Simultaneous Removal of H2S and Organosulfur Compounds from Lique fied Petroleum Gas Using Formulated Solvents: Solubility Parameter Investigation and Industrial Test
2015-06-21ZhangFengShenBenxianSunHuiLiuJichangShangJianfeng
Zhang Feng; Shen Benxian; Sun Hui; Liu Jichang; Shang Jianfeng
(1. State Key Laboratory of Chemical Engineering, East China University of Science and Technology, Shanghai 200237; 2. SINOPEC Zhongyuan Oilfield Puguang Company, Dazhou 635002)
Simultaneous Removal of H2S and Organosulfur Compounds from Lique fied Petroleum Gas Using Formulated Solvents: Solubility Parameter Investigation and Industrial Test
Zhang Feng1; Shen Benxian1; Sun Hui1; Liu Jichang1; Shang Jianfeng2
(1. State Key Laboratory of Chemical Engineering, East China University of Science and Technology, Shanghai 200237; 2. SINOPEC Zhongyuan Oilfield Puguang Company, Dazhou 635002)
The performance of four formulated solvents (labeled as UDS-I, UDS-II, UDS-III, and UDS-IV) for removing methyl mercaptan from liquefied petroleum gas was predicted based on a two-dimensional solubility parameter theory. The calculation results show that UDS-IV has the closest solubility parameter to that of methyl mercaptan as compared with other tested solvents, indicating the strongest affinity and the highest solubility for methyl mercaptan. The industrial tests at a plant for desulfurization of LPG produced from the delayed coker have shown that the UDS solvents have the excellent performance for removal of organosulfur compounds (mainly methyl mercaptan). Although the sulfur loading dramatically increases, the total sulfur content of LPG treated with UDS-IV can be reduced by about 50% in comparison with N-methyl diethanolamine. In addition, UDS-IV has superior regeneration performance and selectivity for sulfur compounds over hydrocarbons. The industrial test and the solubility parameter calculation results are in good agreement with each other.
liquefied petroleum gas; desulfurization solvent; solubility parameter; methyl mercaptan; organosulfur
1 Introduction
Besides H2S, the liquefied petroleum gas (LPG) produced from FCC and delayed coking units contains various organosulfur compounds, e.g., COS, mercaptans, thioethers and disulfides[1]. Among these organosulfur compounds, methyl mercaptan usually accounts for a dominant part. Prior to commercial application of LPG, the sulfur compounds should be carefully removed. The LPG desulfurization process commonly involves alkanolamine treating and subsequent caustic treating. Due to very limited solubility of organosulfur compounds in alkanolamine solvents which have been widely commercially applied, LPG scrubbed with alkanolamines needs further treatment that uses caustic solution in order to remove residual organosulfur compounds to an acceptable level. However, the caustic treating process consumes a large amount of sodium hydroxide and discharges caustic sludge that causes severe environmental problems[2]. The development of an efficient and environmentally friendly desulfurization process for LPG, therefore, is becoming an urgent issue and attracting increasing attention.
The solubility of organosulfur compounds in solvent plays a crucial role in affecting the desulfurization efficiency of alkanolamine treating unit. Formulated solvents composed of alkanolamines and physical solvents (such as N-formyl morpholine, N-methyl pyrrolidone, sulfolane and dimethylsulfoxide) allow simultaneous removal of H2S and mercaptans[3-5], whereas some of them have the disadvantage of higher solubility of hydrocarbons. New formulated solvents based on alkanolamine, the UDS solvents, have been developed in this laboratory and used to treat natural gas with a pretty high efficiency for removal of H2S and organosulfur compounds coupled with a very limited solubility of hydrocarbons[6-7].
The aim of this work is to provide the LPG desulfurization process with a formulated solvent which has an excellent performance for removal of H2S and organosulfur compounds in an attempt to minimize the total sulfur content of LPG. The performance of four UDS solventsin removing methyl mercaptan was studied using the twodimensional solubility parameter theory. In addition, the desulfurization performance of UDS solvents was investigated in an industrial unit for desulfurization of LPG produced from a delayed coker and was compared with that of N-methyl diethanolamine (MDEA).
2 Experimental
2.1 Materials
The UDS solvents were provided by Jiangsu Jinlu Environmental Technology Co., Ltd. The distribution of sulfur compounds in the delayed coker LPG is listed in Table 1. In the LPG feed, methyl mercaptan accounts for 74%—80% of total organosulfur compounds.

Table 1 Distribution of sulfur compounds in delayed coker LPG
2.2 Experimental methods
2.2.1 LPG desulfurization process
Desulfurization test was performed in an industrial plant for treating the delayed coker LPG. The lean solution counter-currently contacts with LPG in the desulfurization tower, and the rich solution leaving the bottom of desulfurization tower is introduced to a flash tank. Then the rich solution after flashing is regenerated in the stripper tower, and the regenerated lean solution is pumped to the top of desulfurization tower to be reused.
Prior to this industrial test, the desulfurization system only contained the aqueous solution of MDEA. The industrial test was carried out through replacing MDEA solvent with the UDS solvents. The mass ratios of additives (the mixture of all components except MDEA in UDS solvents) to MDEA in the desulfurization solution system were increased step by step to reach 1:9, 2:8, 3:7 and 4:6, respectively. Meanwhile, the corresponding amount of water was added in order to keep the solution concentration at 35%—40%. The operating conditions of desulfurization unit were also maintained at almost the same levels.
2.2.2 Analysis
The sulfur contents in LPG were determined using a gas chromatograph equipped with a flame photometric detector[6]. The H2S content in the desulfurization solution was determined by iodometry, and the content of heat stable salts (HSS) in the lean solution was determined using an ion-exchange titration method. The mass ratios of additives to MDEA in the solution were measured using a gas chromatograph equipped with a flame ionization detector.

Figure 1 Desulfurization process for delayed coker LPGT1—Desulfurization tower; T2—Stripper tower; V1—Knockout drum; V2—Flash tank; V3—Solution tank; E1, E2—Heat exchanger; L1—Air cooler; L2, L3—Water cooler; P1, P2, P3, P4—Solution pump.
3 Results and Discussion
The UDS solvents are formulated with alkanolamines (including MDEA), sulfur-containing heterocyclic com-pounds, and a cyclic amine compound for selectively removing various sulfur compounds from hydrocarbon streams. Such particular components can effectively improve the performance of UDS solvents for removing organosulfur compounds, thanks to the increase in the physical solubility of organosulfur compounds, the increased catalytic effect on the hydrolysis of COS, and the enhanced reaction rate of COS with alkanolamines[6-7].
3.1 Solubility parameters
In view of the large proportion of methyl mercaptan in total organosulfur compounds, the enhancement of methyl mercaptan solubility is crucial to the reduction of total sulfur content in the treated LPG. The removal of methyl mercaptan can be attributed to both chemical and physical solubility, in particular the physical solubility[8]. According to the principle of “Likes Dissolve Likes”, the desulfurization solvent which has a closer solubility parameter to that of methyl mercaptan will possess a greater solubility of methyl mercaptan[9]. Therefore, the potential desulfurization performance of UDS solvents was evaluated via applying the solubility parameter theory firstly.
By taking into account the polarity and the association of species involved in mercaptan-solvent system, a twodimensional solubility parameter theory can be used to predict the solubility of methyl mercaptan in different solvents[10]. This theory is derived from the Hildebrand solubility parameter[11], the Hansen three-dimensional solubility parameter[12], the Bagley two-dimensional solubility parameter[13], and the newly defined solubility parameter[14].
The liquid cohesive energy can be divided into physical and chemical interactions[10]. Accordingly, the solubility parameter (δ′) contains two parts, the physical and the chemical components[14]. As a result, the two-dimensional solubility parameter can be expressed by Eq. (1)[10]:
where δ is the Hildebrand solubility parameter, and λ is the square root of liquid internal pressure, δp′and δc′are physical and chemical components of the solubility parameter, respectively.
The solubility parameter of a multicomponent system is calculated using Eq. (2):

where x is the molar fraction, and the subscript i refers to a component in a multicomponent system. δi′is calculated using Equations (3—5)[10]:
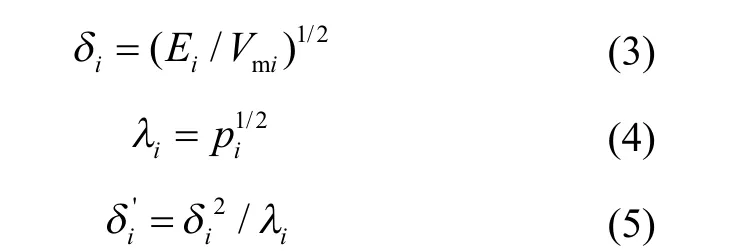
where Ei, Vmiand piare cohesive energy, molar volume and internal pressure, respectively. Eiand Vmiare calculated using the group contribution method[15], and piis calculated using a previously reported method[16].
Furthermore, the physical and the chemical components of the solubility parameter are calculated as follows[10].
For common polar and non-polar liquids, the physical component is defined by Eq. (6):
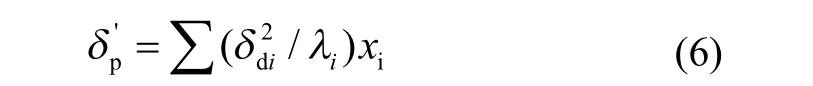
For associating liquids, the chemical component is defined by Eq. (7):

where δdiand δhiare the dispersion and hydrogen-bonding components of the Hansen solubility parameter, respectively. They can also be calculated using the group-contribution method[17].
The difference (Rc) in solubility parameter between methyl mercaptan and the desulfurization solvent is defined by Eq. (8):

where subscripts m and s refer to methyl mercaptan and the desulfurization solvent, respectively.
Two-dimensional solubility parameters of methyl mercaptan and 17 pure solvents are shown in Figure 2, and the distance from one solvent point to the point representing methyl mercaptan indicates the difference in solubility parameter, Rc. And Table 2 lists the values of group contributions involved in the calculation. As compared with alkanolamines (MEA, DEA, MDEA, DIPA), physical solvents and cyclic amines investigated hereby are similar to methyl mercaptan in terms of two-dimensional solubility parameter. And Rc of the functional components whichincreases the methyl mercaptan solubility in the UDS solvents as compared to that of methyl mercaptan is less than 0.66 (MPa)1/4.
Based on the solubility parameters of pure solvents, the solubility parameters of UDS solvents formulated with different proportions are obtained and compared with those of methyl mercaptan, LPG, and MDEA (see Table 3). As shown in Table 3, the difference in solubility parameter between methyl mercaptan and the desulfurization solvent decreases in the following order: MDEA > UDS-I > UDS-II > UDS-III > UDS-IV. Such an order reflects the varying affinity between solvents and methyl mercaptan. The UDS-IV solvent has the closest solubility parameter to that of methyl mercaptan, denoting a strongest affinity and a highest solubility for methyl mercaptan. Additionally, the significant differences in solubility parameters between the UDS solvents and LPG imply that LPG has a low solubility in the UDS solvents. It also means that the UDS solvents show the excellent desulfurization selectivity.
3.2 Industrial test of LPG desulfurization
3.2.1 Sulfur loading

Figure 2 Two-dimensional parameters of methyl mercaptanand pure solvents1—methyl mercaptan; 2—pyridine; 3—3-pyridylcarbinol; 4—pyridinepropanol ; 5—N-methyl pyrrolidone; 6—morpholine; 7—N-formyl morpholine; 8—piperazine; 9—N-hydroxyethyl morpholine; 10—2-piperideneethanol ; 11—triethylene diamine; 12—propylene carbonate; 13—dimethyl sulfoxide; 14—sulfolane; 15—MEA; 16—DEA; 17—MDEA; 18—DIPA.

Table 2 Values of group contributions involved in calculating solubility parameters

Table 3 Solubility parameters of methyl mercaptan, LPG, MDEA and UDS solvents
The sulfur loading in the desulfurization solution, which is defined as the molar ratio of sulfur compounds to effective solvent components, is determined by the sulfurcontent in LPG and mass flow rate ratio of LPG to the solution (Rf). Higher sulfur content in LPG and Rfmean higher sulfur loading in the solution. To accurately evaluate the desulfurization performance of different solvents, it is expected that the desulfurization unit should operate under the same conditions. However, due to the increase in the sulfur content of delayed coker feedstock from 1.5% in MDEA test period to 1.9%—2.2% in the UDS solvents test period, the sulfur content of delayed coker LPG increased significantly (see Table 1). The flow rate of LPG and Rfare shown in Figure 3. The flow rate of LPG and Rfwere approximately 1.3 t/h and 0.31, respectively, during MDEA test period. However, in the UDS solvents test periods, LPG flow rate increased to 1.8—2.9 t/h owing to the increase in upstream LPG yield, and Rfwas maintained at around 0.3 by varying the flow rate of solution in connection to LPG flow rate. As a result, the sulfur loading of solution in the UDS solvents test period was higher than that in MDEA test period.

Figure 3 LPG flow rate and mass flow rate ratio of LPG to solution (Rf)■—Flow rate of LPG;●—RfNote: the measured values for mass ratios of additives to MDEA in UDS-I, UDS-II, UDS-III and UDS-IV were 1.1:8.9, 1.8:8.2, 3.0:7.0 and 4.0:6.0, respectively.
3.2.2 Desulfurization performance
With H2S contents in LPG increasing dramatically from 0.9 v%—1.5 v% for MDEA tests to 1.5 v%—12.0 v% for UDS solvents tests, H2S contents of purified LPG were below 10 mg/m3. Figure 4 shows the performance of MDEA and UDS solvents on removal of total sulfur compounds from the delayed coker LPG feedstock. For MDEA solvent, the total sulfur content in treated LPG was in the range of 2 000—2 300 mg/m3. In the case of UDS-I and UDS-II solvents, there was no remarkable decrease in total sulfur content in the treated LPG. This outcome could be attributed to the excessive sulfur loading resulted from high sulfur content and Rf. When Rfwas reduced to about 0.3, the total sulfur content of LPG treated with UDS-II immediately decreased to 1 300—1 600 mg/m3. In the case of UDS-III, the total sulfur content of treated LPG mainly ranged from 1 100 to 1 300 mg/m3. As for UDS-IV, although the total organosulfur content and the flow rate of LPG increased by 30.4% and 92.3%, respectively, as compared with the case of MDEA, the total sulfur content of treated LPG was reduced to 900—1 200 mg/m3, wherein the decrease in total sulfur content was almost 50% as compared to the case of MDEA.
Owing to their excellent performance for removal of methyl mercaptan, the UDS solvents could significantly reduce the total sulfur content in treated LPG despite the remarkably increased sulfur loading of LPG feedstock. For example, the content of methyl mercaptan was reduced from 2 710.4 mg/m3in the feed LPG to 1 154.6 mg/m3in the purified LPG by using UDS-III. It is observed that the efficiency for removal of organosulfur compounds increased with an increase in the proportion of additives in the formulated solvents. The industrial test results are in good agreement with the calculation results for solubility parameters.

Figure 4 Performance for removal of total sulfur from delayed coker LPG during different test periods
3.3 Regeneration of UDS solvents
During the test, the stripper tower ran steadily, and no foaming behavior of the desulfurization solution was observed. Additionally, the steam consumption per tonof rich solution required by UDS solvents was less than 7.1%—23.5% as compared with the case using MDEA. Table 4 shows the regeneration performance of MDEA and UDS solvents. H2S contents of all lean solutions were less than 1.2 g/L, the corresponding HSS contents were in the range of 2%—3%. All of the results indicate that UDS solvents possess excellent regeneration performance, and especially they show lower regeneration energy consumption than MDEA.

Table 4 Regeneration performance of MDEA and UDS solvents
The compositions of stripped sour gases from rich solutions are shown in Table 5. In each period, the content of total hydrocarbons in stripped sour gas was below 0.65 v%, and such low content of hydrocarbons did not pose a negative influence on the sulfur recovery unit, which was attributed to the good selectivity of the desulfurization solvents for sulfur compounds over hydrocarbons. This also indicates that the solubility of hydrocarbons in UDS solvents was low, and the stripped sour gases from the UDS rich solutions could absolutely satisfy the demand of sulfur recovery unit.

Table 5 Compositions of stripped sour gases from rich solutions
4 Conclusions
The calculation results for solubility parameters show that the UDS-IV solvent has the closest solubility parameter to that of methyl mercaptan, suggesting that the UDS-IV solvent possesses the highest affinity to methyl mercpatan. The results of industrial tests in a delayed coker LPG desulfurization plant showed that H2S contents in LPG treated with MDEA and UDS solvents were all below 10 mg/m3. As the mass ratio of additives to MDEA in UDS solvents increased from 1:9 to 4:6, the total sulfur content in the treated LPG gradually decreased. Although the sulfur loading dramatically increased, the total sulfur content in LPG treated with UDS-IV was reduced by 50% as compared with MDEA. Moreover, the UDS-IV solvent exhibited an excellent regeneration performance along with a decrease of 23.5% in steam consumption per ton of rich solution as compared with MDEA. The contents of H2S and hydrocarbons in stripped sour gas discharged from UDS-IV rich solution were above 80% and below 0.65%, respectively, which could be attributed to its good selectivity for sulfur compounds over CO2and hydrocarbons. These industrial test results agreed well with the calculation results for solubility parameters.
Acknowledgment:The authors are grateful for the financial support from the National Key Science and Technology Project of China (2011ZX05017-005) and the Key Science and Technology Project of Sinopec (“Development and Industrial Application of Sweetening Process for Yuanba Natural Gas” ).
[1] Nielsen R B, Rogers J, Bullin J A, et al. Treat LPGs with amines[J]. Hydrocarbon Processing, 1997, 79(9): 49-59
[2] Tukov G V, Ivanova N N, Sadykov A N, et al. Establishing standards for consumption of caustic soda in treating liquefied gases (LPG) to remove mercaptans[J]. Chemistry and Technology of Fuels and Oils, 1975, 11(11): 869-872
[3] Zong L, Chen C C. Thermodynamic modeling of CO2and H2S solubility in aqueous DIPA solution, aqueous sulfolane-DIPA solution, and aqueous sulfolane-MDEA solution with electrolyte NRTL model[J]. Fluid Phase Equilibria, 2011, 306(2): 190-203
[4] Henni A, Tontiwachwuthikul P, Chakma A. Solubility study of methane and ethane in promising physical solvents for natural gas sweetening operations[J]. J Chem Eng Data, 2006, 51(1): 64-67
[5] Mohammad S, Hadi F, Masih H J. Experimental solubility of hydrogen sulfide and carbon dioxide in dimethylfor-mamide and dimethylsulfoxide[J]. Fluid Phase Equilibria, 2014, 367: 29-37
[6] Zhang J H, Shen B X, Sun H, et al. A study on the desulfurization performance of solvent UDS for purifying high sour natural gas[J]. Petroleum Science and Technology, 2011, 29(1): 48-58
[7] Zhang J H, Shen B X, Liu J C, et al. Absorption selectivity of solvents for organosulfurs in high sour natural gas[J]. Energy Sources, Part A: Recovery, Utilization and Environmental Effects, 2014, 36(8): 822-829
[8] Bedell S A, Miller M. Aqueous amines as reactive solvents for mercaptan removal[J]. Ind Eng Chem Res, 2007, 46(11): 3729-3733
[9] Lin L G, Kong Y, Wang G, et al. Selection and crosslinking modification of membrane material for FCC gasoline desulfurization[J]. Journal of Membrane Science, 2006, 285(1/2): 144-151
[10] Yu C F, Hei E C, Liu G J. Selection of polymer solvents and new two-dimensional solubility parameter[J]. Journal of Chemical Industry and Engineering, 2001, 52(4): 288-294 (in Chinese)
[11] Hildebrand J H, Scott R L. The Solubility of Nonelectrolytes[M]. 3rd ed. New York: Reinhold, 1950: 10-110
[12] Hansen C M. Three Dimensional Solubility Parameter and Solvent Diffusion Coefficient[M]. Copenhagen: Danish Technical Press, 1967: 56-70
[13] Bagley E B, Nelson T P, Scigliano J M. Three-dimensional solubility parameters and their relationship to internal pressure measurements in polar and hydrogen bonding solvents[J]. Journal of Paint Technology, 1971, 43(555): 35-42
[14] Liu G J, Hei E C, Shi J B. A new solubility parameter[J]. Journal of Chemical Industry and Engineering, 1994, 45(6): 666-672 (in Chinese)
[15] Fedors R F. A method for estimating both the solubility parameters and molar volume of liquids[J]. Polymer Engineering & Science, 1974, 14(2): 147-154
[16] Xu Y L, Yu C F, Hei E C, et al. Prediction of internal pressure and values of new solubility parameter for liquids[J]. Journal of Chemical Industry and Engineering, 2000, 51(3): 407-413 (in Chinese)
[17] van Krevelen D W, Te Nijenhuis K. Properties of Polymers: Their Correlation with Chemical Structure; Their Numerical Estimation and Prediction from Additive Group Contributions [M]. 4th completely revised edition. New York: Elsevier, 2009: 189-228
[18] Xu Z L, Qusay F A. Polyethersulfone (PES) hollow fiber ultrafiltration membranes prepared by PES/non-solvent/ NMP solution[J]. Journal of Membrane Science, 2004, 233(1/2): 101-111
[19] Burke J. Solubility parameters: theory and application[J]. The Book and Paper Group Annual, 1984, 3: 13-58
[20] Matsuura T, Blais P, Sourirajan S. Polar and nonpolar parameters for polymeric reverse osmosis membrane materials from liquid chromatographic data[J]. Journal of Applied Polymer Science, 1976, 20(6): 1515-1531
Successful Commercial Production of 1,4-Cyclohexane Dimethanol at Liaoyang Petrochemical Company
The PetroChina Liaoyang Petrochemical Company (LPC) has completed all commercial tests at its self-constructed 200 t/a 1,4-cyclohexane dimethanol (CHDM) unit, while delivering qualified CHDM product with a purity of 99.8% to provide reliable test data for formulating the PDP of an 10-kt/a class CHDM unit.
CHDM is one of two modified monomers for manufacture of polyethyleneglycol terephthalate -co-1,4-cyclohexylenedimethlene phthalate (PETG). In October 2012, LPC had constructed the first in China 100 kt/a PETG copolymer unit to deliver the on-spec product. The staff engaging in the development of a 200 t/a CHDM unit, which serves as the key associated unit of the 100 kt/a PETG copolymer unit, has developed the related CHDM process and the catalyst with the collaboration of the CAS Dalian Institute of Chemical Physics and the China Kunlun Engineering Company. The construction of the 200 t/a CHDM unit was mechanically completed and handed over to the owner by the end of 2013. Beginning from the mid-March 2015, LPC has performed the precommissioning of three major units to manufacture high-purity CHDM product and has accumulated necessary experience for the forthcoming mass production of copolymer products.
date: 2014-07-08; Accepted date: 2014-10-28.
Prof. Shen Benxian, Telephone: +86-21-64252851; E-mail: sbx@ecust.edu.cn.
杂志排行
中国炼油与石油化工的其它文章
- Study on Surface Adsorption and Inhibition Behavior of Corrosion Inhibitors Contained in Copper Foil Rolling Oil
- Sulfur Distribution during Hydrothermal Liquefaction of Lignite, Wheat Straw and Plastic Waste in Sub-Critical Water
- Synthesis of Petroleum Sulfonate Surfactant with Ultra-Low Interfacial Tension in Rotating Packed Bed Reactor
- Effect of Dodecylbenzene Sulfonic Acid Used as Additive on Residue Hydrotreating
- DFT Study of H2Dissociation on MoxSyClusters
- Simulation Optimization and Experimental Study of Cross-Wall Adiabatic Dividing Wall Column Used to Separate Hexane-Heptane-Octane System
