Study of a Novel Fourth-generation Supported Ziegler-Natta Catalyst for Propylene Polymerization: Relationship between Catalyst Structure and Polymerization Properties
2015-06-21LiuTaoLiWeiliXiaXianzhiMaoBingquan
Liu Tao; Li Weili; Xia Xianzhi; Mao Bingquan
(1. College of Materials Science and Engineering, Beijing University of Chemical Technology, Beijing 100029; 2. National Engineering Research Center for Polyolefins, Sinopec Beijing Research Institute of Chemical Industry, Beijing 100013)
Study of a Novel Fourth-generation Supported Ziegler-Natta Catalyst for Propylene Polymerization: Relationship between Catalyst Structure and Polymerization Properties
Liu Tao1,2; Li Weili2; Xia Xianzhi2; Mao Bingquan1,2
(1. College of Materials Science and Engineering, Beijing University of Chemical Technology, Beijing 100029; 2. National Engineering Research Center for Polyolefins, Sinopec Beijing Research Institute of Chemical Industry, Beijing 100013)
This article presents a detailed structural study of a new spherical MgCl2-supported TiCl4Ziegler-Natta catalyst for isotactic propylene polymerization, and researches on the relationship between catalyst structure and polymer properties. The spherical support with the chemical composition of CH3CH2OMgOCH(CH2Cl)2has been synthesized from a new dispersion system and is used as the supporting material to prepare Ziegler-Natta catalyst. The XRD analysis indicates that the catalyst is fully activated with δ-MgCl2in the active catalyst. The far-IR spectrometric results confirm again the presence of δ-MgCl2in the active catalyst. Textural property of the active catalyst exhibits high surface area coupled with high porosity. The high activity in propylene polymerization is mainly ascribed to the full activation and the porous structure of the catalyst. Scanning electron microscopy/energy dispersive spectrometer mapping results indicate a uniform titanium distribution throughout the catalyst particles. Particle size analysis shows that the catalyst has a narrow particle size distribution. The perfect spherical shape, uniform titanium distribution and narrow particle size distribution of the catalyst confirm the advantage of polymer particles production with less fines. The solid state13C NMR and mid-IR spectroscopic analyses indicate that there exists strong complexation between diisobutyl phthalate and MgCl2, which leads to the high isotacticity of polypropylene.
Ziegler-Natta catalyst; MgCl2; propylene polymerization; structure characterization; polymerization properties
1 Introduction
Conventional fourth-generation heterogeneous Ziegler-Natta catalyst consists of a transition metal (usually TiCl4) supported on MgCl2, together with a cocatalyst such as aluminumtriethyl. Electron donors are added to improve the polymer isotacticity[1-2]. Today, close to an astounding 60 million tons of isotactic polypropylene (PP) are produced worldwide annually[3]. For many polymerization processes, in order to achieve steady and smooth operating conditions, it is crucial to make polymers form spherical particles[4]. Therefore, the fundamental task is to prepare the spherical support particles. Many methods can be used to prepare spherical MgCl2support, such as precipitation and emulsion[5], spray crystallization[6-7], as well as spray drying and fast quenching[2]. During the preparation of catalyst, TiCl4is loaded on MgCl2support and thus a porous catalyst is obtained. In order to design and develop new catalysts, it is of great importance to study the structure of a catalyst and to find out how a catalyst works. The understanding of the relationship between structure and properties of a catalyst can give much help to control the polymerization process. It is helpful to seek for a simple method to characterize whether the catalyst is active enough and whether the catalyst is appropriate for polymerization[2].
Very recently, we reported the synthesis of a new spherical support with the chemical composition of CH3CH2OMgOCH(CH2Cl)2[8-12]. In our research, we applied a new dispersion system and the support was obtained from a one-step method at a moderate temperatureof 80 ℃. The development of the new method for preparing spherical support has greatly simplified the support preparation process and saved much energy. Hereupon, the new support was used as support material for manufacture of the Ziegler-Natta active catalyst, and the relationship between catalyst structure and polymer properties was studied.
2 Experimental
2.1 Materials
The chemicals were bought from the Sinopharm Beijing Chemical Reagent Co. Ltd. and were used as received. The manipulations with air and moisture-sensitive compounds were carried out under nitrogen blanketing.
2.2 Synthesis of the support
In a 0.5-L glass autoclave equipped with a mechanical stirrer, 22 g of anhydrous MgCl2, 162 mL of anhydrous ethanol, and 2.25 g of polyvinylpyrrolidone (PVP, MW=10 000) were added and heated at 80 ℃ for 1 hour until MgCl2and PVP were dissolved in ethanol, and then 72.5 mL of epichlorohydrin was added to this system. After 30 minutes of reaction, the liquid was removed and the obtained support particles were washed with n-hexane for three times. The particles obtained were dried under vacuum at room temperature[8-12].
2.3 Preparation of the catalyst
Before the catalyst preparation, the support was first pretreated with titanium ethoxide. In a 300-mL glass reactor, 16 g of support obtained from the above procedure and 150 mL of n-hexane were added, and the mixture was cooled down to -10 ℃. After 30 minutes of cooling, 4 mL of titanium ethoxide was added into this system and the mixture was heated to 60 ℃. After 30 minutes of heating, the liquid was removed and the obtained particles were washed with n-hexane for three times. The obtained particles were dried in vacuo at room temperature. Then, TiCl4(100 mL) was added into a 300-mL glass reactor equipped with a reflux condenser, and the mixture was cooled down to -20 ℃. After 8 g of pretreated support was added, the mixture was heated to 110 ℃. During the heating process, 1.5 mL of diisobutyl phthalate (DIBP) was added to the reactor. After the liquid was removed, the obtained catalyst particles were washed with TiCl4for two times and then with n-hexane for three times. The obtained particles were dried in vacuo at room temperature[8-12].
2.4 Propylene polymerization
Under blanketing with ultra-high purity N2atmosphere, 2.3 L of propylene, 2.5 mmol of aluminum triethyl (dissolved in 5 mL of n-hexane), 0.1 mmol of cyclohexylmethyl dimethoxy silane (dissolved in 1 mL of n-hexane), 10 mg of catalyst, and H2were added into a 5-L stainless steel autoclave in turn. The polymerization was carried out at 70 ℃ for 1 hour, and PP particles were obtained.
2.5 Characterization
A Bruker AXS, D8 Advance powder X-ray diffractometer was used to record the XRD patterns using Cukαradiation. Samples were scanned in the range of 2θ=5°—75°. A thin PE film was used to protect the samples so that the measurement could be performed inertly. The SEM images were taken by a FEI (XL-30) scanning electron microscope. The mid-infrared (mid-IR) spectra of the catalyst sample were measured using a Nicolet Nexus 470 FTIR spectrometer. Measurement was performed by KBr mode under the protection of Nujol mull. The farinfrared (far-IR) spectrum was measured with a Nicolet Magna-IR 750 infrared spectrometer and the sample was also protected by Nujol mull. All solid-state NMR experiments were carried out at 9.4 T on a Bruker Avance III 400 MHz NMR spectrometer with resonance frequencies of 400.13 and 100.60 MHz for1H and13C, respectively. The13C MAS NMR spectra are recorded using a 4 mm MAS probe. The chemical shifts were referenced to tetramethylsilane for1H, and to hexamethylbenzene (HMB) for13C. Repetition times of 50 s for13C were used for single-pulse NMR experiments. For the1H→13C CP/MAS NMR experiments, the Hartmann-Hahn condition was achieved using HMB, with a contact time of 2 ms and a repetition time of 2 s. The surface area of supports and catalysts were determined with nitrogen gas (at -195.6 ℃) by the AutoChem II 2920 automated desorption analyzer from Micrometrics. The particle size distribution was determined by a Mastersizer 2000 particle size analyzer manufactured by Malvern Instruments. The melt mass flow rate (MFR) test was carried out in accordance with the standard test method ASTMD1238-99.
3 Results and Discussion
3.1 Preparation of solid catalyst
Main reaction pathways for catalyst preparation are presented in Figure 1[8]. The first step was the preparation of the new support. The support was obtained from a dispersion system. A certain amount of MgCl2and PVP was dissolved in ethanol at 80 ℃ prior to the addition of epichlorohydrin to this system, and then a dispersion system was formed right after the introduction of epichlorohydrin. In this system, PVP played a critical role to stabilize the spherical particles. Therefore, a perfect spherical support with the chemical composition of CH3CH2OMgOCH(CH2Cl)2was obtained from this system. The second step was the preparation of the catalyst. The support was first pretreated with titanium ethoxide. Then the resulted support was treated with TiCl4, which could convert CH3CH2OMgOCH(CH2Cl)2to MgCl2. This conversion was accompanied by a recrystallization process to form the disordered MgCl2. During the recrystallization process, the inner electron donor DIBP was added to this system, which was coordinated with MgCl2and TiCl4to form isotactic active centers.

Figure 1 Sketch of the catalyst preparation
3.2 Powder X-ray diffraction
Powder XRD pattern of the catalyst is shown and compared with that of anhydrous MgCl2in Figure 2. The XRD pattern of anhydrous MgCl2indicates that it is a cubic close-packed structure (α-MgCl2) (Figure 2a)[13]. The XRD pattern of MgCl2/TiCl4/DIBP catalyst (Figure 2b) shows that the catalyst has a pattern of rotationally disordered δ-MgCl2, viz.: a complex of α-MgCl2and β-MgCl2(hexagonal close-packed structure). These two crystal forms of MgCl2can be identified from the XRD pattern in the range of 30°—35°. The cubic close-packed form has two reflections, namely: (10 -2)+(006) at 30.4° and (104) at 35.2°, while the form of hexagonal close-packed structure gives three peaks, viz.: (002) at 30.6°, (101) at 32.4°, and (104) at 35.2°[1,14-17].
One of the standards for judging whether a catalyst is good enough is to check the XRD pattern of the catalyst. Model experiments have proved that the peak at 28°—40° will be closely related to the degree of activation. As proposed by Galli[14], with the increase of activation time, the peak at 28°—40° will not split. So the shape of this diffraction peak could be an indicator of the catalyst structure. Based on these model experiments, it can be confirmed that the catalyst is fully activated. Sormunen[18]has proposed that one of the requirements for making a good catalyst is that an amorphous phase must be formed on the MgCl2support.
It is noted that, during the addition of TiCl4and internal electron donor, and the subsequent washing with TiCl4, CH3CH2OMgOCH(CH2Cl)2has been converted to disordered δ-MgCl2and that TiCl4is embedded into the lattice of δ-MgCl2and therefore a stable MgCl2·TiCl4complex is formed via the chlorine bridge[17]. This can explain how the active sites are formed to play a key role in the propylene polymerization.

Figure 2 Powder XRD patterns of anhydrous MgCl2(a) and MgCl2/ TiCl4/DIBP catalyst (b)
3.3Element distribution in catalyst particles
Figure 3 shows the element distribution in the crosssection of a catalyst particle. The distribution of three elements (Mg, Cl, and Ti) in the catalyst was analyzed using an energy dispersive spectrometer (EDS) on the SEM microscope. The inorganic components of the catalyst can be considered as microcrystals of MgCl2with Ti compounds embedded into their lattice[1].
Judging by the Cl distribution data (the maximum component of the catalyst), the catalyst particle has a uniform apparent density with no voids in the particle’s center or edge. The distribution of Mg atoms is similar to that of Cl atoms, as expected from the MgCl2crystals. The distribution of Ti atoms is also similar to the distribution of Cl and Mg atoms, and the Ti atoms spread uniformly along the diameter axis. The uniform distribution of Ti atoms in the MgCl2crystals is very important for propylene polymerization. Hutchinson’s[4,19]simulation demonstrated that an uneven distribution of the polymerization sites in the catalyst granule would lead to an even more disastrous effect on polypropylene morphology. Only when the active centers are distributed uniformly, the polymer chains can achieve the same polymerization rate. If not, the stresses in different parts of a polymer particle in the process of polymerization are different and the particles would break up easily. So our obtained catalyst is expected to possess a good performance during polymerization.
3.4 Infrared analysis
The IR spectra of the catalyst are shown in Figure 4. The primary interest of the catalyst system in the mid-IR spectrum was the signals of the internal electron donor (DIBP), especially the C=O and C—O—C bonds. The signals of the asymmetric and symmetric stretching vibrations of C—O—C bonds are present in the mid-IR spectrum of the catalyst sample at 1 308 cm-1and 1 153 cm-1, respectively (Figure 4a). The signal at 1 684 cm-1occurs due to the C=O stretching vibrations of the carbonyl group of the diester. It can be seen from the spectrum that the C=O stretching vibrations of the DIBP in the catalyst have shifted to 1 684 cm-1as compared to 1 725 cm-1of free DIBP. Terano[20]showed that the C=O bond of the TiCl4·DIBP complex appears at 1 650 cm-1and 1 584 cm-1, but that of MgCl2/DIBP —at 1 680 cm-1. This indicates that there is a direct interaction between C=O and MgCl2, which does not occur between the C=O group and TiCl4in the catalyst system. However, a small difference can be observed in the C=O related IR absorption frequency of the catalyst (1 684 cm-1) and that of MgCl2/DIBP mixture (1 680 cm-1), and this indicates that DIBP in the catalyst is in some way complexing with both Mg and Ti species. That is to say, DIBP interacts directly with MgCl2and indirectly with TiCl4. This could be explained by the doublemetal complex-MgTiCl6·4CH3COOC2H5, synthetized by Giannetti and Albizzati[21-22]. The single crystal of this complex was characterized by XRD analyses. The result showed that all of the four CH3COOC2H5molecules were coordinated with Mg atoms, while Ti atoms were coordinated with Mg atoms through the double chlorine bridge. The far-IR spectrum also gives some valuable information of the catalyst. Figure 4b reports the far-IR spectrum of the catalyst. A strong and wide but not split peak was observed at 250 cm-1assigned to υ (Mg-Cl) vibrational mode of the MgCl2polymeric chains, while in the case of the α-MgCl2(whose crystallographic disorder level is smaller than δ-MgCl2and is not suitable to be a supporting material to form active catalyst) this band should appear as split peaks thereby[23]. This also indicates that the MgCl2in the catalyst was fully activated during the catalyst preparation.

Figure 3 SEM photograph of cross-section of a catalyst particle (a); a typical EDS spectrum (b); the element distributions along the arrows (c)
3.5 Solid state13C NMR analysis
Chemical shifts of the carbons in the catalyst system of different modes are shown in Table 1 and compared with neat DIBP in CDCl3. Figure 5 shows the single-pulse13C NMR spectra of the catalyst. The resonance with chemical shift values of 93 was caused by the background signal of the probe. The three narrow resonances with chemical shift values of 14.0, 23.2, and 32.3, respectively, stemmed from the resonance of the carbon atoms of the residual n-hexane during the washing. It can be seen from the line widths of the peaks that the mobility of the DIBP molecule was under serious restrictions. This indicates that DIBP was not loosely adsorbed on MgCl2, but there existed a strong interaction between DIBP and MgCl2. In other words, DIBP molecules were not in free forms. The narrow line widths of n-hexane indicate that n-hexane is simply adsorbed onto the solid catalyst. Another evidence also indicates the complexation between MgCl2and DIBP, as evidenced by the changes in some of the chemical shifts. The resonances of carbon atoms on the diester chains are shifted to lower magnetic fields. For DIBP, the shift is largest for the CH2O carbon atom (ca. 5) and for the carbonyl carbon atom (ca. 6). The large changes observed for DIBP indicates a strong interaction between MgCl2and DIBP. Furthermore, the chemical shifts of the resonance of methyl carbon atoms change a little. This is because the methyl carbon species must be far from thecomplexation center[18,24-26].

Figure 4 The mid-IR spectra (a) and far-IR spectra (b) of the catalyst

Figure 5 The single-pulse13C NMR spectra of the catalyst

Table 1 Chemical shifts of the resonance of carbon atoms in the catalyst system
Figure 6 shows the1H→13C CP/MAS NMR spectra of the catalyst. An asterisk denotes a spinning side band. The catalyst shows two pairs of signals at 75.7, 76.8, and 172.7, 182.5, respectively. The signal at 182.5 is much less intense than the signal at 172.7. The above findings could explain that there are different types of coordination of electron donors onto MgCl2because DIBP is a bidentate. In MgCl2crystal, the electron donor molecules are located at non-equivalent sites. This could take place on different MgCl2crystal phases mono- or bidentally[18,24-26].
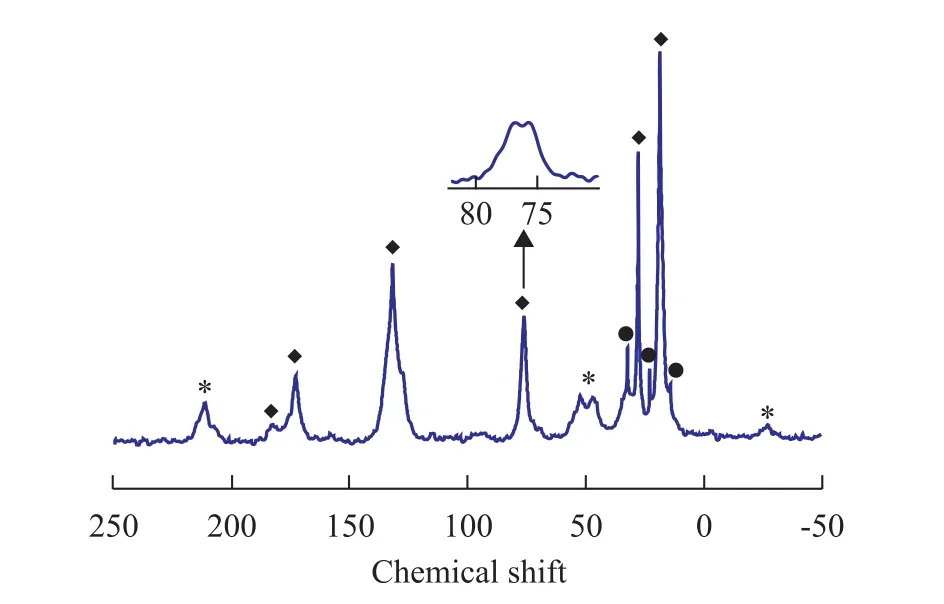
Figure 6 The1H→13C CP/MAS NMR spectra of the catalyst
3.6 Textural properties
The N2adsorption-desorption isotherms and pore size distribution of active catalyst are shown in Figure 7 and presented in Table 2[8]. The surface area of the support is very low, about 6.0 m2/g, while the catalyst has a surface area of 444 m2/g, which is extremely high compared with the previously reported commercial Ziegler-Natta catalysts. High surface area could be attributed to the recrystallization process for converting CH3CH2OMgOCH(CH2Cl)2to δ-MgCl2in the course of active catalyst preparation.

Table 2 Textural characteristics of support and titanated catalyst
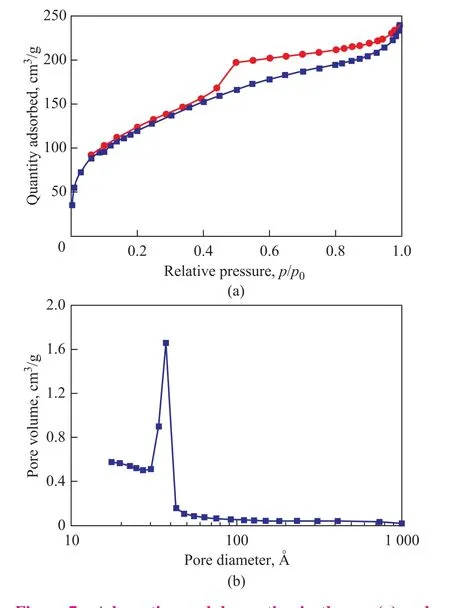
Figure 7 Adsorption and desorption isotherms (a) and pore diameter distribution (b) of MgCl2/TiCl4/DIBP active catalyst
The adsorption pattern of the catalyst indicates a Type-IV isotherm with a H2hysteresis loop[27]. The pore diameter analysis indicates that the major contribution comes from mesopores. As for propylene polymerization, mesopores in the catalyst are the most appropriate ones for providing free access of the monomer to the inner zones. The proper porosity helps the retention of the rubbery phase in heterophasic copolymers[4].
3.7 Propylene polymerization
Propylene polymerization was carried out with different H2volumes, with the results shown in Table 3. The results showed that the catalyst possessed high polymerization activity. The reason leading to high catalyst activity is that the catalyst has been fully activated, and the activated catalyst has the amorphous and porous phase, as discussed in Section 3.2. Compared with the early mechanical activation approaches such as grinding MgCl2with TiCl4and a Lewis base, the new chemical activation method adopted nowadays to produce active catalyst has greatly improvedthe productivity. PP obtained from this new catalyst shows high isotacticity. The high isotacticity is mainly ascribed to the existence of DIBP in the catalyst. As discussed in Section 3.6, the strong interactions between DIBP and MgCl2has been verified by the NMR spectrometric analysis. The existence of a Lewis base, such as DIBP, is necessary for catalyst in order to manufacture PP with high isotacticity, because upon incorporating a proper Lewis base, such as DIBP, to the catalyst, the complexation will poison the atactic sites to improve the isotacticity of polymer. The large downfield shifts for DIBP in the catalyst observed in NMR spectra indicate that DIBP is a strong electron donor. So when the catalyst is treated with TiCl4in the course of preparation, or it is treated with the cocatalyst during the polymerization process, DIBP is not flushed away easily from MgCl2. Therefore, DIBP should be a good agent for controlling isotacticity. With the increase in H2volume, the MFR of PP rises sharply, which indicates that the hydrogen sensitivity of the new catalyst is very good. The hydrogen sensitivity of a catalyst is one of the most important aspects to produce different types of polymers with different molecular weights. Furthermore, a part of the existing PP production devices cannot bear undue pressure, so a catalyst with good hydrogen sensitivity is needed for these devices so that under a relative low pressure different PP grades can be produced.

Table 3 Propylene polymerization under different H2volumes
The screening data of PP particles obtained during polymerization in the presence of new catalyst (Catalyst 2) are shown in Table 4 and are compared with one of most widely used commercial catalysts (Catalyst 1). It can be seen from Table 4 that most of PP particles obtained during polymerization in the presence of catalyst 2 had the size ranging from 0.9 mm to 2 mm and the percentage of PP particles with the size smaller than 0.2 mm was very low. PP particles with a diameter less than 0.2 mm were considered as fines, and the existence of fines will greatly influence the process stability during production. How to reduce the fines during polymerization is one of the most important issues for the application of catalyst. The test results show that the fines generated during the polymerization reaction upon using the new catalyst are much less than those generated from the process using the traditional catalyst. One reason is that the new catalyst particles have a perfect spherical shape. Spherical catalyst is less likely to produce fines than irregular granular catalyst during polymerization reaction when the polymers are subjected to strong collision. Another reason might be that the new catalyst has a uniform active sites distribution, as discussed in Section 3.3. During the polymerization process, the uniform distribution of active sites will produce uniform stress, and the particles are less likely to break up. The third reason is that the new catalyst particles have a uniform size distribution, as shown in Figure 8. The results showed that the diameter of most particles lie in the range of between 20 μm and 90 μm and there are no too small or too large particles. As for the traditional method for preparing the support, it is difficult to control the particle size, so the obtained support particles have a wide range of size distribution. Therefore too small or too large support particles do exist. These improper support particles are generally used to prepare the catalyst with improper size distribution. The improper catalyst will produce much more fines during the polymerization reaction.Catalyst particles with too small diameters will definitely produce fines, meanwhile catalyst particles with too large diameters also can produce fines because the polymerization heat can not easily diffuse and will cause particles to break down. Once the particles are broken, more fines will be produced. While in our experiments, the support was obtained from a new dispersion system and the obtained particles have a uniform size distribution. The catalyst particles with a proper range of diameters will result in less fines during the polymerization process.

Figure 8 Particle size distribution of the catalyst

Table 4 The screen analysis of PP particles
4 Conclusions
The new support with the chemical composition of CH3CH2OMgOCH(CH2Cl)2was obtained from a dispersion system and has been used as support material to prepare Ziegler-Natta active catalyst. The XRD analysis indicated that the catalyst was fully activated and contained δ-MgCl2in the active catalyst. The far-IR spectroscopic results confirmed again the presence of δ-MgCl2in the active catalyst. Textural property of the catalyst exhibited high surface area with high porosity. The high activity of catalyst during propylene polymerization is mainly attributed to the full activation and the porous structure of the catalyst. The SEM/EDS mapping results indicated a uniform titanium distribution throughout the catalyst particle. Particle size analysis showed that the catalyst had a good particle size distribution. The perfect spherical shape, uniform titanium distribution and good particle size distribution led to the reduction of fines during the polymerization process. The solid state13C NMR and middle-IR analyses indicated that there existed a strong complexation between DIBP and MgCl2, which resulted in high isotacticity of the catalyst during propylene polymerization.
Acknowledgements:The authors thank the Sinopec Beijing Research Institute of Chemical Industry for its financial support (No. 5-12ZS0419, 5-10ZS0245, 5-12ZS0270)
[1] Chang M, Liu X, Nelson P J, et al. Ziegler-Natta catalysts for propylene polymerization: Morphology and crystal structure of a fourth-generation catalyst[J]. J Catal, 2006, 239(2): 347-353
[2] Rönkkö H L, Knuuttila H, DeniflP, et al. Structural studies on a solid self-supported Ziegler-Natta-type catalyst for propylene polymerization[J]. J Mol Catal A: Chem, 2007, 278(1/2): 127-134
[3] Kaminsky W. Polyolefins: 50 Years after Ziegler and Natta I[M]. Berlin: Springer-Verlag, 2013
[4] Pasquini N. Polypropylene Handbook, 2nd Edition[M]. Munich: Carl Hanser Verlag, 1996
[5] Collina G, Evangelisti D, Sacchetti M. Magnesium dichloride-ethanol adducts and catalyst components obtained therefrom: Italy, WO 2005063832[P], 2005-07-14
[6] Karbasi A K, Leinonen T, Sormunen P. Method for polymerizing or copolymerizing C4—C40alpha-olefins: Finland, EP 0627449[P], 1994-12-07
[7] Koskinen J, Jokinen P. A polymerization catalyst carrier prepared by spray crystallization: Finland, WO 9319100[P], 1993-09-30
[8] Liu T, Li W L, Xia X Z, et al. A facile one-step method for spherical support preparation of Ziegler-Natta catalyst[J]. J Appl Polym Sci, 2014, 131(21), 10.1002/APP.41014
[9] Xia X Z, Shi C, Li W L, et al. A titanium-containing solid catalyst component used in olefin polymerization: China, CN 201310469927.5[P], 2013-10-10
[10] Li W L, Xia X Z, Liu Y X, et al. A solid component and its preparation method and application: China, CN 201310491641.7[P], 2013-10-18
[11] Li W L, Xia X Z, Liu Y X, et al. A catalyst component used in olefin polymerization and its preparation method, and the catalyst used in olefin polymerization and its application: China, CN 201310491648.9[P], 2013-10-18
[12] Li W L, Xia X Z, Liu Y X, et al. A spherical support used in olefin polymerization catalyst and its preparation method: China, CN 201310491393.6[P], 2013-10-18
[13] Giannini U. Polymerization of olefins with high activity catalysts[J]. Macromol Chem Phys, 1981, 5(1): 216-229
[14] Galli P, Barbè P, Guidetti G, et al. The activation of MgCl2-supported Ziegler-Natta catalysts: A structural investigation[J]. Eur Polym J, 1983, 19(1): 19-24
[15] Dumas C, Hsu C C. Supported propylene polymerizationcatalyst[J]. Polym Rev, 1984, 24(3): 355-386
[16] Zannetti R, Marega C, Marigo A, et al. Layer-lattices in Ziegler-Natta catalysts[J]. J Polym Sci, Part B: Polym Phys, 1988, 26(12): 2399-2412
[17] Barbé P, Cecchin G, Noristi L. The catalytic system Ticomplex/MgCl2[J]. Adv Polym Sci, 1986, 81: 1-81
[18] Sormunen P, Hjertberg T, Iiskola E. A solid-state13C NMR study on heterogeneous Ziegler-Natta catalyst components[J]. Macromol Chem Phys, 1990, 191(11), 2663-2673
[19] Hutchinson R A, Chen C M, Ray W H. Polymerization of olefins through heterogeneous catalysis X: Modeling of particle growth and morphology[J]. J Appl Polym Sci, 1992, 44(8): 1389-1414
[20] Terano M, Kataoka T, Hosaka M, et al. Transition Metals and Organometallics as Catalysts for Olefin Polymerization[M]. Berlin: Springer-Verlag , 1988
[21] Giannetti E, Albizzati E. Ziegler-Natta catalysis by Mg-Ti complexes, 2. Ethylene polymerization by MgTiCl6·4CH3COOC2H5and triisobutylaluminium[J]. Macromol Chem Phys, 1985, 186(5): 907-913
[22] Albizzati E, Giannetti E, Giannini U. Ziegler-Natta catalysis by Mg-Ti complexes, 1. Synthesis, properties and catalytic activity in the polymerization of α-alkenes with MtMt’ Xn·mL complexes[J]. Macromol Rapid Commun, 1984, 5(10): 673-677
[23] Noto V D, Bresadola S. New synthesis of a highly active δ-MgCl2for MgCl2/TiCl4/AlEt3catalytic systems[J]. Macromol Chem Phys, 1996, 197(11): 3827-3835
[24] Abis L, Albizzati E, Giannini U, et al. Cross polarization/ magic angle spinning13C solid state nuclear magnetic resonance of model compounds related to supported Ziegler-Natta catalysts[J]. Macromol Chem Phys, 1988, 189(7): 1595-1601
[25] Chien J C W, Dickinson L C, Vizzini J. Magnesium chloride supported high mileage catalysts for olefin polymerization. XX. Solid state NMR[J]. J Polym Sci, Part A: Polym Chem, 1990, 28(9), 2321-2333
[26] Terano M, Saito M, Kataoka T. Solid-state13C NMR study on the state of the electron donor in MgCl2-supported catalysts[J]. Macromol Rapid Commun, 1992, 13(2): 103-108
[27] Gnanakumar E S, Thushara K S, Gowda R R, et al. MgCl2·6C6H11OH: A high mileage porous support for Ziegler-Natta catalyst[J]. J Phys Chem C, 2012, 116(45): 24115-24122
The Project “Development and Commercial Application of Technology for Ultra-deep HDS of Diesel (RTS)” Passed SINOPEC’s Appraisal
On December 9, 2014 the scientific research project “Development and commercial application of technology for ultra-deep HDS of diesel (RTS)” jointly performed by the SINOPEC Research Institute of Petroleum Processing (RIPP), the Yanshan Petrochemical Branch Company (YPBC), the Maoming Petrochemical Branch Company and the Guangzhou Petrochemical Branch Company has passed in Beijing the technical appraisal organized by the Science and Technology Division of the Sinopec Corp.
The RTS technology by means of the single-stage hydrodesulfurization process flow scheme and non-precious metal-based hydrotreating catalysts operating at different temperatures and space velocities in two reactors can achieve the ultradeep HDS of diesel fraction. In comparison with the traditional hydrotreating technology, the said technology can produce the ultra-low sulfur diesel (ULSD) with improved color of diesel product.
Operation of the RTS technology at YPBC for 14 months has revealed that this unit upon processing a feedstock having a density of 850—880 kg/m3, a cetane index of 40—45, a sulfur content of 5 000—9 000 μg/g and a nitrogen content of 200—800 μg/g can smoothly produce ULSD containing less than 9 μg/g of sulfur and less than 4.7% of polynuclear aromatics, with its color grade (ASTM D1500) not exceeding 0.5. The average energy consumption of the RTS unit is equal to 8.71 kg of SOE per ton of feed (1 kg of SOE= 41.8 MJ). The RTS unit can operate very smoothly to meet the requirements for longcycle production of diesel in compliance with the needs of the national V standard for vehicle exhaust emissions.
date: 2014-09-06; Accepted date: 2014-11-09.
Professor Mao Bingquan, E-mail: maobq.bjhy@sinopec.com.
杂志排行
中国炼油与石油化工的其它文章
- Study on Surface Adsorption and Inhibition Behavior of Corrosion Inhibitors Contained in Copper Foil Rolling Oil
- Synthesis of Petroleum Sulfonate Surfactant with Ultra-Low Interfacial Tension in Rotating Packed Bed Reactor
- Simultaneous Removal of H2S and Organosulfur Compounds from Lique fied Petroleum Gas Using Formulated Solvents: Solubility Parameter Investigation and Industrial Test
- Effect of Dodecylbenzene Sulfonic Acid Used as Additive on Residue Hydrotreating
- Sulfur Distribution during Hydrothermal Liquefaction of Lignite, Wheat Straw and Plastic Waste in Sub-Critical Water
- Simulation Optimization and Experimental Study of Cross-Wall Adiabatic Dividing Wall Column Used to Separate Hexane-Heptane-Octane System
