Effect of Dodecylbenzene Sulfonic Acid Used as Additive on Residue Hydrotreating
2015-06-21SunYudongYangChaohe
Sun Yudong; Yang Chaohe
(College of Chemical Engineering, China University of Petroleum, Qingdao 266580)
Effect of Dodecylbenzene Sulfonic Acid Used as Additive on Residue Hydrotreating
Sun Yudong; Yang Chaohe
(College of Chemical Engineering, China University of Petroleum, Qingdao 266580)
The effect of additive—dodecylbenzene sulfonic acid (DBSA)—on residue hydrotreating was studied in the autoclave. The results showed that the additive improved stabilization of the colloid system of residue, which could delay the aggregation and coke formation from asphaltenes on the catalyst, and make heavy components transformed into light oil. The residue conversion in the presence of this additive increased by 1.94%, and the yield of light oil increased by 1.53% when the reaction time was 90 min. The surface properties of the catalyst in the presence of this additive were better than that of the blank test within a very short time (30 min) and deteriorated rapidly after a longer reaction time due to higher conversion and coke deposition. Compared with the blank test, the case using the said additive had shown that the structure of hydrotreated asphaltene units was smaller and the condensation degrees were higher. The test results indicated that the additive could improve the hydrotreating reactivity of residue via permeation and depolymerization, the heavier components could be transformed into light oil more easily, and the light oil yield and residue conversion were higher for the case using the said additive in residue hydrotreating process.
residue hydrotreating; dodecylbenzene sulfonic acid; product distribution; catalyst properties; asphaltenes structure
1 Introduction
Asphaltene is the most complex and the largest molecule in crude oil. It consists of condensed polyaromatic nuclei carrying alkyl groups and alicyclic systems, and also contains various heteroatoms. Asphaltene transformation is a difficult task and may lead to negative impacts on residue hydrotreating[1-3]. For example, asphaltene is prone to deposition as coke on catalysts via dehydrogenation causing the blockage of catalyst pores, thereby decreasing the catalytic activity and reducing the operating cycle. In the process of coking, asphaltene aggregates from the colloid system of the residue to form the second liquid phase firstly, and then form coke through dehydrogenation[4]. In general, the second liquid phase is considered to be the coke precursor during residue processing, therefore, the key to inhibit coke formation is to prevent the colloid system of residue from destruction and then precipitation in the second liquid phase.
Surfactant can reduce the surface tension of the system through the adsorption of head groups in the surface of asphaltene micelle and the formation of a stable tridimensional alkyl-layer by the terminal groups, which can peptize the asphaltenes in residue better to increase the stability of the colloid system. Therefore, theoretically, the surfactant added into the residue can delay the aggregation and coke formation of asphaltene, promote the transformation of heavy oil to light oil, and play an important role in inhibiting coke formation during residue hydrotreating. In addition, the additives are also able to disperse the formed soluble coke in residue and prevent coke from coagulation.
The asphaltene structure could be changed during the residue hydrotreating process[5]. It is of significance to thoroughly understand the mechanisms and the role of additives in asphaltene hydrotreating reaction by researching the asphaltene structure before and after hydrotreating with or without addition of the additives. Moreover, the coke yield is directly related to the structural composition of asphaltene in residue hydrotreating. The influence of additives on residue hydrotreating process through researching the coke formation, the catalyst properties and the structure changes of asphaltene with or without using additives was mainly explored in this paper.
2 Experimental
2.1 Feedstock and catalyst
The feedstock was atmospheric residue derived from the Suizhong 36-1 crude (SZAR)[6]. The catalyst used in the experiments was FZC-41A, which was produced by the SINOPEC Fushun Petrochemical Research Institute. FZC-41A was a catalyst designed for hydrodecarbonation/hydrodenitrogenation reactions, with its properties shown in Table 1.

Table 1 Properties of the FZC-41A catalyst
2.2 Analytical methods
(1) Carbon, hydrogen, sulfur and nitrogen contents of asphaltenes were determined by a Vario EL III C, N, H, S/O elemental analyzer.
(2) The structural parameters of asphaltenes were calculated by the improved Brown-Ladner (B-L) method[7]according to the data of Proton Nuclear Magnetic Resonance (1H-NMR).1H-NMR data were analyzed by the AV500 NMR spectrometer using CDCl3as the solvent at a resonance frequency SF of 500.13 MHz, a sampling interval D1 of 2s, a sampling time AQ of 1.6 s and a 90opulse power P1 of 13.50 μs.
(3) The surface properties of catalyst before and after hydrotreating were analyzed by the ASAP2020 micropore physisorption analyzer.
2.3 Experimental procedure
The coke yield and the changes in catalyst properties and asphaltene structures were studied at different reaction times in an autoclave with or without using additive. With the reaction time equating to 0, 10, 20, 30, 45, 60, 90, 120 and 180 minutes, respectively, the hydrotreating reactions were investigated at a reaction temperature of 400 ℃, an initial hydrogen pressure of 8.0 MPa, and a catalyst to oil ratio of 1:10. The additive used in the reaction was DBSA at a dosage of 700 μg/g on the basis of our previous research[8]. It was impossible to obtain samples in the process of reaction because the residue hydrotreating reaction was carried out at high temperature and high pressure in a sealed autoclave. Thus, the experiments at each reaction time point were carried out independently and samples were collected at the end of each experiment. It was difficult to determine the precise starting time of the reaction due to the long heating-up procedure of the experiment. The time reached for the given reaction temperature was considered to be the initial point, and was marked as 0 min which actually could commence the reaction. The reaction time of 0 min needed a further study.
3 Results and Discussion
3.1 Product distribution
The product distribution of residue hydrotreating reaction with or without using additive, respectively, is listed in Table 2. It can be seen from Table 2 that coke had already formed at the reaction time of 0 min. On one hand, the reaction had begun before reaching the reaction temperature during the heating process. On the other hand, the asphaltenes could be partially adsorbed on the catalyst surface during the heating process, and they could hardly be desorbed during the catalyst extraction process due to the strong interaction between asphaltenes and catalyst. The coke yield increased with an increasing reaction time. Compared with the coke yield of blank tests, that set of tests using the additive initially (within 0.5 h) showed lower trends, and later showed higher trends.
Additives were able to improve the stability of the residue’s colloid system by increasing the level of asphaltene solubility. They may also depolymerize macromolecules of asphaltenes to smaller molecules and preventing asphaltenes from aggregating and forming coke too fast during residue hydrotreating. The prerequisite of residue hydrotreating was that the feedstock first diffused into the catalyst pores, and then was adsorbed on the hydrogenation centers of the catalyst to take part in the reaction. The aggregation of heavier components did not favor the diffusion of reactants into the catalyst pores, and it was also beneficial to form coke which could deposit on the catalyst, thereby decreasing the activity of the catalyst. Therefore, the light oil yield was increased to a certaindegree by the addition of additives in feedstock due to the fact that additives prevented asphaltenes from aggregating and caused heavier components to decompose to light oil. Light components obtaining hydrogen atoms from heavy components were continuously coupled with some heavy components that were transformed to light components, and the heavy components which had lost hydrogen atoms gradually became heavier and aggregated, which meant that the heavy components became heavier and increased in amount while light oil was increasing. Although the hydrogen-rich environment and the increased stability of system by using the additive may restrain coke formation to a certain extent, the light oil yield was increased together with the inevitable increase in the yields of heavy components and coke deposits due to the fact that the reaction depth of all components was increased at the same time. This was the main reason why the yield of light oil and coke increased together in the presence of additives during residue hydrotreating. However, with the increase in the reaction time, the non-hydrocarbon additive was gradually hydrogenated and the effect of the additive became almost negligible, causing the reaction results with or without using additives to be similar.
3.2 Analysis of catalyst properties
The catalyst is able to markedly influence the results of residue hydrotreating reaction. In the course of residue hydrotreating, the feedstock must diffuse into the catalyst pores and be adsorbed on the hydrogenation centers of the catalyst prior to taking part in the reaction. During this process, various factors may lead to catalyst deactivation and affect hydotreating process. It was shown[9]that the deactivation of the residue hydrotreating catalysts was mainly caused by coke deposition. Coke can greatly reduce catalyst activity by depositing on pore walls, blocking pores and reducing the surface area of catalyst.
The catalysts before and after hydrotreating reaction were analyzed by BET. The pore size distribution of selected catalysts in different states is shown in Figure 1. Changes in pore size, pore volume and surface area with and without the addition of additive were analyzed for comparison (see Table 3).
It can be seen from Fig. 1 that large differences exist between the pore size distribution of catalysts before and after reaction. The pore sizes of fresh catalysts were mainly distributed between 8 nm and 2 nm, however, after hydrotreating reaction the number of 8-nm pores decreased and that of 4-nm pores increased greatly. The differences between pore size distributions were quite small at the reaction times of 1 h and 3 h, which was applicable to both cases with or without addition of additives. This phenomenon was further verified through the conclusion that coking and deactivating of catalysts occurred at the initial reaction stage of the residue hydrotreating process[10-13]. The coke was formed on the catalyst surface at the initial reaction stage due to the strong adsorption of asphalteneson the catalyst. Meanwhile, it may be concluded that catalyst pore blocking was the main reason leading to catalytic activity loss after residue hydrotreating reaction. Pore structures showed clear changes at the reaction time of 0 min (see Table 3), and the surface area, pore volume and pore size decreased significantly at reaction times between 30 min and 60 min. Subsequently, the pore structures showed no significant change with the increase of reaction time. The surface area, pore volume and pore size decreased rapidly with the deposition of heavy components (e.g. asphaltenes) and the formation of coke on the catalyst during residue hydrotreating. Even at the reaction time of 0 min, the pore structure changed remarkably due to the strong adsorption of heavy components on the catalyst during the heating process. When the hydrotreating reaction continued to a certain extent, the coke on the catalysts reached a stable level[14-15]and the pore structure changed slightly.

Table 2 Product distribution of residue hydrotreating with and without additive
Compared to the blank test, the pore structure of the catalyst in the presence of additives performed slightly better within a short reaction time (0-30 min), and became worse with a longer reaction time. Therefore, the role ofadditive for the catalyst stabilization and coke inhibition only took effect at the initial stage of residue hydrotreating. It is consistent with the above conclusion.
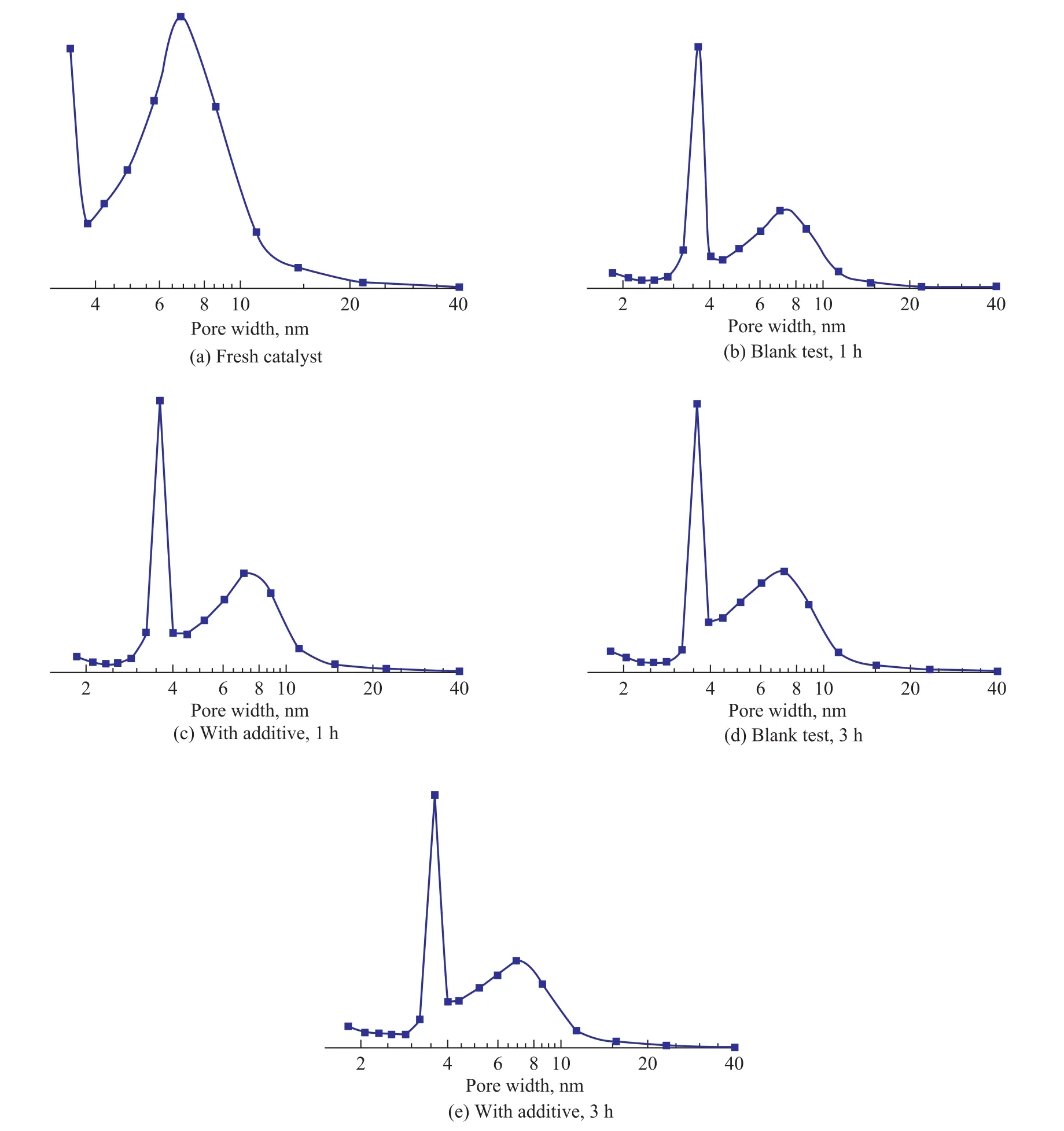
Figure 1 Pore size distribution for different catalysts

Table 3 Surface properties of catalyst before and after hydrotreating with and without addition of additive
3.3 The effect of additive on asphaltene structure
Asphaltenes in the feedstock and hydrotreated residues were separated by the classic liquid chromatography. The obtained asphaltenes were measured by molecular weight determination, elemental analysis,1H-NMR, and other means. The structural parameters of asphaltenes were calculated by the improved B-L method and the structures of asphaltene units were simulated by ChemOffice Ultra 2008 based on the analytical data obtained thereby.
The following calculation and simulation results under the reaction conditions were discussed in an example reaction carried out at a temperature of 400 ℃, an initial hydrogen pressure of 8.0 MPa, a reaction time of 2 h, and a catalyst to oil ratio of 1:10.
The structural parameters of asphaltenes in the feedstock and the hydrotreated residue with or without addition of additives are listed in Table 4. The schematic diagrams of the asphaltene units are shown in Figure 2.
It can be seen from Table 4 and Figure 2 that there was a significant difference between the respective structures of asphaltenes before and after hydrotreating reaction. Compared with the feedstock, it was clear that the molecular weight and structure units of hydrotreated asphaltenes were smaller, but their condensation degree was slightly higher. This observation showed that asphaltene may be transformed into light components through cracking (e. g. chain breaking and naphthenic ring opening, and even unit removing). Furthermore, the condensed ring structure of asphaltenes may polymerize mutually during the process of residue hydrotreating. Chain breaking and polymerization were both capable of increasing the condensation degree of hydrotreated asphaltenes.
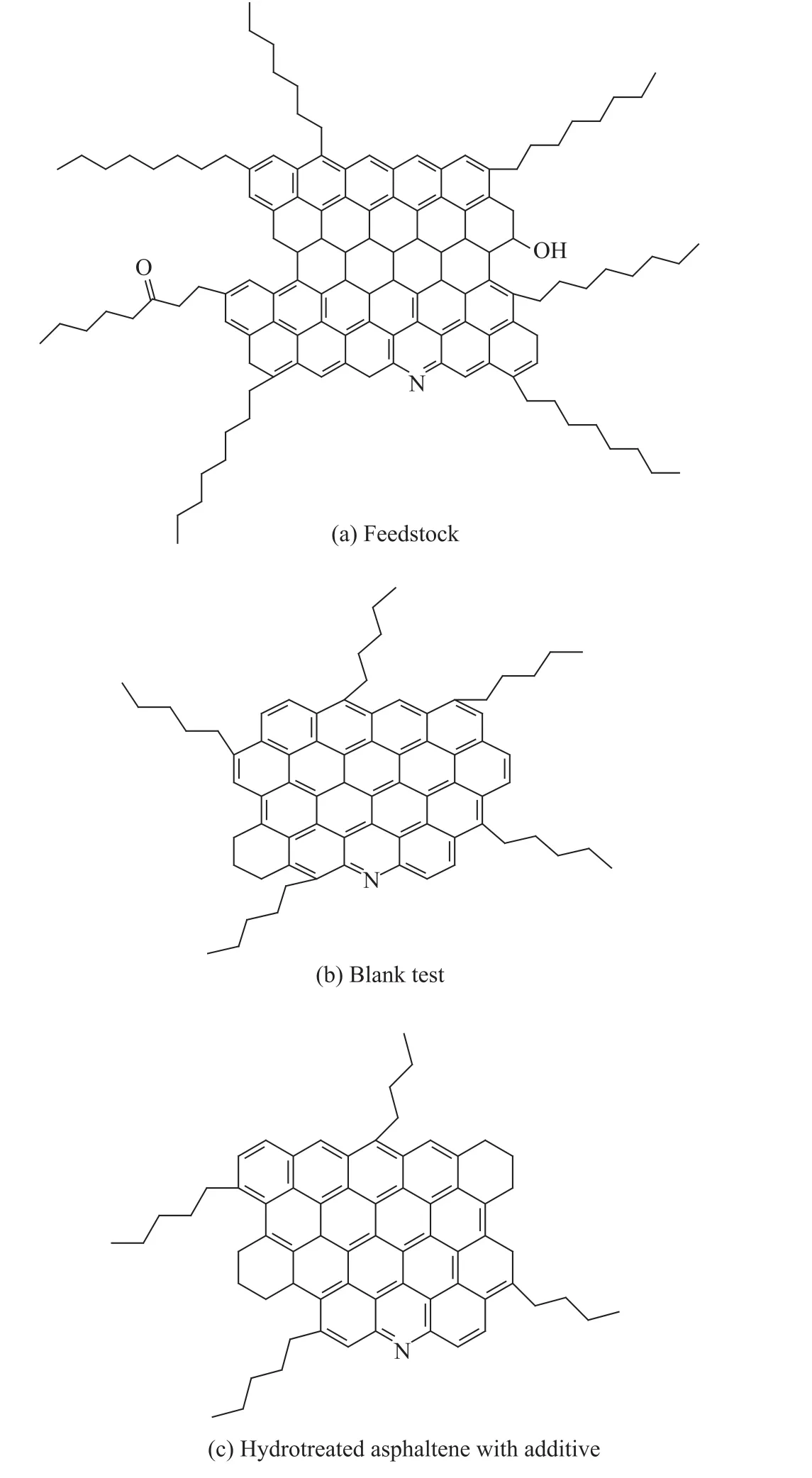
Figure 2 Schematic diagram of asphaltene units
The structure difference between asphaltenes hydrotreated with or without the addition of additive was miniscule. Hydrotreated asphaltene obtained with the addition of additive had slightly smaller structural units, and slightlyhigher ring numbers and condensation degree. The results indicated that asphaltenes reduced the condensation degree through permeation and depolymerization by the action of additives in the feedstock. Permeation and depolymerization are capable of transforming large asphaltene micellae into smaller structural units and promoting the diffusion of asphaltenes into catalyst pores. Along these pathways, the conversion of asphaltenes (including cracking and condensing, especially removal of peripheral alkyl side chains and naphthenic rings) was more complete. So, the light oil yield and conversion rate of residue hydrotreating in the presence of additive were higher than those of the blank tests.
A preliminary study on the effect of additive on residue hydrotreating process was carried out in this paper. The process was studied in an autoclave, and there were some differences in product distribution, reaction conditions and reaction state as compared to that of an industrial unit. More researches had to be done before the commercial application of this technology, which included the optimization of additives, the effect of additive on product quality, the structure and the composition of coke to meet the requirements for the continuous reactor in a real residue hydrotreating process.
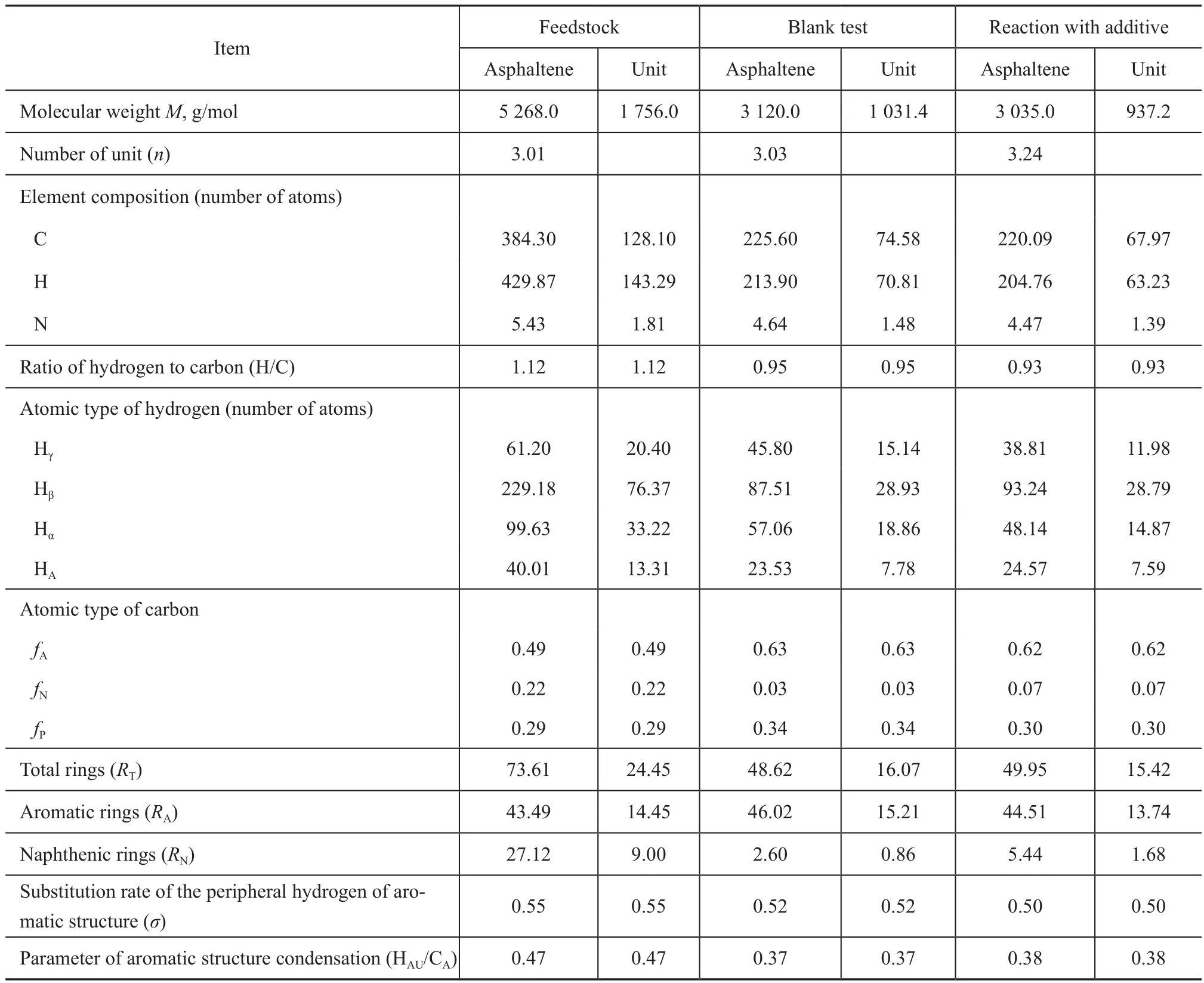
Table 4 Structural parameters of asphaltenes
4 Conclusions
The research on the effect of dodecylbenzene sulfonic acid on residue hydrotreating reaction was carried out in this paper and the conclusions were summarized as follows:
(1) The additive might improve the colloid stability of residue by changing the existing state of asphaltenes. Itwas also capable of delaying aggregation and coke formation of heavy components in hydrotreating reaction, and transforming heavy components into light oil. The additive could improve light oil yield in the process of residue hydrotreating.
(2) The pore structure of the catalysts changed obviously within a short reaction time during the reaction of residue hydrotreating. The surface area, pore volume and pore size of the catalyst were reduced evidently. The surface properties dropped to low levels at reaction times ranging between 30 min and 60 min, and then remained relatively stable in the long period. Compared with a series of blank tests, the surface properties of the catalysts in the presence of the additive were slightly better in shorter reaction time (0—30 min), but became a little worse in a longer reaction time.
(3) There was a significant difference in asphaltene structure before and after hydrotreating. The hydrotreated asphaltene units were smaller than those of protogenous asphaltene, and the hydrotreated asphaltene formed in the reaction with addition of additive showed slightly smaller units and slightly higher condensation degree than those obtained in blank tests. The macromolecules can be easily transformed into light components under the action of additive. In the presence of additive, the light oil yield and residue conversion were higher as compared to the case of residue hydrotreating without addition of additive.
Acknowledgment:Thanks to the financial support provided by the National Natural Science Foundation of China (Grant No. 21376266), the PetroChina Innovation Foundation (Grant No. 2011D-5006-0405) and the Fundamental Research Funds for the Central Universities (Grant No. 27R1104049A).
[1] Gawel I, Bociarska D, Biskupski P. Effect of asphaltenes on hydroprocessing of heavy oils and residua[J]. Applied Catalysis A: General, 2005, 295: 89-94
[2] Ancheyta J, Centeno G, Trejo F, et al. Changes in asphaltene properties during hydrotreating of heavy crudes[J]. Energy & Fuels, 2003, 17:1233-1238
[3] Li Dadong. Processes & Engineering of Hydrotreating[M]. Beijing: China Petrochemical Press, 2004: 1133-1175 (in Chinese)
[4] Xu C M, Yang C H. Petroleum Refining Engineering[M]. 4th Ed. Beijing: Petroleum Industry Press, 2009: 275
[5] Sun Y D, Yang C H, Shan H H, et al. Structure changes of asphaltene in residue hydrotreating[J]. Journal of Petrochemical Universities, 2010, 23(4): 5-9 (in Chinese)
[6] Sun Yudong, Zhang Qiang, Shan Honghong, et al. Effects of ultrasonic treatment on residue properties[J]. China Petroleum Processing and Petrochemical Technology, 2013, 15(4): 14-19
[7] Chen Xiaobo, Li Teng, Liu Yibin, et al. Characterization of nitrogen compounds in vacuum residue and their structure comparison with coker gas oil[J]. China Petroleum Processing and Petrochemical Technology, 2014, 16(3): 33-41
[8] Zhu K. Study on the Effect and Mechanism of Additives in Residue Hydroprocessing[D]. Qingdao: China University of Petroleum, 2011: 22-41 (in Chinese)
[9] Seki Hi, Yoshimoto M. Deactivation of HDS catalyst in two-stage RDS process. II. Effect of crude oil and deactivation mechanism[J]. Fuel Processing Technology, 2001, 69: 229-238
[10] Callejas M A, Martinez M T, Blasco T, et al. Coke characterisation in aged residue hydrotreating catalysts by solidstate13C-NMR spectroscopy and temperature-programmed oxidation[J]. Appl Catal A, 2001, 218(1-2): 181-188
[11] Fonseca A, Zeuthen P, Nagy J B. Assignment of an average chemical structure to catalyst carbon deposits on the basis of quantitative13C-NMR spectra[J]. Fuel, 1996, 75(12): 1413-1423
[12] Matsushita K, Hauser A, MarafiA, et al. Initial coke deposition on hydrotreating catalysts. Part 1: Changes in coke properties as a function of time on stream[J]. Fuel, 2004, 83(7/8): 1031-1038
[13] MarafiM, Stanislaus A. Effect of initial coking on hydrotreating catalyst functionalities and properties[J]. Appl Catal A, 1997, 159(1/2): 259-267
[14] Kam E K T, Al-Shimali M, Juraidan M, et al. A hydroprocessing multicatalyst deactivation and reactor performance model-pilot-plant life test applications[J]. Energy & Fuels, 2005, 19(3): 753-764
[15] MarafiA, Hauser H, Stanislaus A. Deactivation patterns of Mo/Al2O3, Ni-Mo/Al2O3and Ni-MoP/Al2O3catalyst in atmospheric hydrodesulphurization[J]. Catal Today, 2007, 125(3/4): 192-202
date: 2014-06-20; Accepted date: 2014-11-29.
Sun Yudong, Telephone: +86-532-86984702; E-mail: ydsun@upc.edu.cn.
杂志排行
中国炼油与石油化工的其它文章
- Study on Surface Adsorption and Inhibition Behavior of Corrosion Inhibitors Contained in Copper Foil Rolling Oil
- Sulfur Distribution during Hydrothermal Liquefaction of Lignite, Wheat Straw and Plastic Waste in Sub-Critical Water
- Synthesis of Petroleum Sulfonate Surfactant with Ultra-Low Interfacial Tension in Rotating Packed Bed Reactor
- Simultaneous Removal of H2S and Organosulfur Compounds from Lique fied Petroleum Gas Using Formulated Solvents: Solubility Parameter Investigation and Industrial Test
- DFT Study of H2Dissociation on MoxSyClusters
- Simulation Optimization and Experimental Study of Cross-Wall Adiabatic Dividing Wall Column Used to Separate Hexane-Heptane-Octane System
