Study on Zn2SiO4Formation Kinetics and Activity Stability of Desulfurization Sorbent
2015-06-21ZouKangLinWeiTianHuipingXuGuangtongWangLeiXuHua
Zou Kang; Lin Wei; Tian Huiping; Xu Guangtong; Wang Lei; Xu Hua
(SINOPEC Research Institute of Petroleum Processing, Beijing 100083)
Study on Zn2SiO4Formation Kinetics and Activity Stability of Desulfurization Sorbent
Zou Kang; Lin Wei; Tian Huiping; Xu Guangtong; Wang Lei; Xu Hua
(SINOPEC Research Institute of Petroleum Processing, Beijing 100083)
A new industrial S Zorb sorbent (Ni/ZnO-P) was prepared by using the spray drying technique. The other two traditional sorbents (Ni/ZnO-M and Ni/ZnO-H) were prepared in exactly the same way except the use of different silica-alumina binder matrices. The XRD, Rietveld quantitative phase analysis, BET, and laser particle size analysis were employed to characterize their physico-chemical properties. The deactivation mechanism and desulfurization kinetics of sorbent was investigated on a water vapor aging treatment device. It was shown that both the water vapor pressure and reaction temperature significantly could influence the formation rate of inactive Zn2SiO4, which could decrease the sulfur storage capacity of sorbents. The Zn2SiO4content profiles could be fitted into the zero order equation, from which the apparent rate constantkand the activation energyEawere calculated. The matrix P greatly raised theEaof Zn2SiO4formation due to the less bridged hydroxyl silanol groups on its surface, which accounted for the high stability of Ni/ZnO-P. The desulfurization performance of the fresh and aged sorbents showed that the overall average sulfur conversion of Ni/ZnO-P (aged) was 92%, which was close to that of fresh sorbents (95%), and was higher than that of Ni/ZnO-M (aged) (86%) and Ni/ZnO-H (aged) (90%). Based on these findings, the application of Ni/ZnO-P can greatly improve the long-term running stability of the industrial unit.
kinetics, Zn2SiO4, desulfurization activity, sorbent, high stability
1 Introduction
Increasingly strict environmental regulations on exhaust emissions of gasoline-powered transportation vehicles are compelling refiners around the world to produce gasoline with lower sulfur content[1-5]. In Europe and Japan, sulfur content in gasoline is required to be no greater than 10 μg/g[4]. In China, the fourth and fifth versions of gasoline standard, which should come into effect in 2014 and 2018, respectively, also require the sulfur content in gasoline to be no greater than 50 μg/g and 10 μg/g, respectively. The FCC gasoline accounts for more than 70% share in the gasoline pool of China, making itself the key target of sulfur reduction[3-4]. To produce the ultra low sulfur gasoline, the selective hydrodesulfurization technology is most widely used, such as Axens’ Prime G+ and ExxonMobil’s Scanfining, etc.[1-4], although a loss of octane number caused by olefin saturation is still an intrinsic issue of these technologies. Therefore, people have developed several alternative desulfurization technologies, including the reaction adsorptive desulfurization technology – S Zorb[1-4,6-11].
The S Zorb technology was developed by the ConocoPhillips (COP) Company[6-10]and was licensed exclusively to Sinopec in 2007, which has been rapidly developing from then on[12-15]. Today, because of its high sulfur removal efficiency and lower octane loss, twenty-seven S Zorb units have been put into production in China, and the total processing capacity amounts to nearly 40 million tons per annum, making itself one of the most important technologies for manufacture of ultra low sulfur gasoline. In the S Zorb process, hydrogenation of sulfur-containing molecules takes place on a catalysis-adsorption bi-functional sorbent composed mainly of nickel and zinc oxide to release H2S, which is removed in situ by ZnO through the formation of ZnS[16-17]. As a result, H2S can hardly repress the desulfurization reaction, which generally happens in the conventional refining process[1-4]. Then the sorbent is regenerated by calcination in air to recover ZnO again[18-19]. The main chemical reactions are shown below[2,12-13]:

To match the fluidized bed technology, the S Zorb sorbent uses silicon aluminum matrix to shape up[14-15]. However, these silicon aluminum matrixes can react upon ZnO to generate inactive Zn2SiO4under certain conditions, which would significantly reduce the sulfur storage capacity of sorbent, leading to not only the excess sulfur content in gasoline product, but also the unsteady running of the unit as well[11-14,18-19]. Our previous work[14-15]discussed the factors affecting the rate of Zn2SiO4formation, and found out that water vapor was the main reason that led to the formation of Zn2SiO4, from which the ideas of developing high stability sorbent with lower Zn2SiO4formation rate was conceived. By far, we didn’t find any reports on this issue.
In this paper, three S Zorb sorbents were produced using different silica-alumina binder matrices. Their Zn2SiO4formation rates were investigated on a self-built water vapor aging treatment device. The mechanism and kinetics of Zn2SiO4formation on the S Zorb sorbents were discussed. On the basis of above work, we further evaluated the desulfurization performance of fresh and aged sorbents in fixed bed reactor.
2 Experimental
2.1 Catalyst preparation
Three zinc-based sorbents were prepared using different silica-alumina binder matrices. Each sorbent contained the same ratio of matrix and active components ZnO and NiO.
A series of preparation techniques, which included comminution of raw materials, colloidal slurry preparation, spray drying, and calcination, were used to form the sorbent. Silica-alumina binder matrices M, H, and P, corresponded to the commercial S Zorb sorbent Gen IV[10], FCAS-R09[20]and FCAS-II[21], were used to prepare Ni/ ZnO-M, Ni/ZnO-H, and Ni/ZnO-P, respectively. The sorbent Gen IV was developed by the Phillips Company in 2002. The FCAS-R09 and FCAS-II were developed respectively by our group in 2009 and 2012, and have been applied to about 20 industrial units.
2.2 Sorbent characterization
The X-ray powder diffraction (XRD) patterns of the sorbents were recorded on a Rigaku TTR-III instrument. The CuKa radiation (λ=1.540 6 Å) was generated at a tube voltage of 40 kV and a tube current of 250 mA. All scans were measured in a scanning angle (2θ) range of 10°—65othrough each step of 0.02º at a step time of 3s. The samples were crushed to a size of 200 mesh at first. Then, the samples were prepared and tested. During measurements the samples were spun around the vertical goniometer axis to improve the particle statistics and minimize the preferred orientation effects.
The phase identification and Rietveld quantitative phase analysis were carried out using the Jade v7.0 software package developed by Rigaku. The initial structure models for the identified phase were taken from the Inorganic Crystal Structure Database (ICSD) and the International Centre for Diffraction Database (ICDD). In order to stabilize the Rietveld refinement and to avoid spurious correlations, both the structural and chemical constraints were imposed during the Rietveld refinement[22-24].
For conducting the IR transmission studies, self-supporting disks, about 10 mm in diameter, were compacted at about 5 MPa. The self-built IR quartz cell was heated to 500 ℃ and evacuated to 10-3Pa for 3 h to ensure that the physical adsorption of water was completely eliminated. Then, the IR spectra in the range of 4 000—3 000 cm-1were recorded at a resolution of 4 cm-1using a Nicolet 560 Fourier transform infrared spectrometer,in which the spectra were scanned 32 times at room temperature[25].
The N2adsorption and desorption isotherms were measured at -196 ℃ on a Micromeritics ASAP 2010 instrument. The specific surface areas were calculated by the BET method. The particle size distribution of sorbents was measured by a Malvern Mastersizer 2000 instrument in compliance with the ASTM method D4464-10.
The sulfur content of gasoline was characterized in a ThermoEuroglas TS3000 by using the ultraviolet fluorescence analysis.
2.3 Hydrothermal aging experiment
The hydrothermal aging experiment was carried out ina self-designed instrument, including an ATS 3210 split tube furnace and a steam generator (Figure 1). The operating parameters, such as temperature, roasting time, gas flow rate and pressure, were controlled by a Honeywell HC900 controller system. The flow rate of carrier gas (high-purity N2or O2) was accurately adjusted to 50 L/h using a Brooks 5850S thermal mass controller. The sorbent weighing 5 g was heated with water vapor in an alumina salver to different target temperatures at a heating rate of 120 ℃/h. All experiments were carried out under atmospheric pressure. The steam generator and piping were heated and maintained at 250 ℃ and 150 ℃, respectively, in order to avoid condensation of water vapor. The flow rate of water was accurately adjusted using a CASC 2ZB-1L10 double piston micro-pump.

Figure 1 Scheme of self-made hydrothermal aging instrument
2.4 Catalytic activity measurements
Evaluation of catalyst performance was carried out on a bench scale fixed bed reactor, 25 mm in inner diameter, having 16 g of catalyst loading. FCC gasoline containing 1 610 μg/g of sulfur, more than 70% of which was thiophenic sulfur compounds, was used as the feedstock. The evaluation process was divided into three steps, namely: reduction, desulfurization and regeneration. The catalyst was reduced in hydrogen stream at 400 ℃ and 1.4 MPa for 1 h. Desulfurization of the feed lasted for 12 h at 400 ℃and 1.4 MPa and was realized at a mass space velocity of 5 h-1with sampling conducted through every 2 hours. The fluorescence method was employed to analyze the sulfur content in the desulfurized products sampled at different time intervals. Thereafter, the catalyst was regenerated with nitrogen purging at first followed by atmospheric oxidation at 520 ℃. After three cycles, the catalyst activity was stabilized, and was considered as the equilibrium activity.
3 Results and Discussion
3.1 Characterization of fresh sorbents
The physical properties of samples prepared are given in Table 1, which showed similar average particle size (84—88 mm) and size distribution along with the same packed bulk density (1.2 g/cm3). The fine powders with the particle size <20 mm accounted for no more than 0.4%, and particles with grain size in the range of 20—40 mm accounted for about 8%, while particles with grain size greater than 149 mm accounted for about 6%. The BET surface area of Ni/ZnO-M, Ni/ZnO-H, and Ni/ZnO-P was 32 m2/g, 28 m2/g, and 34 m2/g, respectively.
As shown in Figure 2, the XRD patterns of samples showed obvious and strong diffraction peaks of ZnO phase (PDF #01-080-0075) and NiO phase (PDF #01-089-7131). Furthermore, there were no diffraction peaks of silicon and aluminum containing phases, indicating to the amorphous state of SiO2and Al2O3.

Figure 2 XRD patterns of S Zorb sorbent■—ZnO;●—NiO
3.2 Hydrothermal stability of sorbents
The Ni/ZnO-H samples were calcined at 800 ℃ undera high-purity N2stream for 8 h, 16 h, 24 h, and 48 h, respectively. As shown in Figure 3, the XRD patterns of the calcined samples showed the same diffraction peaks as the fresh sorbent. This phenomenon indicatesd that the Ni/ZnO-H was stable at high temperature in dry nitrogen atmosphere.
In contrast, the Ni/ZnO-H sample was further calcined at 800 ℃ for 2 h under a water vapor pressure of 5 kPa, 10 kPa, 20 kPa, 40 kPa, 50 kPa, 70 kPa, and 80 kPa, respectively. The XRD patterns of the hydrothermally treated samples are shown in Figure 4.
The additional weak peaks of Zn2SiO4phase (PDF #01-072-1856) were firstly observed after the hydrothermal treatment under a water vapor pressure of 20 kPa. The reaction proceeds as follows:

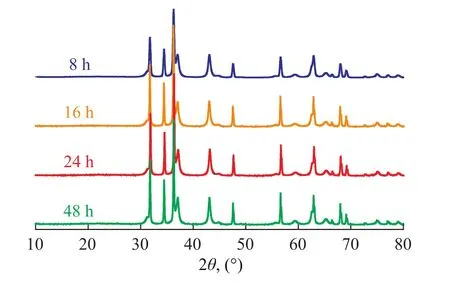
Figure 3 XRD patterns of Ni/ZnO-H calcined at 800℃in high purity N2stream at different time intervals
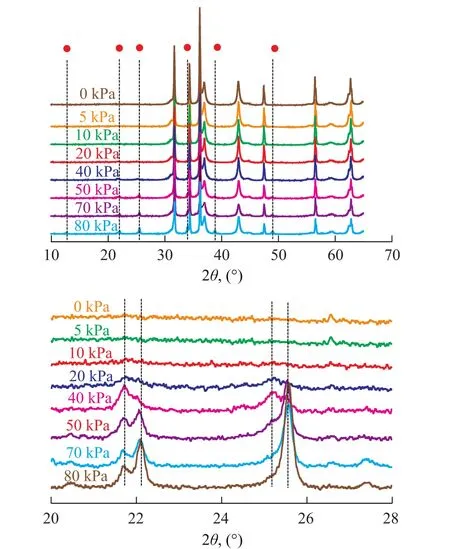
Figure 4 XRD patterns of Ni/ZnO-H calcined under different water pressure values (800℃, 2 h)
When the water vapor pressure rose, the intensity of diffraction peaks of Zn2SiO4phase increased correspondingly. Based on the Rietveld quantitative phase analysis[22], the contents of crystalline Zn2SiO4were about 0.22%, 0.65%, 3.6%, 7.7%, and 11.1% at a vapor pressure of 20 kPa, 40 kPa, 50 kPa, 70 kPa, and 80 kPa, respectively. This phenomenon indicated that the water vapor was one of the most important factors promoting the formation rate of Zn2SiO4phase in the S Zorb sorbents. According to reaction (4), the consumed ZnO phases were about 0.16%, 0.47%, 2.63%, 5.62%, and 8.1%, respectively. The sulfur species in gasoline were removed by ZnO via transformation to ZnS (reaction (1) — (3)). Therefore, the appearance of Zn2SiO4consumed the active ZnO, while decreasing the sulfur storage capacity of sorbents. In addition, the Zn2SiO4could hardly be regenerated in hydrogen stream or air atmosphere, causing the permanent deactivation of sorbents[10-15]. The characteristic diffraction peaks of Zn2SiO4phase were not observed at the water vapor pressure of 5 kPa and 10 kPa, which might be ascribed to the short processing time. As for the formation of crystalline Zn2SiO4during calcination in wet atmosphere, a similar phenomenon had been observed by researchers on the formation of cobalt silicate. According to Puskas[26], the cobalt silicate formation was postulated to be a reaction between migrating silicic acid and hydrated cobalt hydroxide, and water played a vital role in maintaining a sufficiently high equilibrium concentration of silicic acid and metal hydroxide species.
Upon considering the actual situation of industrial units, a water vapor pressure of 20 kPa was applied in further experiments. The Zn2SiO4content profiles of the hydrothermally treated sorbents versus time are shown in Figure 5. The Zn2SiO4content profiles of Ni/ZnO-M, Ni/ZnO-H, and Ni/ZnO-P are close to straight lines and can be fitted into the zero order equation. The similarity may be attributed to the similar physico-chemical properties of the sorbents. According to reaction (4), the apparent reaction rate r can be evaluated by Eq. (5):


Figure 5 The changes of Zn2SiO4contents in sorbents with time at different temperatures■—750 ℃;●—800 ℃;▲—850 ℃
where k is the apparent reaction rate constant, CZnOis the concentration of ZnO, CSiO2is the concentration of SiO2, and the parameters a and b represent the order of reaction. For the zero order reaction, Eq. (5) can be written as:

Eq. (6) indicates the time dependence of reaction, and can be integrated as:

where C0is a constant, CZn2SiO4is the concentration of Zn2SiO4. k can be calculated from the experimental value of CZn2SiO4and t. For all experiments, the fitting coefficients of correlation (R) are greater than 0.982 (Table 2).
For the case of Ni/ZnO-H, k is 0.79%/h, 1.91%/h, and 4.86%/h at temperatures 750 ℃, 800 ℃, and 850 ℃, respectively. The increment of k means that the formation rate of Zn2SiO4increases with an increasing temperature, as evidenced by the cases involving Ni/ZnO-M and Ni/ ZnO-P. The water vapor pressure and temperature are the two important factors affecting the Zn2SiO4formation. In addition, Ni/ZnO-M, Ni/ZnO-H, and Ni/ZnO-P show different hydrothermal stability under the same experimental conditions. It can be seen from the rate constant k at different temperature in Table 2 that the Zn2SiO4formation rate decreases in the following order: Ni/ZnO-M > Ni/ ZnO-H > Ni/ZnO-P, which is in good agreement with the Zn2SiO4content in the sorbents (Figure 5). The different hydrothermal stability of samples is attributed to their different silica-alumina binder matrices.
3.3 Kinetic study on Zn2SiO4formation in samples
More knowledge of the hydrothermal stability depends on the Zn2SiO4formation kinetics. It is well known that lnk versus inverse temperature gives an Arrhenius type plot (Eq. 8)[27-29]:

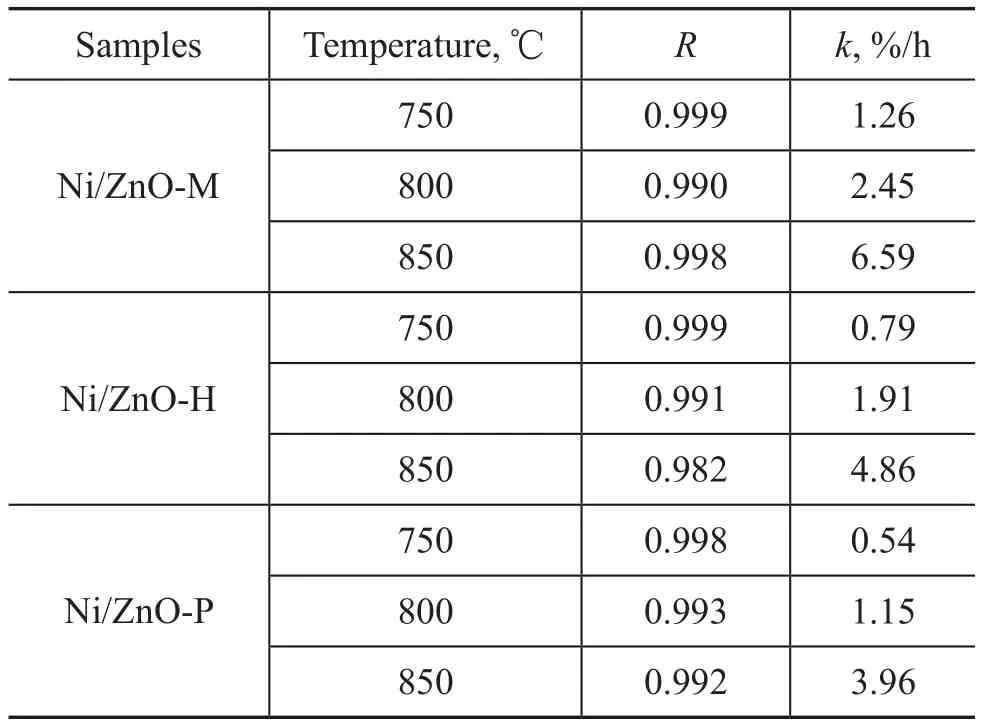
Table 2 Rate constants for the reaction of Zn2SiO4formation in samples at different temperatures and under 20 kPa of water vapor pressure (calculated from Eq. (7))
The apparent activation energy (Ea) calculated is shown in Figure 6. The fitting coefficients of correlation (R) are more than 0.993 at different temperatures (Table 3).

Figure 6 Fitting results of Arrhenius equation
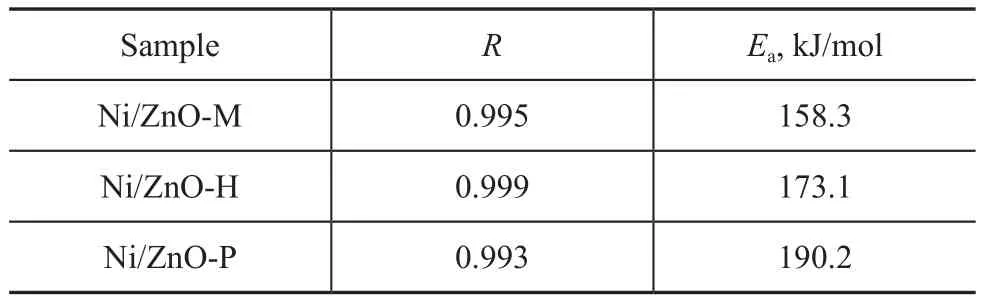
Table 3 Apparent activation energy for the reaction of Zn2SiO4formation in samples at 20 kPa of water vapor pressure (calculated from Eq. (8))
The magnitude of Eaat 20 kPa of water vapor pressure (158.3—190.2 kJ/mol) indicates that the Zn2SiO4formation may be controlled by ionic diffusion, for which similar values (150—200 kJ/mol) are generally observed[17,30]. The Eaincreases in the following order: Ni/ZnO-M < Ni/ ZnO-H < Ni/ZnO-P, which is in good agreement with the hydrothermal stability of the sorbents. The Eaof Ni/ ZnO-M (158.3 kJ/mol) is about 91% of that of Ni/ZnOH (173.1 kJ/mol) and 83% of that of Ni/ZnO-P (190.2 kJ/mol). The obvious difference of Eaindicates that the chosen silica-alumina binder matrix of Ni/ZnO-P remarkably increases its hydrothermal stability. According to previous study[25-26], H2O and SiO2form a transition state of silanol groups (OxSi-(OH)4-x), which can react on ZnO to form amorphous or crystalline zinc silicate. However, there are several types of hydroxyl groups on the surface of SiO2, such as the so-called ‘single free’ (isolated) hydroxyl groups, ‘single geminal’ hydroxyl groups, bridged hydroxyl groups, etc., which possess different nature[31-33]. The infrared spectra between 4 000—3 000 cm-1of the silica-alumina binder M, H, and P are shown in Figure 7. For original samples (Figure 7a), the broad and strong bands at 3 690—3 610cm-1can be attributed to the bridged hydroxyl groups[31-32]. The weak and broad band near 3 493 cm-1can be attributed to the oxygen perturbed hydroxyl groups (SiOH-O)[32]. After having been calcined at 800 ℃ under 20 kPa of water vapor pressure for 8 h (Figure 7 b), the broad flat-topped peak centered at 3 650cm-1(3 690—3 610cm-1) was weakened, especially in samples of H and P. An additional peak at 3 743 cm-1was observed in all samples, which occurred due to the superposition of the ‘single free’ and ‘single geminal’hydroxyl groups[31-33]. With an increasing hydrothermal treatment time, the intensity of peaks at 3 743 cm-1and 3 493 cm-1further increased (Figure 7c). It should be noticed that the changes of hydroxyl groups on the surface of samples M, H, and P were obviously different. The relative intensity of peaks at 3 743 cm-1and 3 493 cm-1decreased in the following order: P > H > M, which is in good agreement with the changes of apparent activation energy of Zn2SiO4formation. An inverse order can be observed at 3 650 cm-1. It can be speculated that the formation of silanol groups on the surface of silica-alumina binder matrix accelerated the rate of ionic diffusion on the solid-solid interfaces and the bridged hydroxyl silanol groups reacted on ZnO to form Zn2SiO4more readily[25-26]. The comparative advantage in hydrothermal stability of Ni/ZnO-P is very useful to deal with the accidents, such as water leaking and the moisture of regenerated atmo-sphere during the operation of industrial equipment.
3.4 Desulfurization performance of sorbents in fixed bed reactor
After three desulfurization-regeneration cycles, the equilibrium activity of sorbents Ni/ZnO-M, Ni/ZnO-H, and Ni/ZnO-P is shown in Figure 8. The sulfur conversion (%) is expressed by Eq. (9), where C0is the sulfur concentration in the feed (μg/g), Ctis the transient effluent sulfur concentration (μg/g) at any time t (h).
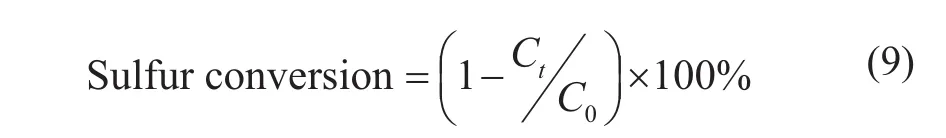

Figure 7 FT-IR patterns of M, H, and P

Figure 8 The desulfurization activity of sorbents in fixed bed reactor■—Ni/ZnO-M(Fresh);●—Ni/ZnO-H(Fresh);▲—Ni/ZnO-P(Fresh);◆—Ni/ZnO-M(Aged);▼—Ni/ZnO-H(Aged); —Ni/ZnO-P(Aged)
It can be seen from Figure 8 that these three fresh sorbents had nearly the same high desulfurization activity and the same trend of sulfur conversion profiles, which might be ascribed to their equal content of active components and closely parallel physicochemical properties, such as surface area, crystal structure, packed bulk density, average particle size, and size distribution (Figure 2 and Table 1). During the initial 2 hours, the feed gasoline sulfur conversion was around 99.5%. Then it slowly dropped because ZnO was gradually turned into ZnS by absorbing the sulfur element from gasoline. After 12 hours of activity test, the sulfur conversion was still higher than 90%, with the overall average sulfur conversion still reaching close to 95%.
To clarify the effect of the newly selected silica-alumina binder matrix on the sorbent performance, these three fresh sorbents were aged at 800 ℃ and under 20 kPa of water vapor pressure for 16 h prior to activity evaluation. The aged samples were correspondingly named as Ni/ ZnO-M (aged), Ni/ZnO-H (aged) and Ni/ZnO-P (aged). The equilibrium activity of the aged sorbents is shown in Figure 8. Upon comparing with the activity of fresh sorbents, the equilibrium activity of these aged sorbents all decreased to different degrees. The equilibrium activity of Ni/ZnO-M (aged) was reduced most quickly. The feed gasoline sulfur conversion on Ni/ZnO-M (aged) was about 97% after 2 hours of operation, and then quickly decreased to 93% at the 4thhour of run, and to 90% at the6thhour of run. Especially after 8 hours of run, a sharp decrease of activity could be observed, and the sulfur conversion was only 73% after 12 hours of run. The overall average sulfur conversion of Ni/ZnO-M (aged) was only 86%. The equilibrium activity of Ni/ZnO-P (aged) reduced most slowly. The feed gasoline sulfur conversion on Ni/ZnO-P (aged) was about 98.6% after 2 hours of operation, which was slightly lower than that of fresh sorbents and was obviously higher than that of Ni/ZnO-M (aged). The difference of sulfur conversion between Ni/ ZnO-P (aged) and Ni/ZnO-M (aged) became bigger with an increasing reaction time, which was equal to about 2%, 2.6%, 3.1%, 4.9%, 11.2%, and 14.1% at the 2ndhour, the 4thhour, the 6thhour, the 8thhour, the 10thhour, and the 12thhour of operation, respectively. It is noticed that the sulfur conversion of Ni/ZnO-P (aged) was 87% after the 12 hours activity test and the overall average sulfur conversion was higher than 92%, which was equal to around 97% of that of fresh sorbent. The sulfur conversion of Ni/ ZnO-H (aged) was about 98.6% after 2 h of operation, which was the same as that of Ni/ZnO-P (aged). The obvious difference of sulfur conversion between Ni/ZnO-H (aged) and Ni/ZnO-P (aged) was firstly observed at the 4thhour of run. Upon comparing with the performance of Ni/ ZnO-P (aged), the sulfur conversion on Ni/ZnO-H (aged) was less by about 0.7%, 1.0%, 2.5%, 3.8%, and 4.8% at the 4thhour, the 6thhour, the 8thhour, the 10thhour, and the 12thhour of run, respectively, in which the similar trend could be observed on the performance of Ni/ZnO-M (aged) and Ni/ZnO-P (aged). However, the sharp decrease of activity was not observed after 8 hours of operation with regard to both Ni/ZnO-H (aged) and Ni/ZnO-P (aged). The sulfur conversion on Ni/ZnO-H (aged) was 82% after the 12-hours activity test, and the overall average sulfur conversion was about 90%, which was equal to around 95% of that of the fresh sorbents. In short, the desulfurization activity of aged sorbents increased in the following order: Ni/ZnO-M (aged) < Ni/ZnO-H (aged) < Ni/ZnO-P (aged).
The XRD patterns of the aged sorbents are shown in Figure 9. It can be seen from Figure 9 that all XRD patterns of the aged sorbents showed strong diffraction peaks of Zn2SiO4phase. However, the different intensity of Zn2SiO4peaks in Ni/ZnO-M (aged), Ni/ZnO-H (aged), and Ni/ZnO-P (aged) indicated that the Zn2SiO4formation rate of three aged sorbents differed greatly from each other. The content of Zn2SiO4in Ni/ZnO-M (aged), Ni/ZnOH (aged), and Ni/ZnO-P (aged) was 25.0%, 14.0% and 8.4%, respectively. Consequently, the active ZnO phase increased to about 18.2%, 10.2% and 6.1%, respectively, which could react on SiO2to form inactive Zn2SiO4phase (Reaction (4)). This phenomenon was directly related to the desulfurization activity of aged sorbents. The Ni/ZnO-M (aged) with a highest Zn2SiO4content possessed a lowest and unstable desulfurization activity. By comparison, the Ni/ZnO-P (aged) sample with a lowest Zn2SiO4content had a higher and stable desulfurization activity.

Figure 9 The XRD patterns of aged sorbents
4 Conclusions
A high stability sorbent and two conventional S Zorb sorbents were prepared by spray drying technique using different silica-alumina binder matrices with the same content of active ZnO and NiO. The physico-chemical properties of sorbents, such as crystal structure, BET surface area, packed bulk density, average particle size and size distribution are similar between each other. The Zn2SiO4formation rates of sorbents increased with an increasing water vapor pressure and reaction temperature, which could lead to the reduction of the sulfur storage capacity of sorbents. The Zn2SiO4content profiles of sorbents can be fitted into the zero order equation. The rate constants k is different for each sorbent due to their different silica-alumina binder matrices. It can be speculated that the formation of silanol groups on the surface of silicon aluminum matrix can accelerate the rate of Zn2SiO4formation and the bridged hydroxyl silanol groups can react on ZnO to form Zn2SiO4more easily. The Ni/ZnO-P with chosen silicon aluminum matrix hasa highest apparent activation energy of 190.2 kJ/mol, which is much higher than that of Ni/ZnO-H (173.1 kJ/ mol) and Ni/ZnO-M (158.3 kJ/mol). Three fresh sorbents showed the excellent and almost the same desulfurization performance. The overall average sulfur conversion on sorbents was close to 95%. After having been aged at 800℃ and under 20 kPa of water vapor pressure for 16 hours, the feed gasoline sulfur conversion on Ni/ZnO-P (aged) decreased slowly and smoothly, and the overall average sulfur conversion was 92%, which was very close to that of fresh sorbents. As regards Ni/ZnO-M (aged) and Ni/ ZnO-H (aged), the feed gasoline sulfur conversion decreased quickly, and the overall average sulfur conversion reached only about 86% and 90%, respectively. The XRD results showed that Zn2SiO4content in Ni/ZnO-M (aged), Ni/ZnO-H (aged), and Ni/ZnO-P (aged) was 25.0%, 14.0% and 8.4% respectively, which could result in their different desulfurization performance. The highest apparent activation energy and desulfurization activity of Ni/ZnO-P was attributed to the chosen silicon aluminum matrix.
Acknowledgements:This work was supported by the Science and Technology Development projects of SINOPEC, China (Nos. 113138, 112008 and 110099).
[1] Song C. An overview of new approaches to deep desulfurization for ultra-clean gasoline, diesel fuel and jet fuel[J]. Catal Today, 2003, 86: 211-263
[2] Babich I V, Moulijn J A. Science and technology of novel processes for deep desulfurization of oil refinery streams: A review[J]. Fuel, 2003, 82(6): 607-631
[3] Eri Ito I J A, Rob V V. On novel process for removing sulphur from refinery streams[J]. Catal Today, 2006, 116: 446-460
[4] Brunet S, Mey D, Perot G, et al. On the hydrodesulfurization of FCC gasoline: A review[J]. Appl Catal A, 2005, 278(2): 143-172
[5] Huang L C, Wang G F, Qin Z F, et al. In situ XAS study on the mechanism of reactive adsorption desulfurization of oil product over Ni/ZnO[J]. Appl Catal B, 2011, 106(1/2): 26-38
[6] Gil J G, Dennis K, Larry R. Next generation sulfur removal technology[C/CD]. NPRA Annual Meeting, AM-00-12, San Antonio, 2000-03-26
[7] Byron J, Peter S, Dennis K, et al. Application of Phillips’ S Zorb process to distillates—meeting the challenge[C/CD]. NPRA Annual Meeting, AM-01-14, New Orleans 2001-03-19
[8] David L, Dennis K, Peter S. Production of ultra-low sulfur gasoline: protecting important stream properties[C/CD]. NPRA Annual Meeting, AM-02-47, San Antonio, 2002-03-18
[9] Paul F M, Warren E, Ron B, et al. S Zorb gasoline sulfur removal technology-optimized design[C/CD]. NPRA Annual Meeting, AM-04-14, San Antonio 2004-03-28
[10] Schmidt R, Sughrue E L. NH3and HCl impact on sulfur removal from E-GasTMgasification streams using S ZorbTMGen. IV[J]. Fuel Process Technol, 2010, 91(6): 582-590
[11] Schmidt R, Tsang A, Cross J, et al. Laboratory simulated slipstream testing of novel sulfur removal processes for gasification application[J]. Fuel Process Technol, 2008, 89(6): 589-594
[12] Qiu L M, Zou K, Xu G T. Investigation on the sulfur state and phase transformation of spent and regenerated S Zorb sorbents using XPS and XRD[J]. Appl Surf Sci, 2013, 266: 230-234
[13] Xu G T, Diao Y X, Zou K, et al. Cause analysis of sorbent deactivation in S Zorb unit[J]. Petroleum Processing Petrochemicals, 2011, 42(12): 1-6 (in Chinese)
[14] Lin W, Wang L, Tian H P. An analysis of the formation rate of zinc silicate in S Zorb sorbents[J]. Petroleum Processing Petrochemicals, 2011, 42(11): 1-4 (in Chinese)
[15] Lin W. Influence of silica precursor and particle size of zinc oxide on the desulfurization activity of S Zorb sorbent[J]. Acta Petrolei Sinica (Petroleum Processing Section), 2012, 28(5): 739-743 (in Chinese)
[16] Ryzhikov A, Bezverkhyy I J, Bellat J P. Reactive adsorption of thiophene on Ni/ZnO: Role of hydrogen pretreatment and nature of the rate determining step[J]. Appl Catal B, 2008, 84(3/4): 766-772
[17] Bezverkhyy I, Ryzhikov A, Gadacz G, et al. Kinetics of thiophene reactive adsorption on Ni/SiO2and Ni/ZnO[J]. Catal Today, 2008, 130: 199-205
[18] Xu L, Zou K, Xu G T, et al. Investigation on structure, composition, and regeneration behavior of industrial S Zorb adsorbent[J]. Petroleum Processing Petrochemicals, 2013, 44(6): 44-48 (in Chinese)
[19] Meng X, Huang H, Shi L. Reactive mechanism and regeneration performance of NiZnO/Al2O3-diatomite adsorbent by reactive adsorption desulfurization[J]. Ind Eng Chem Res, 2003, 52(18): 6092-6100
[20] Long J, Tian H P, Lin W. Desulfurizing adsorbent, preparationmethod and use thereof: US 8202816B2[P]. 2012-06-19
[21] Long J, Tian H P, Lin W. Desulfurizing adsorbent, preparation method and use thereof. US 8222181B2[P]. 2012-07-17
[22] Zou K, Huang N G, Xu G T. Study of Rietveld Quantitative Phase Analysis of S Zorb Sorbent[J]. Acta Petrolei Sinica (Petroleum Processing Section), 2012, 28(4): 598-604 (in Chinese)
[23] Mucusker L B, Dreele R B, Cox D E. Rietveld refinement guidelines[J]. J Appl Cryst, 1999, 32: 36-50
[24] Snellings R, Bazzoni A, Scrivener K. The existence of amorphous phase in Portland cements: physical factors affecting Rietveld quantitative phase analysis[J]. Cement Concrete Res, 2014, 59: 139-146
[25] Zhang X, Xu G T, Huang N G. Formation Conditions of Willemite in S Zorb Sorbent[J]. Acta Petrolei Sinica (Petroleum Processing Section), 2013, 29(4): 619-625 (in Chinese)
[26] Puskas I, Fleisch T H, Full P R, et al. Novel aspects of the physical chemistry of Co/SiO2Fischer-Tropsch catalyst preparations. The chemistry of cobalt silicate formation during catalyst preparation or hydrogenation[J]. Appl Catal A, 2006, 311: 146-154
[27] Zhang Y L, Yang Y X, Han H X, et al. Ultra-deep desulfurization via reactive adsorption on Ni/ZnO: the effect of ZnO particle size on the adsorption performance[J]. Appl Catal B, 2012, 119-120: 13-19
[28] Sedighi M, Keyvanloo K, Towfighi J. Kinetic study of steam catalytic cracking of naphtha on a Fe/ZSM-5 catalyst[J]. Fuel, 2013, 109: 432-438
[29] Mandal P C, Wahyudiono, Sasaki M, et al. Non-catalytic vanadium removal from vanadyl etioporphyrin (VO-EP) using a mixed solvent of supercritical water and toluene: A kinetic study[J]. Fuel, 2012, 92(1): 288-294
[30] Takesue M, Hayashi H, Richard L, et al. Thermal and chemical methods for producing zinc silicate (willemite): A review[J]. Prog Cryst Growth Charact Mater, 2009, 55(3/4): 98-124
[31] Van der Voort P, D’Hamers I G, Vansant E F. Estimation of the distribution of surface hydroxyl groups on silica gel, using chemical modification with trichlorosilane[J]. J Chem Soc Faraday Trans, 1990, 86(22): 3751-3755
[32] Van Der Voort P, D’Hamers I G, Vrancken K C, et al. Effect of porosity on the distribution and reactivity of hydroxyl groups on the surface of silica gel[J]. J Chem Soc Faraday Trans, 1991, 87(24): 3899-3905
[33] Morrow B A, McFarlan A J. Surface vibrational modes of silanol groups on silica[J]. J Phys Chem, 1992, 96(3): 1395-1400
The Project “Third Generation Technology for Selective Hydrodesulfurization (RSDS-III) of FCC Naphtha” Passed SINOPEC’s Appraisal
On December 9, 2014 the scientific research project“Third-generation technology for selective hydrodesulfurization (RSDS-III) of FCC naphtha” jointly undertaken by the SINOPEC Research Institute of Petroleum Processing (RIPP), the Qingdao Refining and Chemical Company (QRCC), the Changling Petrochemical Branch Company (CBPC), and the Shanghai Petrochemical Company (SPC) has passed in Beijing the technical appraisal organized by the Science and Technology Division of the Sinopec Corp.
The RSDS-III technology, which is designed to manufacture gasoline that can meet the China’s national V emission standard, covers the following core techniques, viz.: the technique for regeneration of caustic liquid used in the enhanced fixed-bed Merox unit and the high-activity regenerated Merox catalyst ARC-01, the technique for deep reverse extractive desulfurization by means of caustic liquid, the high-efficiency pipe mixer RSM-01 for reverse extraction, the technique for selective adjustment and control of hydrotreating catalyst, the designated hydrotreating catalyst RSDS-31, and the process capable of inhibiting mercaptan formation and olefins saturation.
The RSDS-III technology has been tested in commercial scale on the FCC naphtha selective HDS units of QRCC, CBPC and SPC to secure favorable results. In comparison with the existing technologies for HDS of FCC naphtha, the RSDS-III technology can guarantee less octane loss during production of gasoline meeting the national V emission standard to be on a par with the international advanced level.
date: 2014-12-26; Accepted date: 2015-01-09.
Lin Wei, Telephone: +86-10-82368891; E-mail address: linwei.ripp@sinopec.com.
杂志排行
中国炼油与石油化工的其它文章
- Study on Surface Adsorption and Inhibition Behavior of Corrosion Inhibitors Contained in Copper Foil Rolling Oil
- Synthesis of Petroleum Sulfonate Surfactant with Ultra-Low Interfacial Tension in Rotating Packed Bed Reactor
- Simultaneous Removal of H2S and Organosulfur Compounds from Lique fied Petroleum Gas Using Formulated Solvents: Solubility Parameter Investigation and Industrial Test
- Effect of Dodecylbenzene Sulfonic Acid Used as Additive on Residue Hydrotreating
- Sulfur Distribution during Hydrothermal Liquefaction of Lignite, Wheat Straw and Plastic Waste in Sub-Critical Water
- Simulation Optimization and Experimental Study of Cross-Wall Adiabatic Dividing Wall Column Used to Separate Hexane-Heptane-Octane System
