脉造影及PCI治疗。术前嚼服300mg阿司匹林、600mg氯吡格雷,穿刺成功后经鞘管内注入肝素5000U,硝酸甘油注射液100µg,PCI术前加注肝素3000~4000U,术中每延长1h即向鞘管内追加肝素1000U。以Judkins法行冠状动脉造影,常规多体位投照,明确冠状动脉病变支数和梗死相关血管(IRA)。所有患者冠状动脉造影图像使用25帧/s记录,PCI操作方法按照标准PCI术实施,导引导管、导丝、球囊的选择根据冠状动脉解剖确定,支架直径按照靶血管正常节段直径1:1,支架长度按照病变血管长度选择。尿激酶组于发现血管血栓负荷重、出现无复流现象(TIMI血流分级<2级)后,即刻将血栓抽吸导管放入血栓内部单次或反复多次给予小剂量尿激酶,即后伴或不伴单次或反复多次血栓抽吸,根据造影TIMI血流情况决定尿激酶用量,每次(5~10)万U,总量不超过50万U。术后口服阿司匹林300mg/d(4周后改为100mg/d)和氯吡格75mg/d(≥12个月),皮下注射低分子肝素1周。
1.2.2 心肌灌注评价 PCI术后即刻进行心肌灌注评价,采用的主要指标包括:①TIMI血流分级。0级:不存在任何超过闭塞处前向血流;l级:存在微弱的超过闭塞处前向血流,但不能完全充盈远端血管床;2级:延迟或缓慢前向血流,能完全充盈远端血管床;3级:正常前向血流,完全充盈远端血管床。②校正TIMI帧数。记录第一帧和最末帧之间的帧数。造影剂完全进入血管的第一帧定义为首帧,造影剂开始进入靶血管末端分支(界标)的第一帧为末帧。前降支的平均帧数比左回旋支、右冠状动脉更长,将左前降支的帧数除以1.7,即为校正的TIMI帧数计数(corrected TIMI frame count,CTFC)。③术后90min ST段回落程度>70%。
1.2.3 观察指标 所有入选患者均在门诊进行30d随访,随访内容包括血常规、肝功能、肾功能、血脂等化验指标,检查包括心脏超声、心电图等。30d随访期间记录各种主要心血管不良事件(major adverse cardiovascular events,MACEs),包括心肌梗死、死亡、心绞痛、再次血运重建等。
1.3 统计学处理 采用SPSS 17.0软件进行数据分析。计量资料若符合正态分布,以表示,两组之间比较采用t检验;不符合正态分布的数据采用中位数表示,两组之间比较采用秩和检验;计数资料用绝对数和百分率表示,两组之间比较采用χ2检验。P<0.05为差异有统计学意义。

表1 两组一般临床基线资料比较Tab.1 Comparison of clinical features between the two groups

表2 两组冠状动脉造影及介入治疗基线资料比较Tab.2 Baseline data in coronary angiography and interventional therapy
2 结 果
2.1 患者基线资料 根据纳入排除标准,共纳入183例患者,其中尿激酶组91例,对照组92例。两组患者临床基线资料比较见表1,造影及介入基线数据见表2,其中尿激酶组患者梗死罪犯血管共植入支架146枚,对照组共植入148枚,均为药物洗脱支架。两组各基线指标比较差异均无统计学意义(P>0.05)。
2.2 两组治疗效果比较 术后即刻TIMI血流比较发现,尿激酶组TIMI Ⅲ级血流患者倒数明显高于对照组,而校正的TIMI帧数则明显低于对照组(21.2±10.7vs29.6±15.3,P=0.000),术后90min ST段回落明显优于对照组(ST段回落>70%患者百分比,93.4%vs82.6%,P=0.025),7d复查心脏超声时左室射血分数(LVEF)改善不明显(53.5%±9.4%vs51.6%±8.7%,P=0.158),仅尿激酶组左室舒张末容积明显小于对照组(98.2±25.7mlvs109.9±29.5ml,P=0.005),但30d随访心脏超声显示尿激酶组LVEF明显高于对照组(56.3%±9.8%vs53.5%±8.1%,P=0.036),且尿激酶组30d MACEs发生率明显低于对照组(4.4%vs13.0%,P=0.038),尤其是心绞痛及心衰的发生率明显下降(表3)。
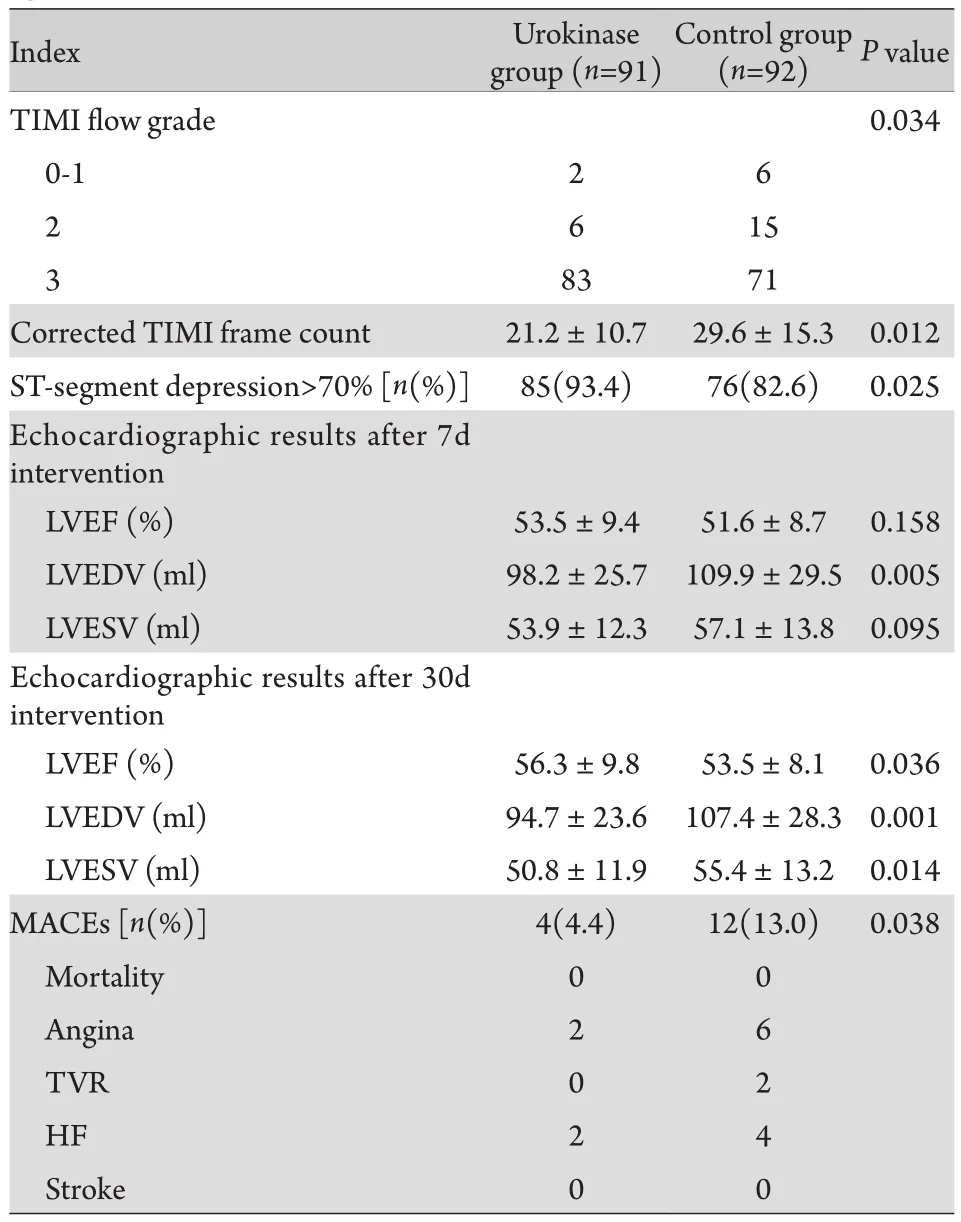
表3 术后相关检查及30d随访比较Tab.3 Comparison of echocardiographic and 30 days followup features
3 讨 论
本研究采用随机对照方法,观察PCI术中于血栓内部单次或反复多次给予小剂量尿激酶处理对急性心肌梗死患者短期预后及心脏功能的影响。结果显示,在PCI术中单次或反复多次给予小剂量尿激酶可明显改善患者PCI术后的心肌灌注,包括即刻TIMI血流、校正TIMI帧数及术后90min ST段回落情况;在改善心肌灌注的基础上,尿激酶组术后30d心脏功能也较对照组明显改善。本组患者均成功开通罪犯血管,尿激酶组患者给予尿激酶后均能在PCI术后6~8h正常解除止血扣,无一例发生穿刺部位或其他部位血肿、明显出血等,随访30d仍然无一例发生明显出血,且尿激酶组30d随访的MACEs明显少于对照组。
急性心肌梗死往往是由于不稳定斑块破裂、血栓形成堵塞血管所致,急诊PCI术可以快速开通罪犯血管、恢复前向血流[10]。然而,部分患者在PCI术后不能有效恢复心肌灌注,其中有些患者不能即刻恢复罪犯血管前向血流,表现为0-1级TIMI血流;部分患者前向血流恢复不充分,表现为TIMI血流2级;还有部分患者虽然前向血流恢复,但从核素或核磁检查来看,心肌的微循环灌注并不能完全有效恢复,仍有一部分心肌处于休眠状态。心肌灌注不良是急性心肌梗死PCI术后预后不良的独立预测因素[11]。此外,研究者还认为,急性心肌梗死患者PCI术后心肌灌注不良的原因很多,但主要原因是球囊扩张及支架植入造成原位血栓碎裂,形成大量微栓子堵塞远端微循环血管[11]。既往国外有研究显示,PCI术后在冠脉内给予小剂量链激酶,可以改善心肌微循环,但在6个月的随访中,并未显示出对左室结构及功能的益处[12],但其后更大样本的进一步研究证实,该方法可以缩小梗死面积、改善左室功能,作者认为这与减少微血管中的纤维蛋白(原)有关[13]。与该研究不同,本研究的给药时间点是PCI术前,效果不佳时在PCI术中再反复多次给药;其次,相对于尿激酶,链激酶的抗原性更强,潜在的药物过敏风险更高[14]。笔者发现,在PCI术中单次或反复多次给予小剂量尿激酶,同样可明显改善患者心肌灌注,30d随访期间未发现明显的尿激酶相关副作用,安全性好,且不良事件发生率明显少于对照组。本研究在PCI术前即给予冠脉内尿激酶治疗的理由包括:①PCI术前给药可能增加药物与血栓接触的时间,当罪犯血管血栓内有尿激酶时,随着介入操作对血栓的破坏,药物可随时与血栓接触从而起到溶栓作用,并且随着反复多次不断溶栓,会达到更好的溶栓效果;②当球囊扩张和支架植入后,药物可随大量微栓塞一起流向远端从而继续发挥作用;③PCI术前单次给药效果欠佳时在术中继续反复多次给药,可使罪犯血管血栓远端维持在溶栓状态,减少微血管堵塞后血栓的继续形成。本研究结果证实这种给药方法对于改善PCI术后的心肌灌注效果明显。
本研究的局限性在于仅观察了30d的随访结果,且某些血液指标也未能纳入具体分析,本方法对于急性心肌梗死患者的远期疗效及安全性还需要进一步长期随访观察;其次,本研究没有与PCI术后立即冠脉内给予尿激酶进行对比,两种给药方法的优劣目前还不能得出结论。
[1]Kushner FG, Hand M, Smith SC,et al. 2009 focused updates: ACC/AHA guidelines for the management of patients with ST-elevation myocardial infarction (updating the 2004 guideline and 2007 focused update) and ACC/AHA/SCAI guidelines on percutaneous coronary intervention (updating the 2005 guideline and 2007 focused update) a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines[J]. J Am Coll Cardiol, 2009, 54(25): 2205-2241.
[2]Liu HW, Wang XZ, Ma YY,et al. Clinical effect of selective thrombus aspiration during primary percutaneous coronary intervention in patients with ST-segment elevation myocardial infarction[J]. Med J Chin PLA, 2015, 40(4): 271-274. [刘海伟,王效增, 马颖艳, 等. ST段抬高型心肌梗死患者直接PCI术中选择性使用血栓抽吸术的疗效观察[J]. 解放军医学杂志, 2015, 40(4): 271-274.]
[3]Xie C, Cong HL, Li XM,et al. Risk factors of depression in coronary heart disease patients who underwent revascularization therapy[J]. Tianjin Med J, 2015, 43(4): 412-415. [解存, 丛洪良, 李曦铭, 等. 冠心病再血管化治疗后发生抑郁的危险因素分析[J]. 天津医药, 2015, 43(4): 412-415.]
[4]Cao Q, Liu JY, Ma SM,et al. The altered plasma adrenomedullin level and its clinical significance after percutaneous coronary intervention[J]. Chin J Pract Intern Med, 2011, 31(5): 370-371.[曹乾, 刘静一, 马淑梅, 等. 经皮冠状动脉介入术后血浆肾上腺髓质素变化及临床意义研究[J]. 中国实用内科杂志, 2011, 31(5): 370-371.]
[5]Kaul S, Ito H. Microvasculature in acute myocardial ischemia: Part Ⅱ: evolving concepts in pathophysiology, diagnosis, and treatment[J]. Circulation, 2004, 109(3): 310-315.
[6]Henriques JP, Zijlstra F, Ottervanger JP,et al. Incidence and clinical significance of distal embolization during primary angioplasty for acute myocardial infarction[J]. Eur Heart J, 2002, 23(14): 1112-1117.
[7]Wang J, Chen YD, Zhi G,et al. Beneficial effect of adenosine on myocardial perfusion in patients treated with primary percutaneous coronary intervention for acute myocardial infarction[J]. Clin Exp Pharmacol Physiol, 2012, 39(3): 247-252.
[8]Chen Y, Wang C, Yang X,et al. Independent no-reflow predictors in female patients with st-elevation acute myocardial infarction treated with primary percutaneous coronary intervention[J]. Heart Vessels, 2012, 27(3): 243-249.
[9]Goodyear MD, Krleza-Jeric K, Lemmens T. The declaration of Helsinki[J]. BMJ, 2007, 335(7621): 624-625.
[10] Eran O, Novack V, Gilutz H,et al. Comparison of thrombolysis in myocardial infarction, global registry of acute coronary events, and acute physiology and chronic health evaluation ii risk scores in patients with acute myocardial infarction who require mechanical ventilation for more than 24 hours[J]. Am J Cardiol, 2011, 107(3): 343-346.
[11] Kaul S. The "no reflow" phenomenon following acute myocardial infarction: Mechanisms and treatment options[J]. J Cardiol, 2014, 64(2): 77-85.
[12] Sezer M, Oflaz H, Goren T,et al. Intracoronary streptokinase after primary percutaneous coronary intervention[J]. N Engl J Med, 2007, 356(18): 1823-1834.
[13] Sezer M, Cimen A, Aslanger E,et al. Effect of intracoronary streptokinase administered immediately after primary percutaneous coronary intervention on long-term left ventricular infarct size, volumes, and function[J]. J Am Coll Cardiol, 2009, 54(12): 1065-1071.
[14] Zhu TQ, Zhang Q, Ding FH,et al. Randomized comparison of intracoronary tirofibanversusurokinase as an adjunct to primary percutaneous coronary intervention in patients with acute stelevation myocardial infarction: results of the ICTUS-AMI trial[J]. Chin Med J (Engl), 2013, 126(16): 3079-3086.
Effects of intracoronary low-dose urokinase injection combined with stent implantation in acute STEMI patients on myocardial perfusion and its influence on short-term prognosis
ZHAO Bei, LIU Li-feng, LIU Ying-qi, PENG You-hua, LIU Li, ZHAO Jing-tao, WANG Shou-li*
Department of Cardiology, 306 Hospital of PLA, Beijing 100101, China
*< class="emphasis_italic">Corresponding author, E-mail: wangsl.63@126.com
, E-mail: wangsl.63@126.com
ObjectiveTo investigate the effects of primary percutaneous coronary intervention (PCI) combined with intracoronary low-dose urokinase therapy on myocardial perfusion and clinical outcome in acute STEMI patients.MethodsFrom Oct. 2011 to Jun. 2013, 183 patients suffering from myocardial infarction with acute ST segment elevation, (STEMI) who had undergone emergent PCI in 306 Hospital of PLA conforming to inclusion criteria were enrolled in the present study. They were randomly assigned to urokinase group (n=91) and control group (n=92). For urokinase group, besides routine interventional treatment, patients were given single or multiple intracoronary injection of 0.05-0.1 million U urokinase immediately before primary PCI, while for control group, patients
routine interventional treatment only. The main indices determined and compared between the two groups included the immediate blood flow grading of thrombolysis in myocardial infarction (TIMI), corrected TIMI frame count, falling degree of ST segment elevation in ECG after intervention, and left ventricular function on the 7th and 30th day after intervention, and also major adverse cardiac events (MACE) on the 30th day after intervention.ResultsThe TIMI Ⅲ blood flow and corrected TIMI frame count were obviously better in urokinase group than in control group (83vs71,P=0.034; 21.2±10.7vs29.6±15.3,P=0.012) immediately after PCI, ant the falling degree >70% of ST segment elevation at 90min after intervention was significantly more marked in urokinase group than that in control group (93.4%vs82.6%,P=0.025). When compared with urokinase group to control group, although no significant difference was found in left ventricular ejection fraction (LVEF) on the 7th day of follow-up (53.5±9.4vs51.6±8.7,P=0.158), the cardiac ultrasound revealed a better outcome of LVEF (56.3±9.8vs53.5±8.1,P=0.036) and a lower MACEs (including death, angina, target vessel revascularization, heart failure andstroke, etc.)(4.4%vs13.0%,P=0.038) on the 30th day of follow-up.ConclusionEmergency PCI combined with single or multiple intracoronary injection of low-dose urokinase before stent implantation may efficiently improve the myocardial perfusion and left ventricular function, and improve the short-term prognosis of acute myocardial infarction patients with due safety.
myocardial infarction; angioplasty, balloon, coronary; urokinase-type plasminogen activator; thrombolytic therapy
R542.22
A
0577-7402(2015)08-0661-05
10.11855/j.issn.0577-7402.2015.08.12
2014-11-20;
2015-03-26)
(责任编辑:张小利)
赵蓓,医学博士,主治医师。主要从事冠心病病理机制及超声影像学诊断方面的研究
100101 北京 解放军306医院心内科(赵蓓、刘利峰、刘瑛琪、彭佑华、刘莉、赵京涛、王守力)
王守力,E-mail:wangsl.63@126.com
