Sulfur Distribution during Hydrothermal Liquefaction of Lignite, Wheat Straw and Plastic Waste in Sub-Critical Water
2015-06-21WangBaofengHuangYaruZhangJinjun
Wang Baofeng; Huang Yaru; Zhang Jinjun
(School of Chemistry & Material Science, Shanxi Normal University, Linfen, Shanxi 041004)
Sulfur Distribution during Hydrothermal Liquefaction of Lignite, Wheat Straw and Plastic Waste in Sub-Critical Water
Wang Baofeng; Huang Yaru; Zhang Jinjun
(School of Chemistry & Material Science, Shanxi Normal University, Linfen, Shanxi 041004)
The distribution and transformation of sulfur in products during hydrothermal liquefaction of lignite, wheat straw and plastic waste in sub-critical water were investigated in an autoclave. The influence of blending ratio, temperature, initial nitrogen pressure, residence time and additives on sulfur distribution was studied systematically. The results showed that most of sulfur existed as organic sulfur and transferred into the residue, and only a small part of sulfur transferred into oil and gas during hydrothermal liquefaction of lignite, wheat straw and plastic waste in sub-critical water. The results also showed that lower temperature (less than 300 ℃) was favorable for obtaining oil with low sulfur content. It can be also seen from the results that the best condition to obtain the oil with low sulfur content should be implemented at a lignite/wheat straw/plastic waste blending ratio of 5:4:1, an initial nitrogen pressure of 3 MPa and a residence time of 30 minutes. Furthermore, the results indicated that adding tourmaline during hydrothermal liquefaction of lignite, wheat straw and plastic waste was beneficial to production of oil with low sulfur content.
lignite, wheat straw, plastic waste, hydrothermal liquefaction, sulfur
1 Introduction
It is well-known that wheat straw and plastic waste possess high heating value and high hydrogen content. However, a majority of the plastic waste and wheat straw are disposed of through landfills or incineration, and only less than 10% of them are being recycled[1-2]. So more and more attention has been paid on how to use plastic waste and biomass efficiently and cleanly. There are many studies on co-processing of coal and biomass, coal and plastic waste, or plastic waste and biomass[3-11], and there are also studies on co-processing of coal, biomass and plastics wastes[12-15]. Moreover, co-liquefaction of coal, biomass and plastic waste could produce oil, so the study on co-liquefaction of coal, biomass and plastic waste is very important. Besides, hydrothermal treatment is an efficient and clean processing method[16-18], so hydrothermal liquefaction of lignite, wheat straw and plastic waste in sub-critical water for manufacture of oil is proposed. However, low grade coals and/or wastes may contain high levels of undesirable elements, such as sulphur, nitrogen, chlorine and other halogens and heavy metals[19-20]. The aim of hydrothermal liquefaction of lignite, wheat straw and plastic waste is to obtain oil with high quality. However, undesirable elements, such as sulfur, will be transferred into the liquefied products during hydrothermal liquefaction of lignite, wheat straw and plastic waste in sub-critical water. How to control more sulfur that is transferred into the residues and gas, and how to control less sulfur contained in the oil products becomes a very important issue. To solve these problems, understanding the transformation behavior and distribution of sulfur during hydrothermal liquefaction is necessary. In this paper, the distribution of sulfur during hydrothermal liquefaction of lignite, wheat straw and plastic waste in sub-critical water was studied.
2 Experimental
2.1 Samples
Jingou lignite (JG), wheat straw (WS) and plastic waste polyethylene terephthalate (PET) used for experiments were obtained from Linfen in Shanxi Province of China. The samples were first air-dried and then crushed, groundand sieved to obtain particles with diameter in the range of 0.15 mm to 0.25 mm. Table 1 shows the results of the proximate and ultimate analysis of Jingou lignite, wheat straw and plastic waste. It can be seen from Table 1 that the sulfur content in Jingou lignite was 2.87%, and the sulfur content in plastic waste (polyethylene terephthalate) was very low. According to the National Standard GB/T 215—2003, the forms of sulfur in Jingou lignite were also determined. The content of inorganic sulfur (sulphate sulfur and pyritic sulfur) was 0.13%, and the content of organic sulfur was 2.74%. Tourmaline was obtained from Lingshou County in Hebei Province of China. Table 2 shows the main composition of the tourmaline.
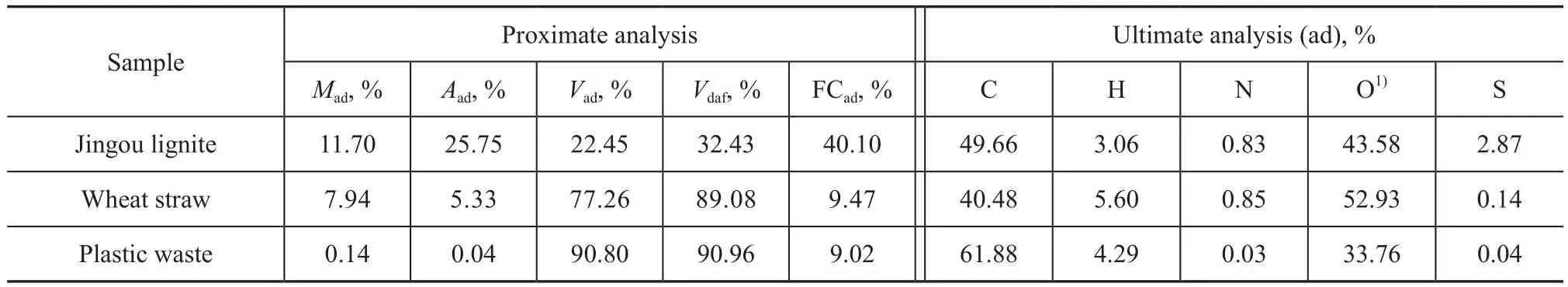
Table 1 Proximate and ultimate analysis of the samples

Table 2 Main composition of tourmaline%
2.2 Experimental setup and procedure
The co-liquefaction experiments were carried out in a 250 mL autoclave. Each time 10.00 g of JG lignite, wheat straw, plastic waste, or mixtures of these three substances were put into the reactor together with 80 mL of deionized water. 0.30 g of tourmaline, Fe2O3or FeS, and 0.12 g of sulfur were added into the reactor to be mixed with the samples. Before the liquefaction experiment, the reactor was filled with nitrogen to the desired initial pressure (2.0—5.0 MPa) and sealed, then the reactor was heated to the desired temperature at a rate of 10 ℃/min by a furnace and then stayed at a specified temperature for a required time. After termination of the reaction, the reactor was cooled down to room temperature and depressurized to atmospheric pressure. The residue was taken out, dried and delivered for further analysis.
2.3 Fractionation of liquefaction products
The gas products were analyzed by an Agilent 7890A gas chromatograph. The solid and liquid products were extracted by hexane, benzene and tetrahydrofuran and then separated into oils (hexane soluble), asphaltenes (hexane insoluble but benzene soluble), preasphaltenes (benzene insoluble but tetrahydrofuran soluble), and residues (tetrahydrofuran insoluble).
The yield of liquefaction products is calculated as:
Residue yield (R) = [wR/ (wJG+wWS+wPET)] ×100%
Other products yield (TC) =100%-R
in which wJGis the mass of Jingou lignite, wWSis the mass of wheat straw, wPETis the mass of the plastic waste, and wRis the mass of the residue.
2.4 Determination of sulfur[21]
The amount of total sulfur in the residue was analyzed according to the National Standard GB/T 214—2007. The organic sulfur content of the residue obtained from hydrothermal liquefaction of lignite, wheat straw and plastic waste was analyzed as follows. Firstly, inorganic sulfur was removed completely by treating the residue with a 1:7 nitric acid solution for 24 h according to the literature description and then was washed with re-distilled water and filtered. Then the residue was dried at 55 ℃ for 24 hours in a vacuum drier and finally analyzed for the organic sulfur content according to the method for determining the total sulfur content of the residue. The content of inorganic sulfur was obtained by difference. The concentration of sulfur-containing gases (H2S and COS) in the gas was measured by a gas chromatograph (Agilent GC-7890A)equipped with a flame photometric detector (FPD).
3 Results and Discussion
3.1 The yield of oil and residue during hydrothermal liquefaction of lignite, wheat straw and plastic waste in sub-critical water
Table 3 shows the yield of residue and other products obtained under different reaction conditions[22]. It can be seen from Table 3 that during hydrothermal liquefaction of lignite, wheat straw and plastic waste in subcritical water at a temperature of 300 ℃, a pressure of 2 MPa and a residence time of 30 minutes, the residue yield decreased with an increasing blending ratio of wheat straw. When the materials blending ratio was 5:4:1, the oil yield was 13.8% and the residue yield reached 53.2%. Moreover, it was also identified that at a reaction pressure of 2 MPa, a materials blending ratio of 5:4:1 and a residence time of 30 minutes during hydrothermal liquefaction, the residue yield decreased when the temperature increased from 260 ℃ to 300 ℃, and when the temperature further increased from 300℃ to 320 ℃, the oil yield and the residue yield all increased, with the oil yield reaching 14.7% and the residue yield equating to 57.5%. At a reaction temperature of 300 ℃, a materials blending ratio of 5:4:1, a residence time of 30 minutes. When the initial nitrogen pressure increased from 2 MPa to 5 MPa, the residue yield also increased and reached 59.2% under a nitrogen pressure of 5 MPa. The oil yield increased when the initial nitrogen pressure increased from 2 MPa to 3 MPa. However, when the initial nitrogen pressure increased from 3 MPa to 5 MPa, the oil yield decreased. Moreover, at a reaction temperature of 300 ℃, a materials blending ratio of 5:4:1 and an initial nitrogen pressure of 2 MPa, the residue yield decreased when the residence time increased from 10 minutes to 60 minutes. The residue yield dropped to 53.8% and the oil yield increased to 18.0% when the residence time was 60 minutes. Furthermore, when such substances as tourmaline, Fe2O3+S, FeS or FeS+S were added into the reactor during hydrothermal liquefaction, the residue yield all increased. In comparison with the oil and residue yields upon addition of the traditional liquefaction catalysts such as Fe2O3+S, FeS or Fe S+S, tourmaline could increase the oil yield and reduce the residue yield.

Table 3 The yield of the residue at different reaction conditions
3.2 Transformation of sulfur species during hydrothermal liquefaction of lignite, wheat straw and plastic waste in sub-critical water
3.2.1 Influence of blending ratio
Figure 1 shows the sulfur fraction of the products and the content of H2S and COS in the gas at different blending ratios of lignite, wheat straw and plastic waste, when the reaction took place at a temperature of 300 ℃, an initial nitrogen pressure of 2 MPa and a residence time of 30 minutes. It can be seen from Figure 1(a) that during hydrothermal liquefaction, the organic sulfur in residue was 83.6%, 87.1%, 85.9% and 87.2%, respectively, when the materials blending ratio was 5:1:4. 5:2:3, 2:1:1 and 5:4:1, respectively, with the inorganic sulfur content in residue reaching only 2.6%, 2.9%, 2.4% and 3.5%, respectively, and sulfur content in other products (gas and oil) equatingto 13.8%, 10%, 11.7% and 9.3%, respectively. Moreover, the content of sulfur in other products was the lowest at a materials blending ratio of 5:4:1. It can be seen from Figure 1(b) that the content of H2S and COS in gas reached a highest value when the materials blending ratio was 5:4:1, denoting that the materials blending ratio of 5:4:1 was the proper ratio to obtain the oil product with a lowest sulfur content.
3.2.2 Influence of reaction temperature
Figure 2 shows the sulfur fraction of the products and the content of H2S and COS in the gas obtained at different reaction temperatures under conditions including a materials blending ratio of 5:4:1, an initial nitrogen pressure of 2 MPa, and a residence time of 30 minutes. It can be seen from Figure 2(a) that during hydrothermal liquefaction of lignite, wheat straw and plastic waste in sub-critical water, when the temperature was 280 ℃, the organic sulfur content in residue reached a highest value of 96.8%, while the inorganic sulfur content in residue was the lowest, which was equal to only 1.3%; and the content of sulfur in other products (oil and gas) was 1.9%. When the temperature increased to 300 ℃, the organic sulfur content in residue decreased, while the sulfur content in other products (oil and gas) increased obviously. When the temperature increased to 320 ℃, the organic sulfur content in residue increased while the sulfur content in other products (oil and gas) decreased. The result indicated that a lower temperature (less than 300 ℃) was conducive to transferring sulfur into the residue instead of oil and gas.
It can be seen from Figure 2(b) that during hydrothermal liquefaction, when the reaction temperature increased, the content of H2S and COS in gas also increased, and when the temperature reached 320 ℃, the content of H2S andCOS in gas was 7.4 ppm and 4.9 ppm, respectively, which were all very low. Therefore a conclusion could be drawn that a lower temperature (less than 300 ℃) was favorable for obtaining oil with low sulfur content.

Figure 1 Sulfur fraction of the products and the content of H2S and COS in the gas at different blending ratios

Figure 2 Sulfur fraction of the products and the content of H2S and COS in the gas at different temperatures
3.2.3 Influence of reaction pressure
Figure 3 shows the sulfur fraction of the products and the content of H2S and COS in the gas at different nitrogen pressures under conditions covering a materials blending ratio of 5:4:1, a reaction temperature of 300 ℃, and a residence time of 30 minutes. It can be seen from Figure 3(a) that the fraction of organic sulfur in residue also increased (with the exception of an initial nitrogen pressure of 4 MPa) when the initial nitrogen pressure increased from 2 MPa to 5 MPa, the fraction of organic sulfur in residue was about 96%, the fraction of inorganic sulfur in residue was about 3%. The fraction of sulfur in oil and gas was equal to only about 1%, when the initial nitrogen pressure was 5 MPa. Moreover, when the initial nitrogen pressure was 3 MPa, the fraction of sulfur in oil and gas was the lowest, which was only about 0.8%. Figure 3(b) shows that the content of H2S and COS in gas was really very low when the initial nitrogen pressure increased from 2 MPa to 5 MPa. Furthermore, when the initial nitrogen pressure was 2 MPa, the content of COS in gas was the highest, and the content of H2S was only lower than that obtained at 3 MPa. The content of COS decreased with an increasing nitrogen pressure. When the initial nitrogen pressure was 3 MPa, the content of H2S in gas was the highest, while the content of COS in gas was the lowest. Because the content of H2S and COS was all very low, it was evident that 3 MPa was an optimal pressure to obtain oil with lower sulfur content.
3.2.4 Influence of residence time
Figure 4 shows the sulfur fraction of the products and the content of H2S and COS in the gas at different residence times under conditions covering a blending ratio of lig-nite, wheat straw and plastic waste of 5:4:1, a temperature of 300 ℃, and an initial nitrogen pressure of 2 MPa. It can be seen from Figure 4 (a) that when the residence time increased from 10 minutes to 60 minutes, the fraction of organic sulfur and inorganic sulfur in residue and the fraction of sulfur in other products (gas and oil) all changed irregularly. When the residence time was 30 minutes, the fraction of sulfur in other products (gas and oil) was the lowest. It can also be seen from Figure 4(b) that when the residence time was 30 minutes, the content of H2S in gas was the highest, while the content of COS in gas was the lowest.
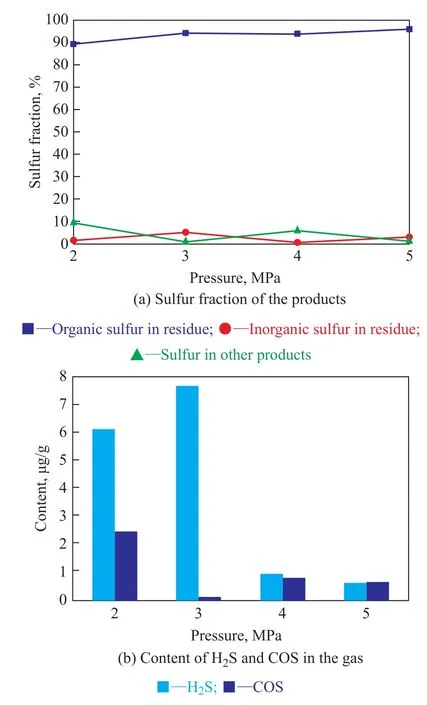
Figure 3 Sulfur fraction of the products and the content of H2S and COS in the gas under different pressures

Figure 4 Sulfur fraction of the products and the content of H2S and COS in the gas at different residence times
3.2.4 Influence of adding tourmaline
Figure 5 shows the sulfur fraction of the products and the content of H2S and COS in the gas when tourmaline was added at a lignite, wheat straw and plastic waste blending ratio of 5:4:1, a temperature of 300 ℃, an initial nitrogen pressure of 2 MPa, and a residence time of 30 minutes. It can be seen from Figure 5 (a) that during hydrothermal liquefaction, the addition of tourmaline could increase the fraction of organic sulfur in residue and reduce the sulfur in other products (oil and gas).
It can be seen from Figure 5 (b) that adding tourmaline also increased the content of H2S and COS in gas, which meant that the addition of tourmaline during hydrothermal liquefaction of lignite, wheat straw and plastic waste was also beneficial to formation of oil with low sulfur content. This result might be attributed to two reasons. One reason was that during hydrothermal liquefaction, tourmaline could react on sulfur in oil, making more sulfur transferred into gas, and the other reason might be associated with the physical adsorption of sulfur by tourmaline, so more organic sulfur was transferred into the residue.

Figure 5 Sulfur fraction of the products and the content of H2S and COS in the gas after adding tourmaline
4 Conclusions
The transformation of sulfur during hydrothermal liquefaction of lignite, wheat straw and plastic waste in subcritical water at different blending ratios, reaction temperatures, initial nitrogen pressures and residence time was investigated. The results showed that during hydrothermal liquefaction, most of the sulfur existed as organic sulfur and transferred into the residue, and only a small part of sulfur transferred into oil and gas. The experimental results also showed that lower temperature (less than 300 ℃) was favorable for manufacture of oil with low sulfur content. Besides, the test results also showed that the best conditions for obtaining oil with low sulfur content covered a blending ratio of lignite, wheat straw and plastic waste of 5:4:1, an initial nitrogen pressure of 3 MPa and a residence time of 30 minutes. Furthermore, the results indicated that adding tourmaline during hydrothermal liquefaction of lignite, wheat straw and plastic waste was beneficial to manufacture of oil with low sulfur content.
Acknowledgements:Financial support to this work provided by the Research Fund for the Doctoral Program of Higher Education for New Teachers of China (20091404120002), the Shanxi Province Science Foundation for the Youth of China (2011021008-1) and the Soft Science Program of Shanxi Province (2011041015-01) was gratefully acknowledged.
[1] Kannan P, Shoaibi A Al, Srinivasakannan C. Energy recov-ery from co-gasification of waste polyethylene and polyethylene terephthalate blends [J]. Computers & Fluids, 2013, 88: 38-42
[2] Wu Chunfei, Williams P T. Pyrolysis–gasification of postconsumer municipal solid plastic waste for hydrogen production [J]. Int J Hydrogen Energy, 2010, 35 (3): 949-957
[3] Sharma S, Ghoshal A K. Study of kinetics of co-pyrolysis of coal and waste LDPE blends under argon atmosphere [J]. Fuel, 2010, 89(12): 3943-3951
[4] Yang Tianhua, Kai Xingping, Sun Yang, et al. The effect of coal sulfur on the behavior of alkali metals during co-firing of biomass and coal [J]. Fuel, 2011, 90 (7): 2454-2460
[5] Montiano M G, Barriocanal C, Alvarez R. Effect of the addition of waste sawdust on thermoplastic properties of a coal [J]. Fuel, 2013, 106: 537-543.
[6] Howaniec N, Smolinski A. Effect of fuel blend composition on the efficiency of hydrogen-rich gas production in co-gasification of coal and biomass [J]. Fuel, 2014, 128: 442-450
[7] Brebu M, Ucar S, Vasile C, et al. Co-pyrolysis of pine cone with synthetic polymers [J]. Fuel, 2010, 89 (8): 1911-1918
[8] Karaca F, Bolat E. Coprocessing of a Turkish lignite with a cellulosic waste material: 1. The effect of coprocessing on liquefaction yields at different reaction temperatures [J]. Fuel Process Technol, 2000, 64 (1/3): 47-55.
[9] Pei Xiaokai, Yuan Xingzhong, Zeng Guangming, et al. Coliquefaction of microalgae and synthetic polymer mixture in sub- and supercritical ethanol [J]. Fuel Process Technol, 2012, 93 (1): 35-44
[10] Haykiri-Acma H, Yaman S. Interaction between biomass and different rank coals during co-pyrolysis [J]. Renewable Energy, 2010, 35 (1): 288-292
[11] Emami-Taba L, Irfan M F, Daud W M A W, et al. Fuel blending effects on the co-gasification of coal and biomass -A review [J]. Biomass Bioenerg, 2013,57:249-263
[12] Pinto F, Franco C, Andre´ R N, et al. Effect of experimental conditions on co-gasification of coal, biomass and plastics wastes with air/steam mixtures in a fluidized bed system [J]. Fuel, 2003, 82 (15/17): 1967-1976
[13] A znar M P, Caballero M A, Sancho J A, et al. Plastic waste elimination by co-gasification with coal and biomass in fluidized bed with air in pilot plant [J]. Fuel Process Technol, 2006, 87 (5): 409-420
[14] L iu Kui, Meuzelaar H LC. Catalytic reactions in waste plastics, HDPE and coal studied by high-pressure thermogravimetry with on-line GC/MS [J]. Fuel Process Technol, 1996, 49 (1/3):1-15
[15] Mastellone M L, Zaccariello L, Arena U. Co-gasification of coal, plastic waste and wood in a bubbling fluidized bed reactor [J]. Fuel, 2010,89 (10): 2991-3000
[16] Brunner G. Near critical and supercritical water. Part I. Hydrolytic and hydrothermal processes [J]. The Journal of Supercritical Fluids, 2009, 47 (3): 373-381.
[17] Wang Yun, Wang Hui, Lin Hongfei, et al. Effects of solvents and catalysts in liquefaction of pinewood sawdust for the production of bio-oils [J]. Biomass Bioenerg, 2013, 59: 158-167
[18] Akhtar J, Amin N A S. A review on process conditions for optimum bio-oil yield in hydrothermal liquefaction of biomass [J]. Renew Sust Energ Rev, 2011, 15 (3):1615-1624
[19] Pinto F, André R N, Franco C, et al. Co-gasification of coal and wastes in a pilot-scale installation. 2: Effect of catalysts in syngas treatment to achieve sulphur and nitrogen compounds abatement [J]. Fuel, 2010, 89(11): 3340-3351.
[20] Liu Lijuan, Fei Jinxia, Cui Mingqi, et al. XANES spectroscopic study of sulfur transformations during co-pyrolysis of a calcium-rich lignite and a high-sulfur bituminous coal [J]. Fuel Process Technol, 2014, 121: 56-62
[21] Wang Baofeng, Zhao Shuguang, Huang Yaru, et al. Effect of some natural minerals on transformation behavior of sulfur during pyrolysis of coal and biomass [J]. J Anal Appl Pyrol, 2014, 105: 284-294
[22] Wang Baofeng, Huang Yaru, Zhang Jinjun. Hydrothermal liquefaction of lignite, wheat straw and plastic waste in sub-water for oil: Product distribution[J]. J Anal Appl Pyrol, 2014, 110: 382-389
date: 2014-07-21; Accepted date: 2014-10-18.
Wang Baofeng, Telephone: + 86-357-2051192; E-mail: wangbaofeng1234@sina.com.
杂志排行
中国炼油与石油化工的其它文章
- Study on Surface Adsorption and Inhibition Behavior of Corrosion Inhibitors Contained in Copper Foil Rolling Oil
- Synthesis of Petroleum Sulfonate Surfactant with Ultra-Low Interfacial Tension in Rotating Packed Bed Reactor
- Simultaneous Removal of H2S and Organosulfur Compounds from Lique fied Petroleum Gas Using Formulated Solvents: Solubility Parameter Investigation and Industrial Test
- Effect of Dodecylbenzene Sulfonic Acid Used as Additive on Residue Hydrotreating
- DFT Study of H2Dissociation on MoxSyClusters
- Simulation Optimization and Experimental Study of Cross-Wall Adiabatic Dividing Wall Column Used to Separate Hexane-Heptane-Octane System
