Soil microbial activity and nutrients of evergreen broad-leaf forests in mid-subtropical region of China
2015-06-05ZhangquanZengSilongWangCanmingZhangHongTangXiquanLiZijianWuJiaLuo
Zhangquan Zeng•Silong Wang•Canming Zhang•Hong Tang•Xiquan Li•Zijian Wu•Jia Luo
Soil microbial activity and nutrients of evergreen broad-leaf forests in mid-subtropical region of China
Zhangquan Zeng1•Silong Wang2,3•Canming Zhang1•Hong Tang4•Xiquan Li1•Zijian Wu1•Jia Luo1
To better understand the effects of forest succession on soil microbial activity,a comparison of soil microbial properties and nutrients was conducted between three forest types representing a natural forest succession chronosequence.The study compared a pine(Pinus massoniana)forest(PF),a pine and broadleaf mixed forest (MF)and an evergreen broadleaf forest(BF),in the Yingzuijie Biosphere Reserve,Hunan Province,China. Results showed that soil nutrients in the MFand BFplots were higher than in the PFplots.The range in microbial biomass carbon followed a similar pattern with BFhavingthe greatest values,522–1022 mg kg-1,followed by MF368–569 mg kg-1,and finally,PF193–449 mg kg-1.Soil nutrients were more strongly correlated with microbial biomass carbon than basal respiration or metabolic quotient.Overall,forestsuccession in the study site improved soilmicrobialproperties and soilfertility,which in turn can increase primary productivity and carbon sequestration.
qCO2·Soil microbial biomass C· Soil nutrient
Introduction
During succession forest ecosystems undergo a series of directional changes in community structure as well as chemical and microbial properties(Bakker et al.1997; Sheil 2001).Although there have been many studies on microbial properties in different forest ecosystems(Aikio et al.2000;Wang and Wang 2008;Wang et al.2010; Susyan et al.2011;Wang et al.2011),processes and changes that occur during forest succession are poorly understood,particularly in the subtropicalzones of China. Subtropicalzones were originally primary forests thathave been disturbed by human activities during the past centuries.Over the last few decades,secondary forest succession has resulted in an increase in the growth of young subtropical forests through the rapid conversion of abandoned and degraded lands in mountainous areas.This naturalreforestation provides a unique modelofa‘‘reverse transformation’’of ecosystems,allowing us to observe changes in the content and quality of soil nutrients and microbial components.
Microbial biomass carbon(C)is an important index of biological status in soil since it is both a source of labilenutrients and an agent of transformation and cycling of organic matter(Sicardi et al.2004).Forest succession influences microbial processes associated with carbon and nitrogen cycling in forest ecosystems due to differences in quality,quantity and decomposition rates of litter,which in turn affects the availability of resources for microbial growth(Mo et al.2003;Jiang et al.2009).Forest succession also impacts soil nutrients and microbial community structure,which affects soil microbial biomass C(MBC) utilization efficiency.Microbialmetabolic quotient(qCO2), described as the ratio of respired C to MBC,is considered an index for evaluating C utilization efficiency of soil microbes measured during a short-term incubation(Wardle and Ghani1995).Soilbasalrespiration is influenced by C input(Landi et al.2006)and microbial biomass.Consequently,highly productive forestecosystems willhave high soilbasalrespiration and decomposition rates owing to the large C input from the forest floor and root systems. Vegetation composition also affects soil basal respiration by altering soil microclimate and structure,litter quality, and soil microorganisms(Yan et al.2004).It is reasonable to expectthatthe greater biodiversity in pine and broadleaf mixed forest and evergreen broadleaf forest will increase tree productivity,and thus C inputs into soils,leading to increased microbial respiration.However,the effect of forest succession on microbial biomass and metabolic status in subtropicalforestsoils remains largely unknown. In this study,we measured and compared soilnutrients and microbial activity in three forest types representing three succession stages of forest recovery from pine forest(PF), to pine and broadleaf mixed forest(MF),and finally to evergreen broadleaf forest(BF).Our aim was to quantify changes in soil nutrients and microbial activity at each of these three stages of forestsuccession.
Materials and methods
Site description
The study area is located in the Yingzuijie Biosphere Reserve(26°46′–26°59′N,109°48′–109°58′E),Hunan Province.The reserve is home to 1798 recorded native species of higher plants and lies atthe transition zone between the Yunnan–Guizhou plateau and the mountains on the southern side of the Yangtze River.Altitude of the reserve ranges from 270 to 938 m above mean sea level.The climate of this region is humid mid-subtropical monsoon, with a mean annual temperature of 15.9°C,from 1990 to 2010.The mean annual precipitation was 1400 mm,of which 76%occurred between April and August.
In the reserve,there are three types ofnaturalvegetation communities:PF,MFand BF,ranging in age from 40 to 60 years.They represent a chronosequence of succession stages from pioneer to climax vegetation communities (Peng and Wang 1995).The soil texture is classified as a clay loam.The soilis predominantly derived from slate and shale,and classified as an Oxisol under the USDA taxonomy.From May to July 2010,three 20 m×20 m plots were established at each foresttype.
Soil sampling and analysis
Soil samples were collected at a depth of 0–10 and 10–20 cm from each plot.Each composite sample consisted of ten randomly collected sub-samples at the same depth in each plot.In total,six composite samples were taken in each forest type at two soil depths.Each sample was divided into two parts.One was sieved through a 2-mm mesh immediately and then stored at 4°C until analysis for microbialbiomass and basalrespiration for up to 3 days.The second part was air-dried,and ground.
Soilorganic C(SOC)and totalnitrogen were determined using Vario-MAX C/N auto-analyzer(made in Germany; Elementar).NH4+–N and NO3-–N were extracted with 1 mol L-1KCl solution,and then the filtered solution was measured by colorimetry.Totalphosphorus(P)wasanalyzed using the molybdate blue method afterdigestion with sulfuric acid and perchloric acid.Available P was measured with Olsen method(Olsen and Sommers1982).Fresh soilpH was measured using a portable pHmeter.Chloroform fumigationextraction method was used to estimate soil MBC(Wu etal. 1990).Basal respiration was determined by measuring CO2evolution(Chen and Xu 2004).Metabolic quotient or qCO2(μg CO2–C released mg-1biomass C h-1)was calculated as theratio ofbasalrespiration and MBC.Themicrobialquotient was calculated as the ratio of MBC to totalorganic C.
Statisticalanalysis
One-way analyses of variance(ANOVA)were used to test for significant differences in soil nutrients and microbial properties between PF,MFand BF.The least significant differences(LSD)were calculated when treatments were significantly different.The relationships between soil microbial properties and nutrients were analyzed.Analyses were performed with SPSS release 13.0,and the significant level was fixed at0.05.
Results
Soil nutrient
The concentrations of soil nutrients at both depths were slightly higher in the MFand BFplots compared to the PFplots(Table 1).However,significant differences in soil nutrients were only found in SOC,total N and DOC between plots.SOC in the BFplots was significantly higher than the MFand PFplots at both depths.Total N at each depth in the MFand BFplots was significantly higher than in the PFplots.At 0–10 cm soil depth,dissolved C increased in the following order:MF<PF<BF.SoilpH was significantly higher at a depth of 10–20 cm in the BFand MFplots than in the PFplots.

Table 1 Concentrations of nutrients in forestsoilunder Pinus(PF),Pinus mixed with broadleaf(MF)and under evergreen broadleaf(BF)in the Yingzuijie Biosphere Reserve,subtropical China
Soil microbialproperties
There were significantdifferences between plots in allsoil microbial properties except for basal respiration(Fig.1). Soil basal respiration ranged from 1.12 to 1.36 mg CO2–C kg-1h-1at 0–10 cm and 0.57 to 0.85 mg CO2–C kg-1h-1at 10–20 cm.MBC varied with forest succession and soil depth.MBC ranged from 449 to 1022 mg kg-1at 0–10 cm and 193 to 522 mg kg-1at 10–20 cm(Fig.1).The values of MBC at each depth increased in the following order:PF<MF<BF.The values ofqCO2ranged from 1.33 to 2.97 mg CO2–CgCmic-1h-1(Fig.1)and decreased in the following order at both depths:PF>MF>BF.The microbial quotient in the BFand MFplots was significantly higher compared to the PFplots.
Relationships between soil microbialproperties and nutrients
MBC and microbial quotient responded similarly to stand composition.There were significantly positive relations between MBC and SOC,TN,NO3-–N and available P in the forest soils(p<0.05).The relationships between basal respiration and dissolved C,NO3-–N and available P were significant.The qCO2was significantly negatively correlated with SOC and total N(Table 2).
Discussions
Forest succession can improve soil properties and increase fertility(Campbell et al.1994).The restoration of soil fertility by succession is a complicated ecological process which is affected by many biotic and abiotic variables.Soil nutrients,especially available nutrients,are closely linked to plant biomass and are often used as indicators of soil fertility(Moria 2002).Total N concentration in PFwas significantly lowerthan otherforesttypes,indicating thatN may be a limiting factor for pine(Pinus massoniana) growth.With forestsuccession,soilchemicaland microbial concentrations in the MFand BFplots were elevated compared to the PFlikely due to increased litter inputs and low incorporation rates of surface litter into the soil.Some research has suggested thatthe amountoflitterfallin MFor BFis much higher than in pure coniferous forests(Chen and Wang 2004).Additionally,the quality of litter controls decomposition rates,which in turn affects nutrientrelease into forestsoils.Our results provide evidence thatnutrients returning to soil through litter degradation were higher in MFor BFthan in PF,suggesting that the quality of litter is improved by the colonization ofbroadleafspecies.The shift in species composition during forest succession alters soil pH.Conifers dominate early successional stages inputting litter that contains large amounts of phenolic compounds and lignin yielding acid residues,lowering soil pH and slowing decomposition rates(Scholes and Nowicki 1998).
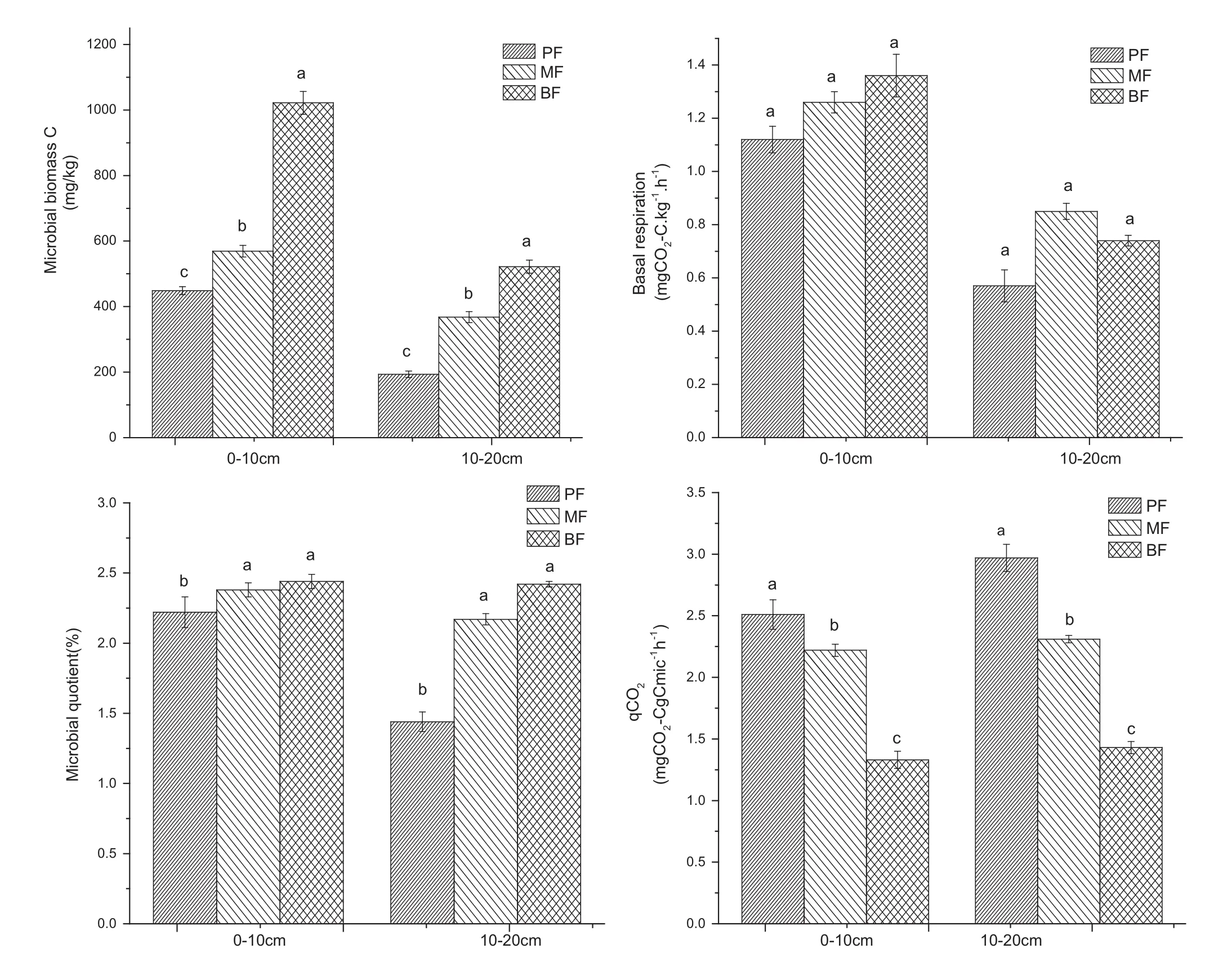
Fig.1 Microbialbiomass C,basalrespiration,microbialquotientand qCO2in forestsoilunderpine forest(PF),pine and broadleafmixed forest (MF)and evergreen broadleaf forest(BF)plots at 0–10 and 10–20 cm depths
The values of MBC in PF,MFand BFwere within the reported range(530–822 mg kg-1)for the other soils studied in the subtropics(Yi et al.2005),but were lower than the reported range(102–2073 mg kg-1)for various temperate and tropical forest soils(Hernot and Robertson 1994).The MBC values recorded in the PFplots in the presentstudy were lower than thatof the MFand BFplots. This indicates an increase in microbial biomass during succession.Microbial biomass appears to be closely linked to aboveground plant productivity in many ecosystems (Zak et al.1994)suggesting that the biomass of microbes depends directly on inputs ofcarbon to the soil.Ourresults supportthis theory as microbialbiomass was greatestin the more productive BFplots(Zeng et al.2013).Our results also indicate that the changes in MBC across the chronosequence are similar to changes observed in SOC. Diaz-Ravina etal.(1988)showed thatsoilwith low organic C has less microbial biomass,and vice versa.Since total soil C and MBC are intimately correlated(Insam and Domsch 1988),changes in totalsoil C resultin comparable changes in MBC.The more productive BFor MFplots showed an increase in soil C because of the increased C input from roots and litter resulting in increased available carbon.Soil MBC has been reported to be significantly influenced by litter diversity(Bardgett 1999).
Microbial metabolic quotient,qCO2,is the ratio of respired C to biomass C.It is considered an index for evaluating substrate utilization efficiency of soil microbial communities(Insam 1990).It is a sensitive indicator of stress and is used to assess the process of soildevelopment or degradation(Insam et al.1989).The more efficiently microorganisms function,the more substrate C is incorporated into biomass and less C per unit biomass is lost through respiration,resulting in a low metabolic quotient (Behera and Sahani 2003).Thus,the higher qCO2in the PFplots reflects a decrease in the efficiency of substrate utilization by the soilmicrobialcommunity.The low microbial C utilization in PFwas consistent with an earlier reportby Mu(2004),who found thatsoilCO2efflux in a mixed forest was significantly lower than in a deciduous,and coniferous forest.A possible explanation for this discrepancy is achange in soil pH.Anderson and Domsch(2010)reported that there was an increased qCO2and a decreased Cmic–Corg ratio under acidic conditions.Some broadleaf trees in the MFor BFplots have been shown to decrease soil pH. However,this effect is not appreciable and requires continued long-term research(Anderson and Domsch 2010).
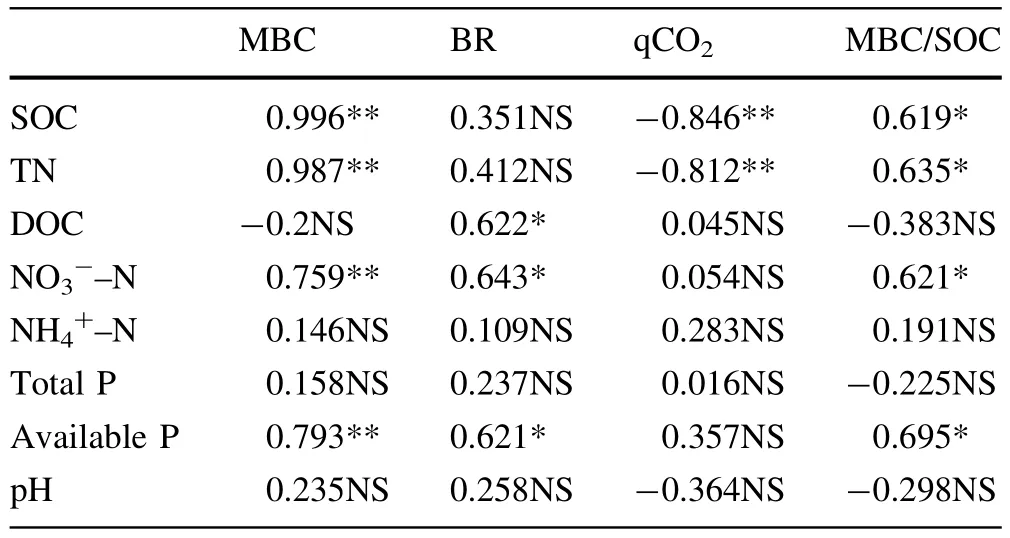
Table 2 Pearson correlation coefficients of soil nutrients and microbial properties under the pine forest(PF),the pine and broadleaf mixed forest(MF)and the evergreen broadleaf forest(BF)in the Yingzuijie Biosphere Reserve,subtropical China
Conclusions
The chronosequence of PFto MFto BFresulted in an increase in SOC,total N,NO3-–N,available P,and MBC. Forest succession affects soil microbial biomass through changes in forest structure and biodiversity.The contribution of MBC to SOC was lowestin PFsites.In addition, MBC was strongly correlated to SOC,total N and available P.It could be concluded thatsuccession from PFto MFto BFresulted in an increase in soil microbial biomass and soil fertility.In other words,the increase of soil nutrients indicates the restoration of degraded forest soil.To gain a more complete understanding,future research of the impacts of forestsuccession on soilmicrobialactivity should incorporate the influence of changing abiotic factors. Overall,our research suggests that forest management practices such as enclosures,selective cutting and promoting by shrubs should be reformed to accelerate the succession ofconiferous forestinto evergreen broad-leaved forestin order to restore soil fertility.
AcknowledgmentsWe thank Deng Shijian for help in instructing and collecting samples and Yu Xiaojun for analyses for some parameters,and other colleagues who participated in the field work.We would also like to thank Alison Beamish at the University of British Columbia for her assistance with English language and grammatical editing of the manuscript.
Aikio S,Va¨re H,Stro¨mmer R(2000)Soil microbial activity and biomass in the primary succession ofa dry heath forest.SoilBiol Biochem 32:1091–1100
Anderson TH,Domsch KH(2010)Soil microbial biomass:the ecophysiological approach.Soil Biol Biochem 42:2039–2043
Bakker SA,Jasperse C,Verhoeven JTA(1997)Accumulation rates of organic matter associated with different successional stages from open waterto carrforestin formerturbaries.PlantEcol129:113–120
Bardgett RD(1999)Shine A.Linkages between plantlitter diversity, soil microbial biomass and ecosystem function in temperate grasslands.Soil Biol Biochem 31:317–321
Behera N,Sahani U(2003)Soil microbial biomass and activity in response to Eucalyptus plantation and natural regeneration on tropical soil.For Ecol Manag 174:1–11
Campbell BM,Frost P,King JA,Mawanza M,Mhlanga L(1994)The influence of trees on soil fertility on two contrasting semiarid types at Matopos,Zimbabwe.Agrofor Syst 28:159–172
Chen CY,Wang SL(2004)Ecology of mixed plantation forest. Science Press,Beijing,p 3(in Chinese)
Chen CR,Xu ZH(2004)Soilcarbon and nitrogen pools and microbial properties in a 6-year-old slash plantation of subtropical Australia:impacts of harvest residue management.For Ecol Manag 206:237–247
de la Moria PJ(2002)Soil quality:a new index based on microbiological and biochemical parameters.Biol Fertil Soils 35:302–306
Diaz-Ravina M,Caraballas T,Acea MJ(1988)Microbialbiomass and activity in four acid soils.Soil Biol Biochem 20:817–823
Hernot J,Robertson GP(1994)Vegetation removalin two soils ofthe humid tropics:effect on microbial biomass.Soil Biol Biochem 26:111–119
Insam H(1990)Are the soil microbial biomass and basal respiration governed by the climatic regime?SoilBiolBiochem 22:525–532
Insam H,Domsch KH(1988)Relationship between soil organic carbon and microbial biomass on chronosequences of reclamation sites.Microb Ecol 15:177–188
Insam H,Parkinson D,Domsch KH(1989)The influence of microclimate on soilmicrobialbiomass level.Soil Biol Biochem 21:211–221
Jiang JP,Xiong YC,Jiang HM,Ye DY,Song YJ,Li FM(2009)Soil microbial activity during secondary vegetation succession in semiarid abandoned lands of Loess Plateau.Pedosphere 19(6):735–747
Landi F,Valori J,Ascher J,Renella G,Falchini L,Nannipieri P (2006)Rootexudates effects on the bacterial communities,CO2evolution,nitrogen transformations and ATP content of rhizosphere and bulk soils.Soil Biol Biochem 38(3):509–516
Mo JM,Brown S,Peng SL,Kong GH(2003)Nitrogen availability in disturbed,rehabilitated and mature forests of tropical China.For Ecol Manag 175:573–583
Mu S(2004)Respiration of soils under temperate deciduous, coniferous and mixed forest.Acta Pedologia Sinca 41(4):564–570(in Chinese)
Olsen SR,Sommers LE(1982)Phosphorus.In:Page AL,Miller RH, Keeney DR(eds)Methods ofsoilanalysis.Part2.Agronomy no. 9.American Society of Agronomy,Madison,pp 403–430
Peng SL,Wang BS(1995)Forest succession at Dinghushan, Guangdong,China.Chin J Bot 7(1):75–80(in Chinese)
Scholes MC,NowickiTE(1998)Effects ofpines on soilproperties and processes.In:Richardson DM(ed)Ecology and biogeography of Pinus.Cambridge University Press,Cambridge,pp 341–353
Sheil D(2001)Long-term observations of rain forestsuccession,tree diversity and response to disturbance.Plant Ecol 155:183–199
Sicardi M,Garcia-Prechac F,Frioni L(2004)Soil microbial indicators sensitive to land use conversion from pastures to commercial Eucalyptus grandis(Hill ex Maiden)plantations in Uruguay.Appl Soil Ecol 27:125–133
Susyan EA,Wirth S,Ananyeva ND,Stolnikov EV(2011)Forest succession on abandoned arable soils in European Russia—impacts on microbial biomass,fungal-bacterial ratio,and basal CO2respiration activity.Eur J Soil Biol 47:169–174
Wang QK,Wang SL(2008)Soilmicrobialproperties and nutrients in pure and mixed Chinese plantations.J For Res 19(2):131–135
Wang SL,Zhang WD,Sanchez F(2010)Relating net primary productivity to soil organic matter decomposition rates in pure and mixed Chinese fir plantations.Plant Soil 334:501–510
Wang B,Zhu B,Liu G,Xue S(2011)Changes in soil physicochemical and microbiological properties during natural succession on abandoned farmland in the Loess Plateau.Environ Earth Sci62:915–925
Wardle DA,Ghani A(1995)A critique of the microbial metabolic quotient(qCO2)as a bioindicator of disturbance and ecosystem development.Soil Biol Biochem 27:1601–1610
Wu J,Joergensen RG,Pommerening B,Chaussod R,Brookes PC (1990)Measurementof soilmicrobialbiomass C by fumigationextraction:an automated procedure.Soil Biol Biochem 22:1167–1169
Yan SK,Wang SL,Yu XJ(2004)Effect of mixtures with alders on soilfauna in plantation forestof Chinese fir.Chin JApplEnviron 10:462–466(in Chinese)
Yi ZG,Yi WM,Zhou LX,Wang XM(2005)Soil microbial biomass of the main forests in Dinghushan Biosphere Reserve.Ecol Environ 14(5):727–729(in Chinese)
Zak DR,Tilman D,Parmenter RR,Rice CW,Fisher RM,Vose J, Milchunas D,Martin CW(1994)Plant production and soil microorganisms in late-successional ecosystems:a continentalscale study.Ecology 75:2333–2347
Zeng ZQ,Wang SL,Zhang CM,Gong C,Hu Q(2013)Carbon storage in evergreen broad-leaf forests in mid-subtropicalregion of China atfour succession stages.J For Res 24(4):677–682
7 January 2014/Accepted:16 May 2014/Published online:26 May 2015
©Northeast Forestry University and Springer-Verlag Berlin Heidelberg 2015
Projectfunding:This work was supported by International Science& Technology Cooperation Program of China(2012DFB30030), Strategic Priority Research Program of the Chinese Academy of Sciences(XDA05050205),Natural Science Foundation of Hunan province(2015JJ6050);Hunan forestry science and technology program(XLK201417),Youth Innovation Fund of Hunan Academy of forestry(2013LQJ08),and The Twelfth Five-Year Plan in national science and technology forthe environmentfield(2012BAC09B03-4).
The online version is available at http://www.springerlink.com
Corresponding editor:Chai Ruihai
✉Silong Wang slwang@iae.ac.cn
1Hunan Academy of Forestry,Hengshan Station of Forest Ecology,Changsha 410004,China
2Huitong Experimental Station of Forest Ecology,State Key Laboratory of Forest and Soil Ecology,Institute of Applied Ecology,Chinese Academy of Sciences,Shenyang 110164, China
3Huitong National Research Station of Forest Ecosystem, Huitong 418307,China
4College of Environment and Life Science,Kaili University, Kaili556011,China
杂志排行
Journal of Forestry Research的其它文章
- Management of pests and diseases of tropical sericultural plants by using plant-derived products:a review
- Gamma generalized linear model to investigate the effects of climate variables on the area burned by forest fire in northeast China
- Diversity,abundance,and structure of tree communities in the Uluguru forests in the Morogoro region,Tanzania
- Brazilian savanna re-establishment in a monoculture forest: diversity and environmental relations of native regenerating understory in Pinus caribaea Morelet.stands
- Carbon storage and sequestration rate assessment and allometric model development in young teak plantations of tropical moist deciduous forest,India
- Use of infrared thermal imaging to diagnose health of Ammopiptanthus mongolicus in northwestern China
