Preparation of Overbased Calcium Alkylbenzene Sulfonate for Formulating Complex Sulfonate Grease
2015-06-21LiuYinong
Liu Yinong
(SINOPEC Research Institute of Petroleum Processing, Beijing 100083)
Preparation of Overbased Calcium Alkylbenzene Sulfonate for Formulating Complex Sulfonate Grease
Liu Yinong
(SINOPEC Research Institute of Petroleum Processing, Beijing 100083)
Six kinds of alkylbenzene sulfonic acids were selected to prepare the sulfonates S1—S6. Among them, the sulfonates S3, S4, and S6could be incorporated into lubricating grease with good performance in comparison with the grease produced from commercial sulfonates T106-1 and T106-2. The optimized conditions for synthesis of the sulfonates S3, S4, and S6were explored by using different mass ratios of methanol, water and the type of copromoters. It was found out that the appropriate conditions for synthesis of the sulfonate S3included a methanol to M (M is the total mass of alkylbenzene sulfonic acid and base oil) mass ratio of 16%, a water to M mass ratio of 4%, and a copromoter A to M mass ratio of 2%; the appropriate conditions for synthesis of the sulfonate S4included a methanol to M mass ratio of 24%, a water to M mass ratio of 2%, a copromoter B to M mass ratio of 2%; and the optimized conditions for synthesis of the sulfonate S6included a methanol/ M mass ratio of 8%, a water/M mass ratio of 4% and a copromoter B/M mass ratio of 2%. The new sulfonates S3, S4, and S6produced under the optimized conditions exhibited higher TBN and better antiwear property in camparison with the previous products. Grease samples G9, G10, and G11 were prepared with new sulfonates S3, S4, and S6successfully and exhibited improved water stability and high temperature performance.
complex sulfonate grease; overbased calcium sulfonate; optimization
1 Introduction
Compared with other types of lubricating greases, the calcium sulfonate complex grease has excellent high temperature performance, water resistance ability, antiwear property, extreme pressure (EP) property and anticorrosion property thanks to its very high dropping point and excellent mechanical stability under a wide range of conditions, and has been known as the excellent grease in this century. Although calcium sulfonate complex grease was developed as early as in the 1960s, it was not widely applied in various working fields until Muir and Blokhuis improved its synthesis technology in 1985[1]. Since then, many upgraded calcium sulfonate complex greases had been developed by Muir and other researchers by introducing calcium borate and 12-hydroxystearic acid and decreasing the dosage of thickener[2-6]. With the development of domestic steel industry and other sectors of machine building industry, more and more calcium sulfonate complex grease is needed.
The production process of calcium sulfonate complex grease involves such starting materials as calcium alkylbenzene sulfonate, base oil, the converting agent (such as acetic acid, propionic acid, alcohol), water, calcium hydroxide, calcium borate and 12-hydroxystearic acid that are mixed in a vessel and heated for a certain time, and during this process the calcium carbonate in the micelle of sulfonate is gradually converted from an amorphous state to calcite, when the corresponding infrared absorption peak of carbonate shifts from 863 cm-1to 881 cm-1, and furthermore, after about 1 hour, the diluted mixture changes into a thick grease to corroborate the completion of conversion process[2,7].
It is generally realized that the conversion of calcium alkylbenzene sulfonate to complex grease is closely related to the quality of calcium alkylbenzene sulfonate and the technology for grease conversion. If the technology for conversion of calcium sulfonate complex grease is the same, the conversion outcome, in particular the quality of grease, is greatly determined by the quality of calcium alkylbenzene sulfonate[3-4].
It is clear that the critical step in the production of calcium complex grease is the conversion of calcium alkylbenzene sulfonate from a Newtonian fluid to a grease, when the amorphous calcium carbonate particles are converted into calcite. It was reported that some domestic calcium alkylbenzene sulfonates could not be converted into grease at all although the conversion process was the same. Even the process for conversion to grease was successful, the grease could not implement the same performance as other calcium alkylbenzene sulfonate greases did[2,7].
The reason could be that the calcium alkylbenzene sulfonate, which was synthesized by alkylbenzene sulfonic acids originally prepared from monoalkylbenzene or dialkylbenzene with different alkyl carbon chain lengths and isomers, was mainly used for formulating lubricating oil for detergency, dispersity and acid neutralization, and might not be suitable for the production of calcium alkylbenzene sulfonate grease.
In order to solve the above-mentioned problems, the calcium alkylbenzene sulfonates were synthesized firstly with alkylbenzene sulfonic acids which were prepared from alkyl benzene containing different mass ratios of monoalkylbenzene and dialkylbenzene, and then the proper sulfonates were selected by evaluating the performance of sulfonate grease after the conversion of sulfonate into grease and finally, the conditions for preparation of calcium alkylbenzene sulfonate were investigated, and the optimized synthesis conditions were then identified.
2 Experimental
2.1 Raw materials
Six kinds of alkylbenzene sulfonic acids were selected, with their properties listed in Table 1.
CaO was provided by the Jiangsu Changshu Calcium Oxide Company with the following properties: a CaO purity of >95%, an impurity (CaCO3) content of <1.5%, a content of other impurities <2.0%, and a content of 100 mesh particles > 90%.
The No.120 solvent was a commercial product with a distillation range of between 80—120 ℃. Methanol was produced by the Shangxi Pingyuan Fertilizer Plant with a purity of 99% or more. The copromoter A, copromoter B and copromoter C were all commercial products. Carbon dioxide was a commercial product with a purity of 99.5% or more. The kinematic viscosity of 150SN base oil was 5.221 mm2/s at 100 ℃ and 30.21 mm2/s at 40 ℃. The kinematic viscosity of 150BS base oil was 31.34 mm2/s at 100 ℃and 566.8 mm2/s at 40 ℃.
2.2 Analytical methods
The kinematic viscosity, turbidity, active components content, TBN (total base number) and elements content of sulfonate were measured by test methods: GB/T 265 (Determination of kinematic viscosity of petroleum products and calculation of dynamic viscosity), SH/T 0028 (Measurement of turbidity of lubricating oil detergents), SH/T 0034 (Measurement of active components in additives), SH/T 0251(Measurement of total base number in petroleum products--Method of potentiometric titration by perchloric acid), and SH/T 0749 (Measurement of elements content in lubricating oil and additives), respectively.
2.3 Experimental process
Synthesis of calcium alkylbenzene sulfonate: Synthesis of calcium alkylbenzene sulfonate included two steps. At first, the solvent, methanol, calcium oxide, base oil, and copromoters were mixed in a flask for a certain time, and then, sulfonic acid was added and blended for a certain time. The calcium alkylbenezene sulfonate formed thereby was collected in a form of reverse micelle in oil. After that, carbon dioxide was introduced into the flask, in which calcium carbonate was coated by calcium alkylbenzene sulfonate as soon as the amorphous calcium carbonate was formed in situ, and the overbased sulfonatewas formed eventually.

Table 1 properties of alkylbenzene sulfonic acids
Chemical equations for synthesizing calcium alkylbenzene sulfonate are shown below. Neutralization:

Preparation of calcium sulfonate complex grease: At first, calcium alkylbenzene sulfonate was added into a vessel at a mass ratio of 40%—60% based on the mass of all reactants, and the 150BS base oil was added at a mass ratio of 40%—60% based on the mass of all reactants, and then a measured amount of converting agent, water, calcium hydroxide, calcium borate and 12-hydroxystearic acid was also mixed in the vessel, prior to preheating all the reactants to 83—115 ℃, when the calcium carbonate in the micelle of sulfonate was gradually converted from amorphous particles to calcite, as evidenced by the shift of the corresponding infrared absorption peak of carbonate from 863 cm-1to 881 cm-1. About one hour later, the diluted mixture was changed into a thick grease, and the conversion process was completed.
3 Results and Discussions
3.1 Exploration on synthesis of sulfonate
Usually the sulfonate for formulating lubricating oil was synthesized by alkylbenzene sulfonic acid containing different mass ratios of C12-30monoalkyl chains and C12-30dialkyl chains[8]. In order to synthesize the alkylbenzene sulfonate for formulating the grease, six kinds of alkylbenzene sulfonates were synthesized from alkylbenzene sulfonic acids containing different mass ratios of C12-30monoalkyl chains and C12-30dialkyl chains when the mass ratio of methanol to M (M is the total mass of alkylbenzene sulfonic acid and base oil) was 20% and the mass ratio of water to M was 5%, with the copromoter A also added. The properties of sulfonates along with two commercial sulfonates are listed in Table 2.
The sulfonate greases were prepared from the above sulfonates according to the same conversion conditions, with the results listed in Table 3.
It can be found out from Table 3 that five sulfonate samples could be converted to greases smoothly except for the sulfonate S2. Among the seven grease products, the grease G-4 exhibited the best roll stability with the remaining water equating to 63 mL, while the grease G-6 exhibited the best high temperature performance, with a cone penetration differential of 36. Compared with the performance of greases G-7 and G-8, the overall performance of greases G-3, G-4 and G-6 was more outstanding. Greases G-1 and G-5 were knocked out because the high temperature performance of G-1 and the water stability of G-5 were poor.
As the sulfonate with high TBN had more content of micellized carbonate which was beneficial to the antiwearproperty of grease[8], and also the sulfonate with low viscosity was easy to be handled in grease conversion process, the alkylbenzene sulfonic acids A3, A4 and A6 were used as raw materials to synthesize the new sulfonates S3, S4 and S6 with high TBN and low viscosity. Meanwhile the mass ratio of methanol, water and type of copromoters were investigated to cope with the synthesis of new sulfonates S3, S4 and S6. The new sulfonate greases were prepared and their performance was evaluated.

Table 2 Properties of synthesized sulfonates
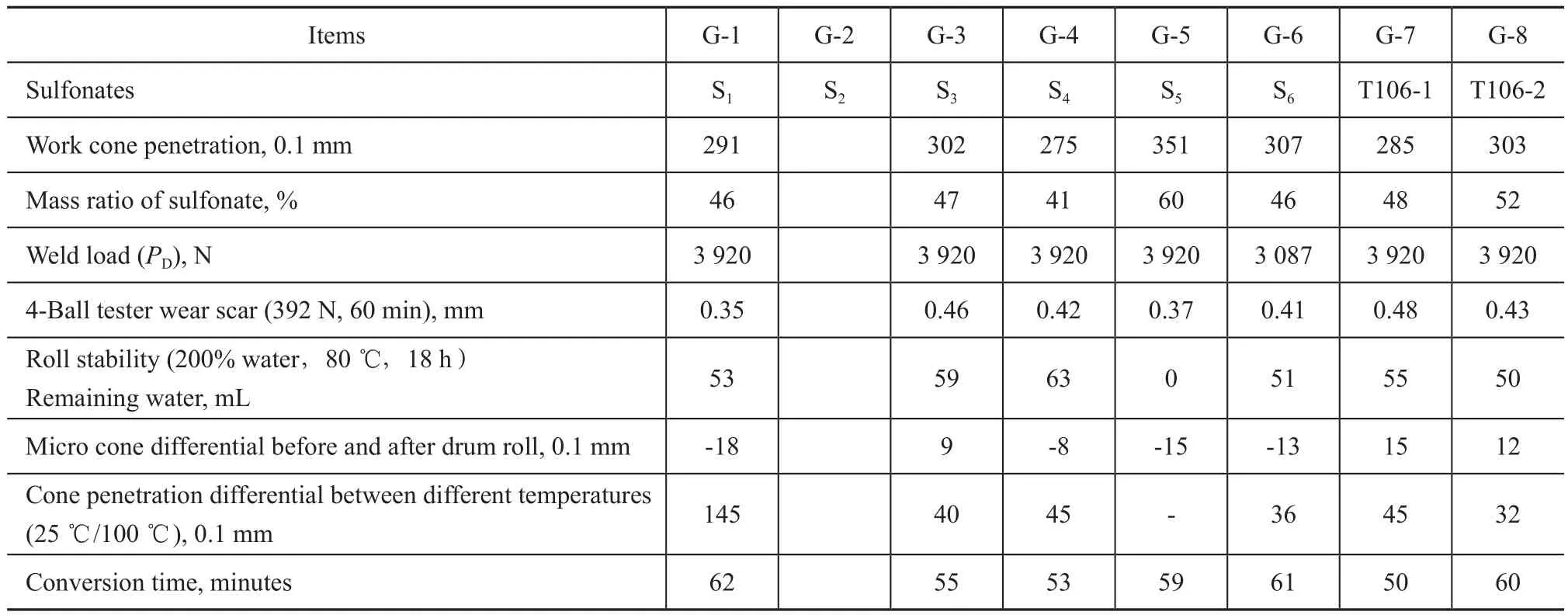
Table 3 Properties of complex sulfonate greases
3.2 Synthesis of new sulfonate S3
The new sulfonate S3was synthesized when the mass ratio of methanol to M was 12%, 16%, 24%, and 32%, respectively, the mass ratio of water to M was 2%, 4%, and 6%, respectively, and the mass ratio of copromoter (A, or B, or C) to M was 2%. The prepared sulfonates were tested on their TBN, turbidity, and kinematic viscosity at 100 ℃, with the results listed in Tables 4-6.
It can be seen that the new sulfonate S3exhibited a higherTBN of 370 mgKOH/g, a lower viscosity of 185 mm2/s and a turbidity of 121 when the mass ratio of methanol to M was 16%. The sulfonate S3exhibited a higher TBN of 375 mgKOH/g, a lower viscosity of 172 mm2/s and a lower turbidity of 102 when the mass ratio of water to M was 4%. The sulfonate S3also exhibited a higher TBN of 371 mgKOH/g, a turbidity of 101 and a higher viscosity of 183 mm2/s when the copromoter A was used. Thus, the optimized conditions for synthesis of new sulfonate S3specified that the mass ratio of methanol, water and copromoter A to M was equal to 16%, 4% and 2%, respectively.

Table 4 Influence of the mass ratio of methanol on the property of sulfonate S3

Table 5 Influence of the mass ratio of water on the property of sulfonate S3
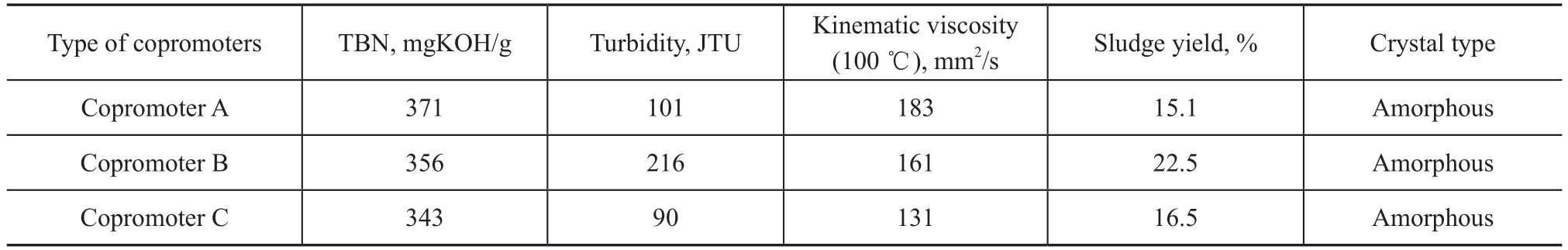
Table 6 Influence of the type of copromoters on the property of sulfonate S3
3.3 Synthesis of new sulfonate S4
The new sulfonate S4was synthesized when the mass ratio of methanol to M was set at 16%, 24%, and 32%, respectively, the mass ratio of water to M was 2%, 4%, and 6%, respectively, and the mass ratio of copromoter (A, or B, or C) to M was 2%. The prepared sulfonates were evaluated on their TBN, turbidity and kinematic viscosity at 100 ℃, with the results listed in Tables 7-9.
It can be seen that the new sulfonate S4exhibited a higher TBN of 375 mgKOH/g, a turbidity of 118 and a viscosity of 125 mm2/s when the mass ratio of methanol to M was 24%. The sulfonate S4exhibited a higher TBN of 375 mg-KOH/g, a higher turbidity of 150 and a viscosity of 121 mm2/s when the mass ratio of water to M was 2%. The sulfonate S4also exhibited a TBN of 371 mgKOH/g, a turbidity of 130 and a lower viscosity of 116 mm2/s when the copromoter B was used. Thus, the optimized conditions for synthesis of new sulfonate S4specified the mass ratio of methanol, water and copromoter B to M to be 24%, 2% and 2%, respectively.
3.4 Synthesis of new sulfonate S6
The new sulfonate S6was synthesized when the mass ratio of methanol to M was 8%, 12%, and 20%, respectively, the mass ratio of water to M was 0, 4%, and 8%, respectively, and the mass ratio of copromoter (A, or B, or C) to M was 2%. The prepared sulfonate samples were evaluated on their TBN, turbidity and kinematic viscosity at 100 ℃, with the results listed in Tables 10—12.
It can be seen that the new sulfonate S6had a TBN of 391 mgKOH/g, a lower turbidity of 134 and a lower viscosity of 263 mm2/s when the mass ratio of methanol to M was 8%. The sulfonate S6had a TBN of 402 mgKOH/g, a turbidity of 139 and a lower viscosity of 257 mm2/s when the mass ratio of water to M was 4%. The sulfonate S6also had a higher TBN of 396 mgKOH/g, a higher turbidity of 157 and a lower viscosity of 230 mm2/s when the copromoter B was used. Thus, the optimized conditions for synthesis of the sulfonate S6specified the mass ratio of methanol, water and copromoter B to M to be 8%, 4% and 2%, respectively.

Table 7 Influence of the mass ratio of methanol on the property of sulfonate S4
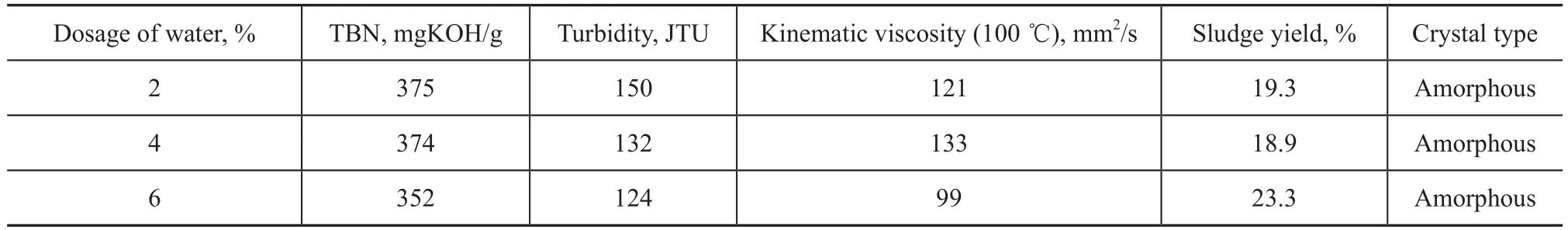
Table 8 Influence of the mass ratio of water on the property of sulfonate S4

Table 9 Influence of the types of copromoters on the property of sulfonate S4

Table 10 Influence of the mass ratio methanol on the property of sulfonate S6

Table 11 Influence of the mass ratio of water on the property of sulfonate S6
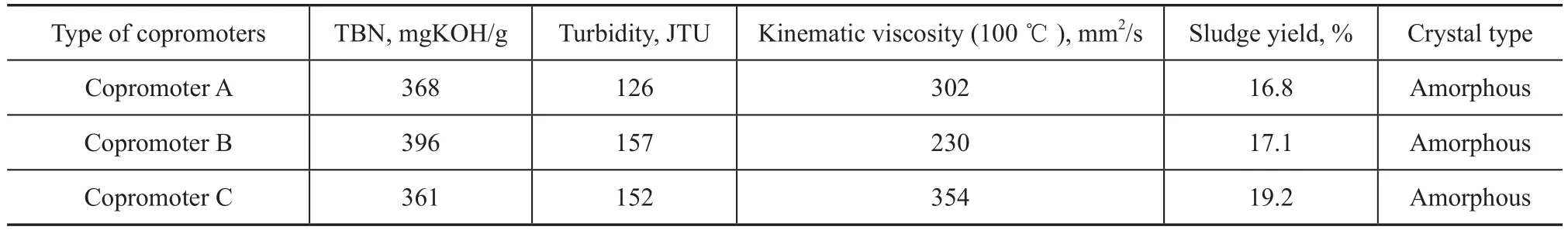
Table 12 Influence of the type of copromoters on the property of sulfonate S6
3.5 Synthesis of new sulfonates S3, S4, and S6and greases G-9, G-10, and G-11 under the optimized conditions
New sulfonates S3, S4, and S6were synthesized according to the above-mentioned optimized conditions, with the results listed in Table 13.
It can be seen from Table 13 that new sulfonates S3, S4, and S6which were synthesized under the optimized conditions had a higher TBN of 386.7 mgKOH/g, 381.7 mgKOH/g, and 409 mgKOH/g, a lower turbidity of 108, 113, and 137 and a lower viscosity of 165.5 mm2/s, 123 mm2/s, and 225 mm2/s, respectively, in comparison with the original properties of sulfonates S3, S4, and S6as depicted in Table 2. Thus, greases G-9, G-10 and G-11 were prepared by using the new sulfonates S3, S4, and S6, with the results listed in Table 14.
It can be seen from Table 14 that the complex greases G-9, G-10, and G-11 demonstrated a shorter conversion time of 45 minutes, 40 minutes and 42 minutes, respectively, along with improved performance in comparison with greases G-3, G-4 and G-6, which had a conversion time of 55 minutes, 53 minutes and 61 minutes, respectively, as shown in Table 3. For instance, the roll stability of greases G-3, G-4 and G-6 was 59 mL, 63 mL and 51 mL, respectively, according to the measurement of remaining water, while greases G-9, G-10, and G-11 increased the values to 65 mL, 65 mL and 57 mL respectively, which indicated that the new sulfonate greases had improved water stability. Meanwhile, the cone penetration differential of greases G-3, G-4 and G-6 decreased from 40, 45, and 36 to 30, 25, and 27, respectively, which meant that the new sulfonate greases had improved high temperature performance. Besides, the new greases exhibited the same antiwear property and other performance indicators as the previous ones as evidenced by data in Table 3.
3.6 Discussions
Through the research activities carried out above, it couldbe found out that the structures and components of sulfonates used for grease differed from those of sulfonates used for lubricating oil formulation. The sulfonates used for grease should have short conversion time, good water stability, antiwear property and high temperature performance at the same time. In order to produce the above mentioned sulfonate, suitable alkyl benzene sulfonic acid and preparation process are important. The proper mass ratio of monoalkylbenzene sulfonic acid and dialkylbenzene sulfonic acid to all alkyl benzene sulfonic acids is the key factor. If the mass ratio of monoalkylbenzene sulfonic acid exceeds 70% or decreases to 40% or less, it would not be in favor of the conversion process of sulfonate and the properties of sulfonate grease would deteriorate. Meanwhile, the preparation process of sulfonate would have influence on TBN, turbidity and viscosity of sulfonate, which would affect the converting ability of sulfonate and the properties of grease to a great extent. The sulfonate with high TBN, low turbidity and viscosity would be beneficial to the sulfonate grease in terms of water stability and high temperature performance. Thus, the ideal strategy for development of sulfonate for grease manufacture should be to select a proper alkyl benzene sulfonic acid at first, and then to optimize the preparation method of sulfonate.
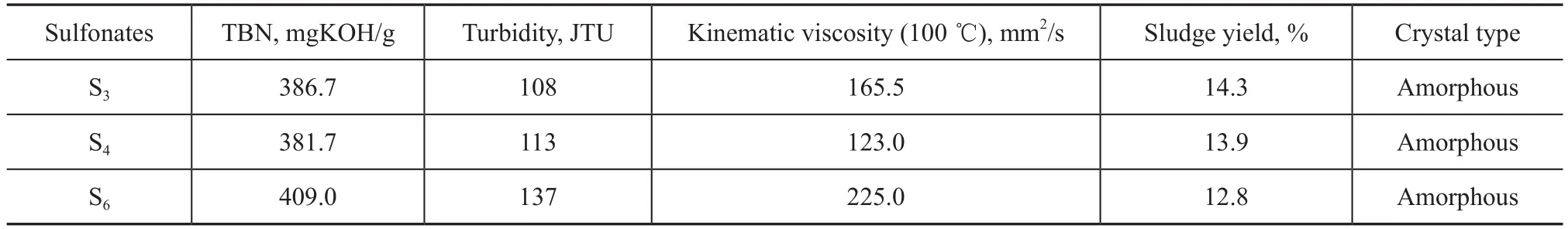
Table13New sulfonates S3, S4, and S6synthesized under the optimized conditions

Table 1 4 Properties of complex sulfonate greases
4 Conclusions
(1) Six kinds of alkylbenzene sulfonic acids were selected to produce the sulfonate, and sulfonates S3, S4, and S6could be converted to lubricating greases with good performance in comparison with those produced from commercial sulfonates T106-1, and T106-2.
(2) Optimized conditions for synthesis of sulfonates S3, S4, and S6were explored, and the proposed conditions for synthesis of sulfonate S3included a methanol/M mass ratio of 16%, a water/M mass ratio of 4% and a copromoter A/M mass ratio of 2%. The proposed conditions for synthesis of sulfonate S4included a methanol/M mass ratio of 24%, a water/M mass ratio of 2% and a copromoter B/M mass ratio of 2%. The optimized conditions for synthesis of sulfonate S6included a methanol/M mass ratio of 8%, a water/ M mass ratio of 4% and a copromoter B/M mass ratio of 2%.
(3) The new sulfonates S3, S4, and S6produced under the optimized conditions had high TBN, low turbidity and low viscosity as compared to the previous products.
(4) Greases G9, G10, and G11 produced from the new sulfonates S3, S4, and S6exhibited good grease conversion property, improved water stability and high temperature performance.
[1] Wayne Mackwood. Calcium Sulfonate Complex Grease—25 Years Young[J]. Lubricating Oil, 2013, 28(3): 4-16
[2] Yi J G, Structure, components and application of sulfonate complex grease[C]// Proceedings of the Eleventh Annual Conference of Society of Automotive Engineers of China, Shanghai, 2004: 190-197 (in Chinese)
[3] Papke L. Process for preparing overbased metal sulfonates: The United States, US4995993[P]. 1991
[41 Ron M, Ronald J W M. High performance calcium borate modified overbased calcium sulfonate complex greases: The United States, US4560489[P]. 1985
[5] Mansot J L. Colloidal antiwear additives, tribological behavior of colloidal additives in mild wear regime, Colloidal and Surface[J]. Physics and Chemical Aspects, 1993, 75: 25-31
[6] Ronald J M, Ken N. Oil soluble calcite overbased detergent and engine oils containing same: WO, 0004113[P]. 2000
[7] Liu Q L, Rong Z L. High based sulfonate complex grease and its preparation method: China, CN1414076[P]. 2003
[8] Zhang J H, Han C N. Modern Lubricating Oil and Fuel Additives[M]. Beijing: China Petrochemical Press, 1990: 239 (in Chinese)
date: 2014-05-28; Accepted date: 2014-11-29.
Liu Yinong, Telephone: +86-10-82368875; E-mail: liuyinong.ripp@sinopec.com.
杂志排行
中国炼油与石油化工的其它文章
- Study on Surface Adsorption and Inhibition Behavior of Corrosion Inhibitors Contained in Copper Foil Rolling Oil
- Synthesis of Petroleum Sulfonate Surfactant with Ultra-Low Interfacial Tension in Rotating Packed Bed Reactor
- Simultaneous Removal of H2S and Organosulfur Compounds from Lique fied Petroleum Gas Using Formulated Solvents: Solubility Parameter Investigation and Industrial Test
- Effect of Dodecylbenzene Sulfonic Acid Used as Additive on Residue Hydrotreating
- Sulfur Distribution during Hydrothermal Liquefaction of Lignite, Wheat Straw and Plastic Waste in Sub-Critical Water
- Simulation Optimization and Experimental Study of Cross-Wall Adiabatic Dividing Wall Column Used to Separate Hexane-Heptane-Octane System
