Carbon storage and sequestration rate assessment and allometric model development in young teak plantations of tropical moist deciduous forest,India
2015-06-05KaushalendraKumarJha
Kaushalendra Kumar Jha
Carbon storage and sequestration rate assessment and allometric model development in young teak plantations of tropical moist deciduous forest,India
Kaushalendra Kumar Jha1
Carbon(C)sequestration through plantations is one of the importantmitigation measures for rising levels of carbon dioxideand othergreenhouse gasesin the atmosphere. This study aimed to assess C stocks and their sequestration rate,and to develop allometric models for estimation of C stocking in age-series young teak(Tectona grandis)plantations(1,5,11,18,24 and 30 years)by using biomass and productivity estimation and regression,respectively.These plantations were raised in tropicalmoistdeciduous forests of Kumaun Himalayan tarai.TotalC stocks estimated for these plantationswere 1.6,15.8,35.4,39.0,61.5 and 73.2 Mg ha-1, respectively.Aboveground and belowground C storage increased with increasing plantation age;however,the range of their percentage contribution showed little variation (87.8–88.2 and 11.7–12.7%,respectively).The rate of C sequestration forthese respective plantations was 1.06,6.95, 5.46,5.42,3.39 and 5.37 Mg ha-1a-1.Forty percentof the aboveground annual storage was retained in the tree while 60%wasreleased in the formoffoliage,twigs,and fruitlitter. In the case oftotal(tree)annualproduction,43%wasretained while 57%was released as litter including root.C stock,C sequestration rate,accumulation ratio(1.4–18.1),root:shootC ratio(0.61–0.13)and production efficiency(0.01–0.18)were comparable to some previous reports for other species and forests.These data could be usefulin deciding the harvesting age foryoung teak with respectto C storage and sequestrationrate.Fourallometric modelsusing linearregression equations were developed between biomass(twice the C stock)and diameter,girth,and heightof the tree atdifferentages.The diametermodelwas found more suitable for C stock prediction in similarareas.
Age series·Biomass·Carbon budget·DBH· Productivity·Production ratios·Regression equations· Tectona grandis
Introduction
An issue of globalconcern today is the rapidly increasing level of carbon dioxide(CO2)in the atmosphere and its potential to change the world climate.The concern over rising levels of CO2and othergreenhouse gases(GHGs)in the atmosphere was addressed at the third meeting of the United Nations Framework Convention on Climate Change (UNFCCC 2008)in Kyoto.The results of this convention led to an agreement to reduce rising levels of CO2and other GHGs in the atmosphere and the Kyoto protocol proposed carbon(C)reduction through decreasing fossil fuel emission or accumulating C in vegetation and soil (Oelbermann et al.2004).Further,CoP 7 of UNFCCC in Bonn,Germany,in July 2001 decided to include afforestation and reforestation as an effective way to reduce atmospheric C by building up terrestrial C stocks.In this context,the quantity of C storage at a given time and the rate of sequestration become important along with the varying capacity of tree species to sequester C.C accumulation or rising CO2mitigation could be achieved by planting millions of hectares of new forests(Dixon et al. 1994;Dabas and Bhatia 1996;Kraenzeletal.2003)of,for example,long rotation species such as teak.
Teak(Tectona grandis)is one of the five major planting species in the world(Krishnapillay 2000;Sreejesh et al. 2013).Due to a rising trend in the demand for teak products (Behaghel1999),plantations may create a huge C sink in the future.Forest plantation teak has ten times higher growth than naturalforestteak(Mohdar and Zuhaidi2005)and the duration of retention of C in teak wood products is longer than formany othertimberspecies(Eliyanietal.2005).Teak has traditionally been a long-rotation species planted in forests over large areas in several tropical countries,including India.Private enterprises grow teak and offer shares to the public for investment,promising high returns within periods of 20–30 years.Market forces and economic pressures have encouraged shortrotations and have reduced the commercial rotation age of teak to 15 years(Keh 1997; Keogh 2000).Although young plantations can sequester relatively large quantities of C(Sreejesh et al.2013),apprehensions regarding growth projections have yet to be ruled out since there is a lack of data on the accumulation pattern of biomass and production potential(Jha and Singh 1999)or C sequestration in young teak plantations.
The measurement of sequestered C at any point in time would need the non-harvesting method of biomass assessment since the harvesting method,although more accurate,is time consuming and cumbersome(Ketterings et al.2001).A common method of biomass assessment is through the use of allometric equations which relate the biomass of trees to easily obtainable nondestructive parameters(Ketterings et al.2001;Singh et al.2011;Kridiborworn et al.2012;Russo et al.2014)such as diameter, girth,and height.Forms of allometric equations vary widely but the one most commonly used is the linear equation(Dudley and Fownes 1992).
The aim of this study was to analyze C sequestration in teak plantations by assessing biomass and productivity of young trees and their compartmentalization of C in different tree components.I compared the results with the same or with other species in similar or different growth zones.This study also aimed to develop and assess the suitability of regression-based models for quantifying relationships between C stock(biomass)and tree variables of diameter,girth,and height.
Materials and methods
Study site
The study was executed in teak plantations of 6 ages(viz., 1,5,11,18,24 and 30 years)in the tarai region of Kumaun Himalaya.The study sites were at29°3′to 29°12′N latitude and 79°20′to 79°23′E longitude at elevations of 230–280 m(Fig.1;Jha 2014).The natural vegetation of this area is‘‘alluvialsavannah woodland—type 3/1S1,with some pockets of moist Tarai Sal—type 3c/C2c’’(Champion and Seth 1968).The climate is subtropical and monsoonalwith long dry(8 months)and shortwetseasons.Ten year data(1985–1995)show that mean monthly temperature ranged from 14.4 to 31.3°C and annual average rainfall was 1593 mm.Average humidity in the locality was 65%.The soilofthis region is a Typic Hapludollwith a fine loam texture(Kumar and Sharma 1990).Jha(2014) and Jha and Singh(1999)provide more detailed descriptions of the study site.
Carbon stock estimation
Estimates of biomass are essential for estimating productivity and C fluxes,which provide means of assessing C sequestration in wood,leaves and roots(Cooper 1983; Chambers et al.2001;Specht and West 2003).C stock estimation was done through the destructive method of biomass estimation and the use of a conversion factor (Gower et al.2001;Nowak and Crane 2002;Terakunpisut etal.2007).Harvesting and measurements were carried out in September 1992 for herbs,October 1992 for shrubs and November1992 for trees,and were followed by laboratory works.
Biomass estimation for trees followed Lodhiyal et al. (1995).Imeasured tree diameteratbreastheight(DBH)of all trees in all the six sample plots using metallic calipers. Tree girth(GBH)was also measured using metallic tape at 1.37 m from ground level(in a 1 year old plot at 50 cm heightsince this is considered equivalentto breastheightin very young trees).Trees were then categorized into DBH classes of 00–5;5.1–10;10.1–15;15.1–20;20.1–25; 25.1–30;30.1–35;35.1–40;40.1–45;45.1–50;50.1–55 cm and three representative trees from each ofthe classes in all plantations were harvested at ground level.In a couple of cases,however,due to inadequacy of samples only one tree was harvested.The length of the harvested trees(tree height,H)was measured using a metallic field tape.The fresh weight of tree components(trunk,bark,branches, twigs,leaves,dug out stump roots and lateral roots)was determined using a field balance.Fresh samples of each componentwere weighed and broughtto the laboratory for drying in an oven at 60°C to constant weight.Using this fresh weight/dry weightfactor,the dry weightfortrees was estimated.The data were subjected to regression analysis (y=a+bx,where y is the dry weight in kg and x is the diameter in cm)(Chaturvedi and Singh 1982).The mean diameter value for each diameter class was used in the regression equation of the different components to obtain an estimate of mean biomass.This was multiplied by the density in that diameter class.For shrub biomass the total number of stems of shrubs was counted in each plantation.All shoots coming up from ground level were treated as independent individuals.The green weight of above and below ground parts was estimated.The samples were dried in an oven at 60°C to constant weight and the biomass value for each species was calculated by multiplying itby density per hectare.The monolith method was used for estimating the herbaceous biomass in each plantation.All samples in five 50 cm×50 cm monoliths were segregated by species.They were weighed,dried and multiplied by density per hectare.The summed values of allspecies were taken as the herb biomass of the stand.
Fig.1 Location of the study area(Jha 2014)
The biomass of trees,shrubs and herbs was summed to calculate the stand biomass,which was reduced to C stock by a C factor.Forherbs and shrubs the globalfactor0.5,as suggested in UNFCCC guidelines(Lamlom and Savidge 2006;Takimoto et al.2008;Basuki et al.2009)was used, but for trees(Tectona grandis)the regional and speciesspecific factor(0.52)reported by Jha(2005)was used to obtain a more accurate estimation of C stock atthe present study site.
Carbon sequestration rate
C sequestration rate(CSR)was estimated through the net annual productivity(NAP)assessment and the use of a conversion factor.The NAP assessmentprotocolof Bargali etal.(1992)was followed,which included measurementof DBH of all the trees of the sample plots in May 1992(B1) and May 1993(B2).Annual biomass accumulation was calculated(ΔB=B2-B1)using the allometric equation (y=a+bx)developed in biomass studies.To obtain NAP,annual litter fall(leaves,twigs and fruits)and root necromass(15%of rootbiomass as reported by Singh and Srivastava 1984 in dry deciduous teak)were added to it.
Two to three average sized shrubs selected in May 1992 were re-measured and harvested for biomass calculation.By subtracting average shrub biomass of 1992 from average shrub biomass of 1993 the net average shrub biomass was quantified for each species.These were multiplied by their respective densitiesto estimate the totalannualshrub biomass by stand.For herbs,netproductivity was estimated by summation of species peak biomass in the month of September 1993 assuming itwas equalto netproductivity ofherbs.
Net annual productivity of trees,herbs and shrubs was summed up to estimate stand NAP which was converted to C sequestration rate by using the factormentioned in the C stock estimation section.
Allometric models
Allometric models are important for estimation of biomass and C storage in terrestrial ecosystems(Litton and Kauffman 2008).Several studies have used biometric parameters like GBH,DBH,H and age as independent variables for developing biomass regression equations(Singh and Singh 1991;Bargali et al.1992;Karmacharya and Singh 1992; Lodhiyaletal.1995;Segura and Kanninen 2005;Litton and Kauffman 2008;Chaturvedi et al.2010;Feldpausch et al. 2011;Alvarez etal.2012;Chaturvedietal.2012;Lima etal. 2012;Chaturvedi and Raghubanshi 2013;Durkaya et al. 2013;Jansons et al.2013).Few reports on teak provide a sufficient number of regression equations between biomass and biometric parameters especially in young age-series plantations.In the present study four models,e.g.,(1)biomass(y)=intercept(a)+slope(b)×DBH,(2)biomass (y)=intercept(a)+slope(b)×H,(3)biomass(y)=intercept(a)+slope(b)×DBH2×H,and(4)biomass (y)=intercept(a)+slope(b)×GBH were formulatedand compared.R2was used as the indicator of goodness of fit.
Results and discussion
Carbon stock
Shrub C stock had a very low contribution,varying from 0.003 Mg ha-1at 30 years to 0.122 Mg ha-1at 5 years. Herbaceous C storage was even lower ranging between 8×10-8and 10×10-5Mg ha-1.These estimates being very low,tree C stock accounted for virtually allofthe stand C stock.The C stock data of different tree components at different ages are listed in Table 1.Tree C stock was 1.6, 15.8,35.4,38.9,61.5 and 73.2 Mg ha-1,respectively,for1, 5,11,18,24,and 30 yearsold plantations.Plantation age was directly proportionalto total,aboveground,and belowground C stocks.However,the percentage contribution of these stocks to the total stock of the tree did notvary widely with increasing age(exception 1 year old plantation).The ranges were 87.8–88.2%(above ground),and 11.7–12.7%(below ground).The belowground C stocking was comparable to Eucalyptus forest(10–12%;Feller 1980)and temperate forest(8–15%;Whittaker and Woodwell 1971;Nihlgard 1972;Larsen etal.1976)but much higher than Indonesian forest(5.6%;Herteletal.2009).
The percentage contribution of bark,twig and foliage was greater in younger trees and declined with maturity.At older ages crown development was less as compared to girth increment and C accumulation was concentrated in trunk wood.This trend supported the findings of Lodhiyal et al.(1995)who reported increased proportions of C in trunks and coarse roots with increasing age.
Literature review revealed variations in C stocks at differentagesin T.grandis plantationsgrown in varioustropical localities(Table 2).Aboveground C stock in the present study differed considerably ata similar ages even within the same regions in the country.The results of C assessmentby Singh and Gupta(1993)and Karmacharya and Singh(1992) were lower than those of the present study while those of George and Verghese(1992)and Chandrashekara(1996) were higher.Edapho-climatic factors at these study sites indicated that soil condition,precipitation and temperature, in combination orindependently,were relatively pooratthe former sites and better atthe latter,showing their influence on C sequestration.This conformed the hypothesis of Brown and Lugo(1982)and Murphy and Lugo(1986a)that forest biomass(C)in the tropics increased with increasing precipitation.Watanabe etal.(2009)recorded thatprecipitation influenced the heightand aboveground biomass(C)ofteak. This was further corroborated by Hase and Foelster(1983), Lugo and Figueroa(1985),Zech and Drechsel(1991)and Karmacharya and Singh(1992)who reported thatin addition to rainfall,temperature and soil characteristics also played important roles in biomass(C)accretion of T.grandis.Sequestered C data outside India further suggested that Panamanian,Venezuelan,Ghanaian and Nigerian conditions were more suited to teak growth as compared to India(Hase and Foelster 1983;Nwoboshi1984;Ola Adams 1983;Kraenzel etal.2003;Watanabe etal.2009).
Carbon sequestration rate
C sequestration rates(CSRs)in different plantations are listed by tree component in Table 1.Total tree CSRs at 1, 5,11,18,24 and 30 years were 1.06,6.95,5.46,5.42,3.39 and 5.37 Mg ha-1a-1,respectively.The proportions of aboveground and belowground production with changing age were similar.Aboveground contribution ranged from 84 to 90%in retained annual production compared to 92–95%in released annual production.Below ground ranges of retained and released production were 10–15 and 4–7%,respectively.Increasing amounts of released annual production were recorded with increasing plantation age. The percent contribution of different plant parts towards total annual sequestered C showed a wider range of variation.Bark ranged from 1.9 to 5.7%(3.1%),trunk 21.7–29.2%(26.1%),branch 2.7–4.5%(3.8%),twigs 6.3–12.0%(9.2%),foliage 47.1–55.3%(50.6%),fruits 0.3–1.9%(1.1%),above ground 92.9–95.7%(93.9%), stump root 2.0–3.9%(3.1%),lateral root 2.1–3.7% (2.8%)and below ground 4.2–7.1%(6.0%).
CSRs of important plantations and forest types are compared in Table 3.The CSR of 30 years old teak (5.38 Mg ha-1a-1)recorded in the presentstudy washigher than tropicaldry forest(Chaturvedietal.2011)butcloserto many other plantation species and forests such as Tectona grandis of other regions(Faruqui 1972;Sharma and Naik 1989;Karmacharya and Singh 1992),Cryptomaria japonica (Tadaki et al.1965),Populus deltoides(Kaul et al.1983), montane rain forest(Jordan 1971),Oak-Pine forest(Whittaker and Woodwell1968).The value from the presentstudy also fell within the global range of tropical dry forest (4–10.5 Mg ha-1a-1)reported by Murphy and Lugo(1986b) and centralHimalayan forests(5–10 Mg ha-1a-1)by Rana etal.(1989)butwaslowerthan CSRsforwettropicalforests (6.5–14 Mg ha-1a-1)reported by Murphy and Lugo(1986b) and regenerating teak forest(6.75–15.2 Mg ha-1a-1)reported by Swain and Bahera(1997).
C stock and CSR ratios
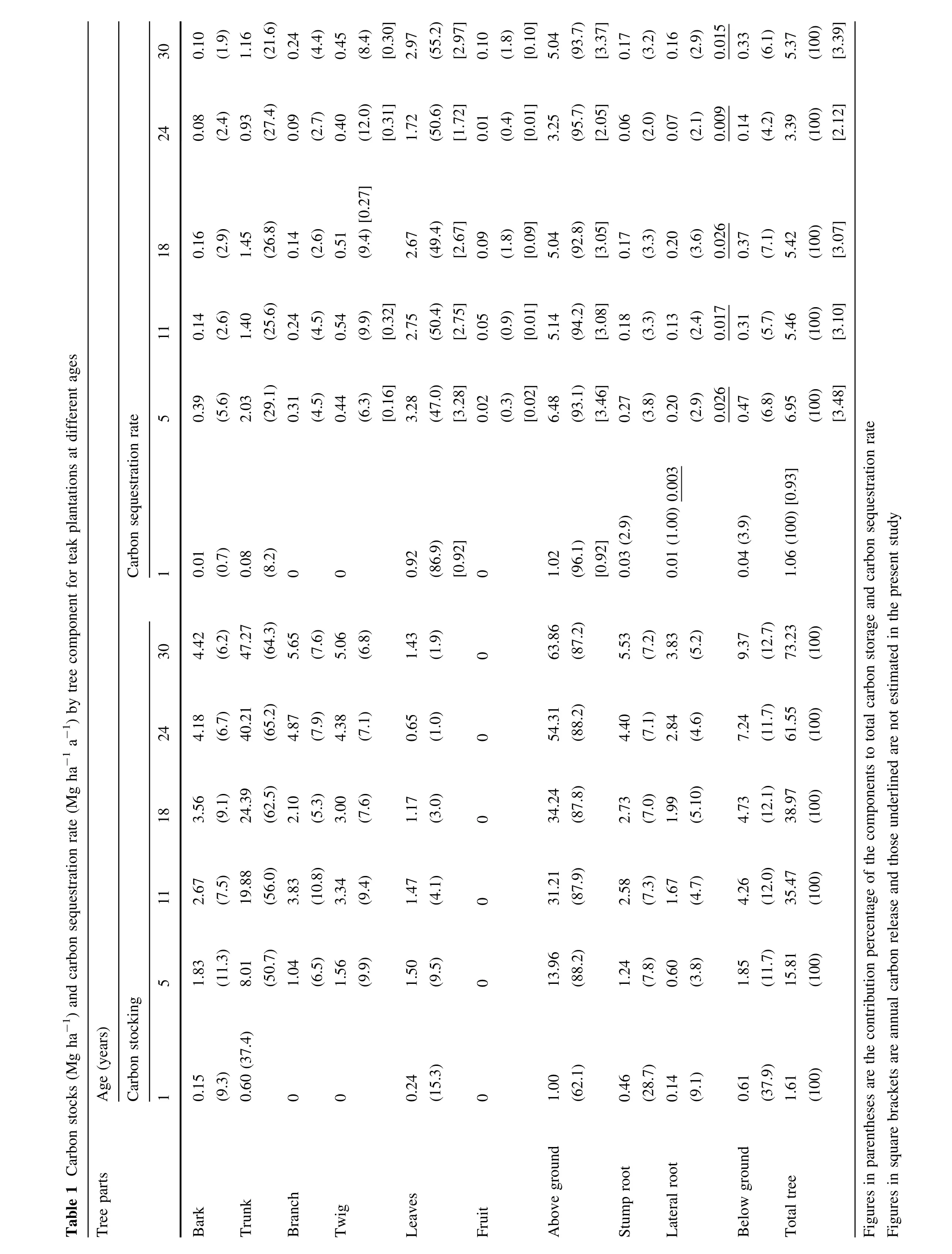
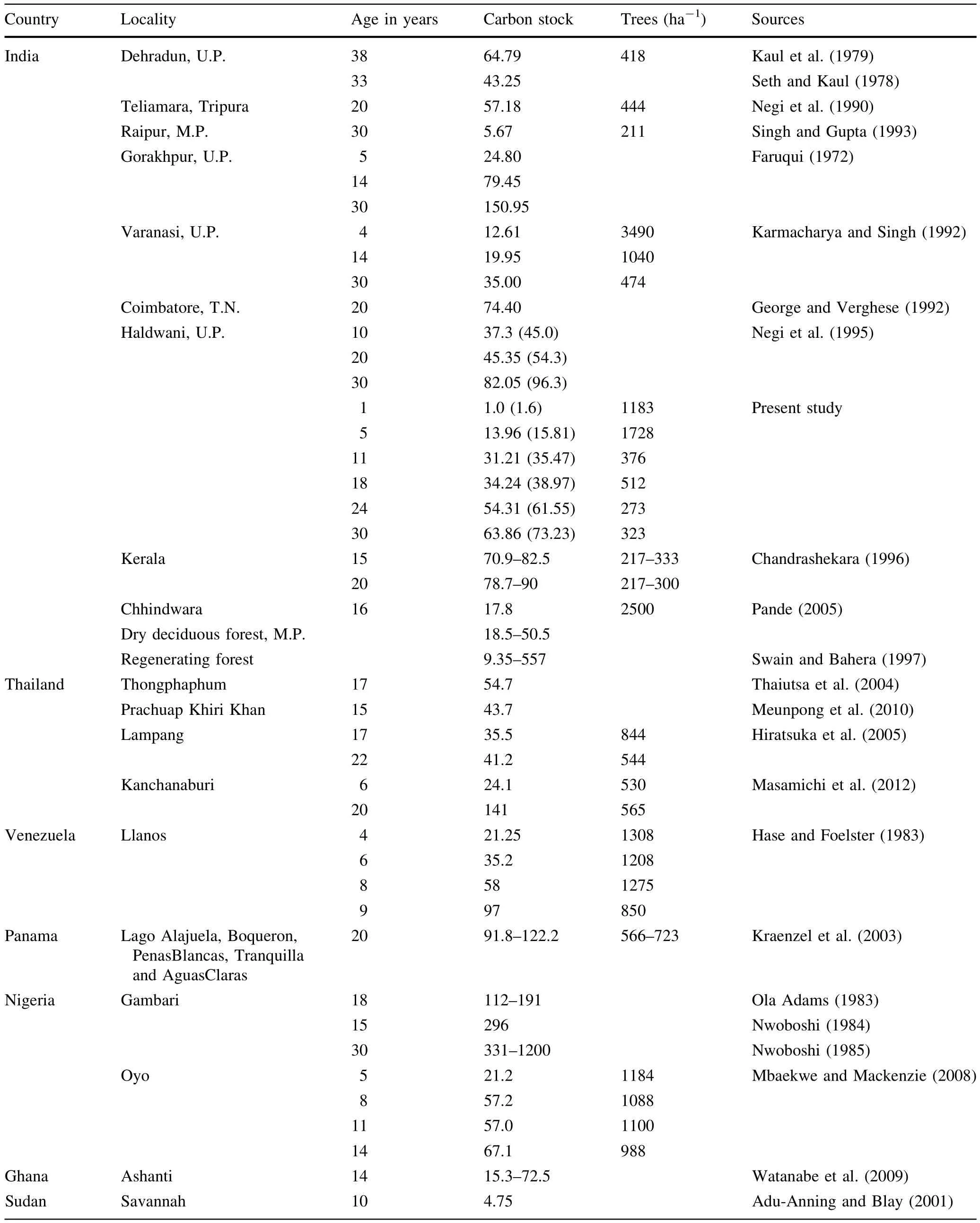
Table 2 Above ground carbon stocks(Mg ha-1)of teak plantations in various teak growing indigenous and exotic areas
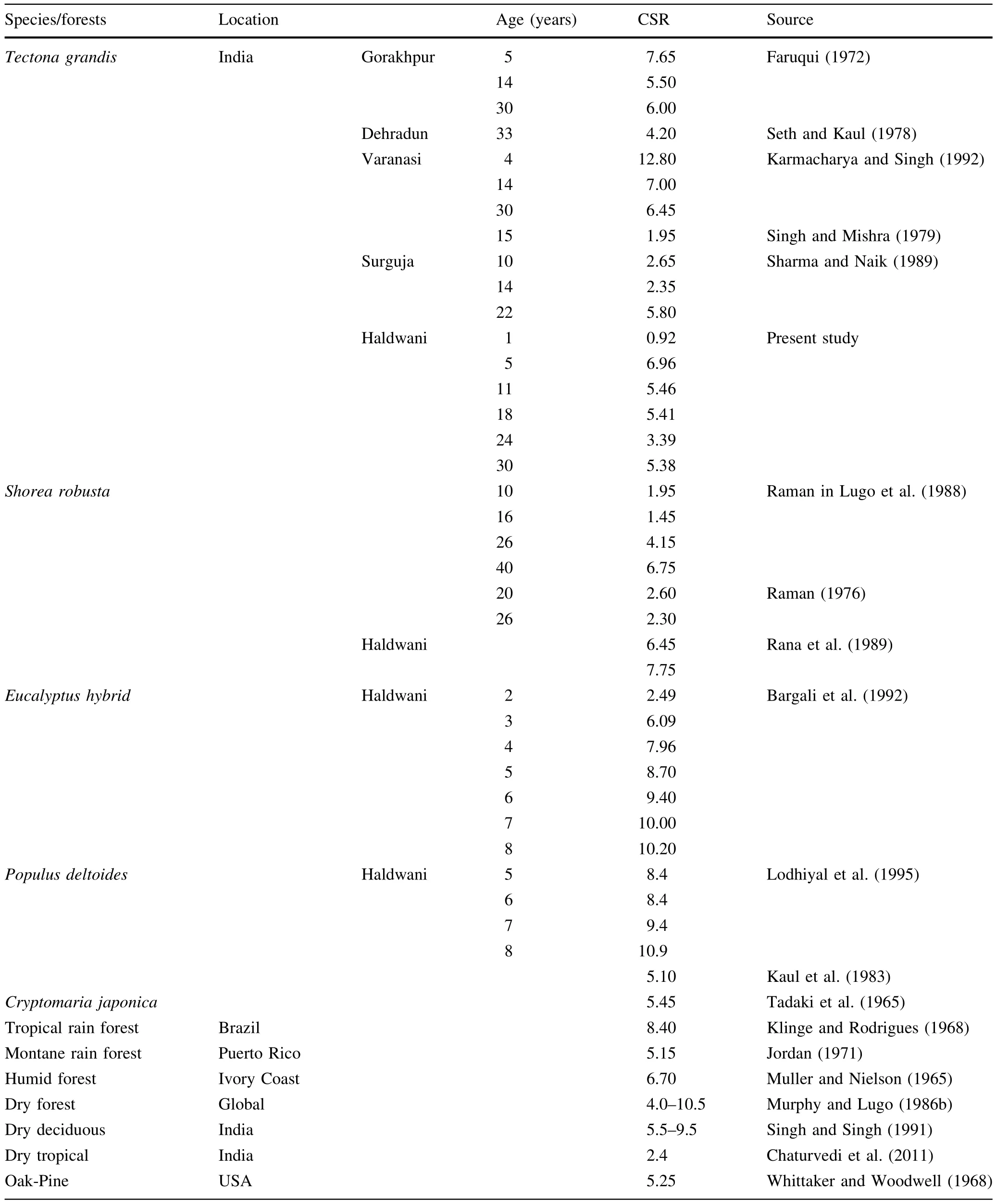
Table 3 Comparative statement of carbon sequestration rate(Mg ha-1a-1)by plantation age,in different regions,species and forests
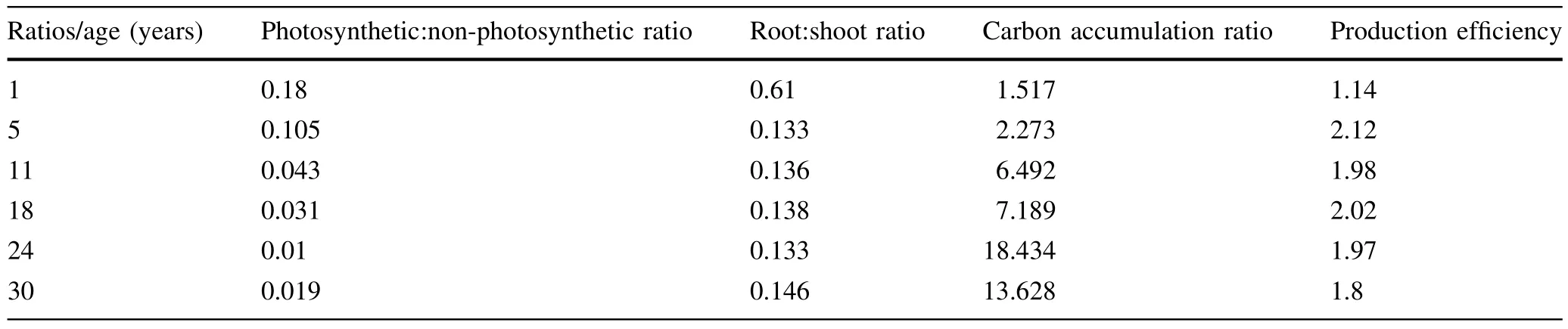
Table 4 Carbon stock and carbon sequestration rate ratios(photosynthetic:non-photosynthetic ratio,and root:shootratio ofcarbon stock,carbon accumulation ratio,annual total carbon/leaf carbon or production efficiency ratio)in age series teak plantations
Photosynthetic:nonphotosynthetic ratio(PNR),root:shoot ratio(RSR)and carbon accumulation ratio(CAR)expressed in terms of C stock and C sequestration rate are listed in Table 4.PNR,ranging between 0.01 and 0.18, declined with increasing plantation age in the present study.This indicated that the structural building of commercial components,and thereby C storage,was higher as compared to the foliage when the tree aged.Similarresults were reported by Bargali et al.(1992)for Eucalyptus, Lodhiyal et al.(1995)for Populus and Karmacharya and Singh(1992)for T.grandis.Gaucheretal.(2005)reported that a higher allocation of photosynthetic tissue(or carbon content)was found in younger seedlings of sugar maple and birch.
RSR ranged from 0.61 to 0.13 in the presentstudy.After an initial peak at the age of 1 year it plateaued from 5 to 30 years of age.This curve was a little differentfrom that of Prasad and Mishra(1984)for dry deciduous teak where a decreasing slope from seven to 20 years(0.40–0.10) changed to an increasing slope from 30 to 50 years (0.33–0.38)and then decreased from 65 to 120 years (0.32–0.26).For Nigerian teak,Nwoboshi(1984)reported RSR as 0.12 at the age of 30 years,similar to the present study.RSRs ofthe presentstudy were wellwithin the range of short rotation forest trees(0.94–0.14)such as Populus, Salix and Alnus as reported by Dickman and Pregitzer (1992).However,30 years old teak did not compare with 30 years(0.25)old Alnus nepalensis plantation(Sharma et al.2002),20 years(0.27)and 39 years(0.21)artificial Larix leptolepis,and 139 years(0.47)mature L.gmelinii forests(Kajimoto et al.1999).This could be due to different growing conditions of the stands.Moreover,the age of stand and nutrient status of soil(Scarascia-Mugnozza et al.1997;Kajimoto et al.1999;Sharma et al.2002; Agren and Franklin 2003;Greenwood et al.2007;Lim etal.2013),spacing oftrees and wateravailability(Barton and Montagu 2006)have been recognized as factors affecting rootbiomass accumulation.Wang etal.(2008)and Luo et al.(2012)suggested that RSR also depended on forest type and climate.
Biomass(C)accumulation ratio is used to characterize the production condition in communities(Whittaker 1966; Whittaker and Woodwell1969).A higher C accumulation ratio(CAR)suggests more production of timber or C sequestration.In the present study,total CAR ranged between 1.5 and 18.4,increasing with plantation age.This was supported by Bargali et al.(1992),Karmacharya and Singh(1992),Lodhiyal et al.(1995,2002)and Sharma et al.(2002)in Eucalyptus,T.grandis,Populus deltoides, Dalbergia sissoo and Alnus nepalensis,respectively.The C accumulation ratio of 30 years old moist deciduous teak in the present study was higher than that for dry deciduous teak(Karmacharya and Singh 1992),sodic land Casuarina (Rana etal.2001),dry deciduous forest(Singh and Mishra, 1979)and pine forest(Chaturvedi and Singh 1987).
The production efficiency values(the netproduction per unitweightofleaf,Johnson and Risser1974)in the present study expressed in terms of annual sequestered tree and leaf C were 1.14,2.12,1.98,2.02,1.97 and 1.80 for1,5,11, 18,24 and 30 years,respectively.This showed an initial peak and then a decreasing slope,similar to the finding of Sharma et al.(2002)for Alnus-cardamum plantation. Karmacharya and Singh(1992)reported a decreasing trend with increasing age of a teak stand.The above reported values were much lower as compared to Karmacharya and Singh(1992)and Sharma et al.(2002)for T.grandis (2.12–2.96)and A.nepalensis(3.6–6.3),respectively.
Allometric models for C stock estimation
I initially used pooled data from all plantations of different ages(1–30 years)to formulate linear regression equations for biomass assessment(bark,branch,trunk,twig,foliage, above ground,stump root,lateral root,below ground and totaltree).Allten equationswere non-significant(p>0.05). Therefore,polynomial regression equations were used to analyze the same parameters.All resulting equations for biomass estimation for one to 30 years old plantations were highly significant(p<0.01).The equations are presented in Figs.2 and 3 along with the scatter diagrams.

Fig.2 Scatter diagram of above ground biomass of different tree components with polynomial regression equations.All equations were significant(p>0.01).y Biomass in kg ha-1and x diameter in cm.Pooled data from all six age series plantations(1,5,11,18,24,30 years)
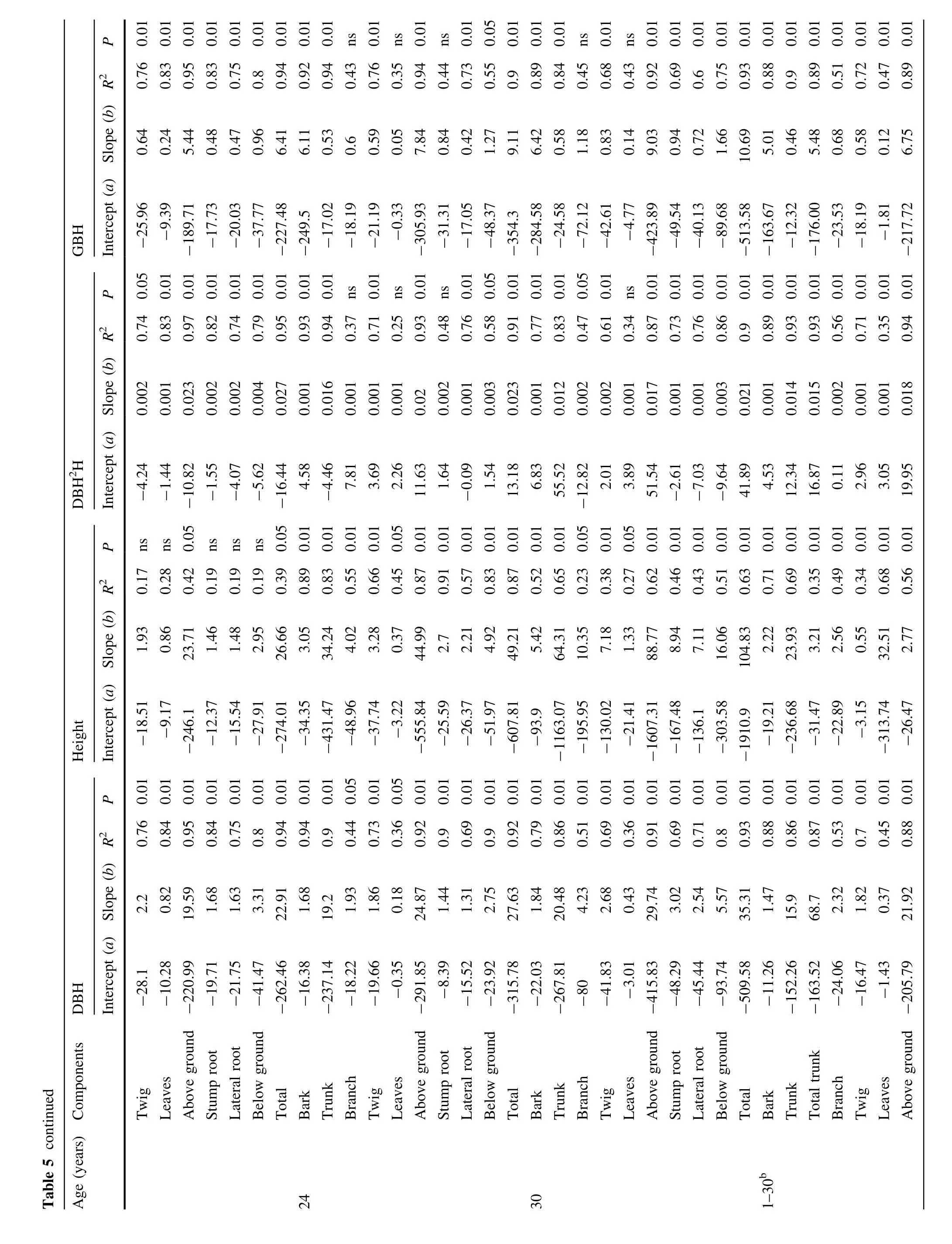
Linear regression equations(y=a+bx)were used for estimation oftree biomass components atdifferentageswith independentbiometric parameterssuch as DBH,height(H), DBH2H and GBH.The constants and other corresponding values are presented in Table 5.These equations could be modified to y=(a+bx)/2 for estimation of C storage.All four different models had 68 linear regression equations each.In model(1)where DBHwastheindependentvariable, equations for 5,11,18,24 and 30 years plantations were significant.Only equations for 1 year old plantations were not significant.In model(2)where H of the tree was the estimator,almostallequationsfor11,24 and 30 yearsstands were significant.The numberofnon-significantequations in this modelwas greater,around 50%in the remaining stands of 1,5 and 18 years.In model(3)where both DBH and H were incorporated,most equations for 11,18,24 and 30 years plantations were significant.In model(4)where GBH was the independent variable,most equations were significant for 5,11,18,24 and 30 years plantations.The proportions of the number of significantand non-significant equationswere60 versus8,51 versus17,51 versus17 and 53 versus15 in DBHmodel(1),Hmodel(2),DBH2Hmodel(3) and GBH model(4),respectively.Although the numbers of non-significantequations were equalin the H and DBH2H modelstheirdispersion through age classesin the lattercase wasgreater.The numberofequationssignificantat p>0.01 was in the order DBH(57)>GBH(51)>DBH2H (47)>H(41).Thus,the situation improved formodel(3)in comparison to model(2)as regardsthe numberofsignificant equations.Model(4)wasan improvementoverthesetwo but model(1)wasbeston thisaccount.The lack ofimprovementover DBH-based regressions resulting from inclusion of H along with DBH in the present study differed from the finding of Kumar et al.(1998)for multipurpose trees,Lim etal.(2013)for Japanese Cedar,Komiyama etal.(2000)for secondary mangrove forest and the general observation of Nihlgard(1972)that DBH2H resulted in stronger correlation.However,Nelson et al.(1999)suggested that DBH alone is a very good predictorofspecies-specific regression (up to 95%accuracy for Eucalyptus,Kuyah et al.2013). Negietal.(1995)used various linear models incorporating DBH and H as the variables,and also found thatDBH alone gave reasonably precise values of biomass in T.grandis plantations and tropicalhumid forest(Segura and Kanninen 2005).The findings of Chaturvediand Raghubanshi(2013) were similarfortropicaldry forest.They suggested using the diameter-only model,which might be more practical and equally accurate when applied to stands outside their study area.

Fig.3 Scatter diagram of below ground biomass of different tree components and tree biomass with polynomial regression equations. All equations were significant(p>0.01).y Biomass in kg ha-1 and x diameter in cm.Data were pooled from allsix age series plantations (1,5,11,18,24,30 years)
All24 equations for 1 year plantation,6 each in the four models,were non-significant(p>0.05).This could be due to very little variation in crop diameter and low numbers of samples for regression(only one diameter class and three tree samples).Itcould also be possible thatdiameter 50 cm above ground might not accurately represent DBH in teak and does not reflect a good relationship with biomass. Nonetheless,the equations having significant correlations from any model can be used for the purpose of biomass (C)prediction.Since height measurement is too cumbersome,time spent in the field could be greatly reduced by eliminating height measurements in stands that are relatively homogeneous(Dudley and Fownes 1992).In this study it proved feasible to estimate,non-destructively and using DBH(or GBH as the second preference)measurements alone,the biomass ofdifferenttree components fora commercially valuable species like T.grandis in young plantations growing in comparable edapho-climatic conditions.Itis also feasible to assess C stocking(1/2 biomass) by using these equations.
Conclusions
The present study revealed the following aspects of the functioning of young teak plantations:(1)C storage increased through 30 years of age,though CSR peaked at around 5 years;(2)Although production efficiency was greatest at 5 years,CAR peaked at 24 years;(3)plantation teak in moist deciduous forest regions,although it accumulated C at a slower rate than teak in high rainfall zones and tropical countries,showed much better commercialprospects than teak grown in drier areas of northern and central India;(4)DBH(or GBH)was the bestparameter for C stock prediction using linear regression for young teak plantation.


The above conclusions might help in choosing silviculturaloptions and harvesting ages to achieve optimum C sequestration in young plantations.CSR and CAR were greater in higher DBH classes,therefore,forest management should pay more attention to the protection of older age teak trees.The results presented here can also be useful for the assessmentof the actualand potentialrole of young teak plantations in reducing atmospheric CO2.Regression models will serve the purpose of C stock prediction of standing crops in similarareas withoutdestruction oftrees.
AcknowledgmentsThe contributions of Prof.R.P.Singh,Kumaun University,Nainital,and Prof.J.S.Singh,BHU,Varanasi,for the conceptualization and suggestions regarding the presentation of the problem,respectively,are appreciated.
Adu-Anning C,Blay DJ(2001)Ensuring sustainable harvesting of wood:impactofbiomass harvesting on the nutrientstress ofteak woodlot stand in the Sudan savanna.Ghana J For 10:17–24
Agren GI,Franklin O(2003)Root:shoot ratio,optimization and nitrogen productivity.Ann Bot 92:795–800
Alvarez E,Duque A,Saldarriga J,Cabrera K,Salas G,Valle I,Lema A,Moreno F,Orego S,Rodriguez L(2012)Tree above-ground biomass allometries for carbon stocks estimation in the natural forest of Colombia.For Ecol Manag 267:297–308
Bargali SS,Singh SP,Singh RP(1992)Structure and function of an age series of Eucalyptus plantations in Central Himalaya.I.Dry matter dynamics.Ann Bot 69:405–411
Barton CVM,Montagu KD(2006)Effect of spacing and water availability on root:shootratio in Eucalyptus camaldulensis.For Ecol Manag 221:52–62
BasukiTM,van Laake PE,Skidmore AK,Hussin YA(2009)Allometric equations for estimating the above-ground biomass in tropical lowland Dipterocarp forests.For EcolManag 257:1684–1694
BehaghelI(1999)The state ofteak(Tectona grandis L.f.)plantations in the world.Plantations de tec dans le monde.Bois et Foret de Tropique 262:18(Internet document).http://bft.cirad.fr/pdf/ res262.pdf.Accessed 15 Aug 2012
Brown S,Lugo AE(1982)The storage and production of organic matter in tropical forests and their role in global carbon cycle. Biotropica 14:161–187
Chambers JQ,dos Santos J,Rebeiro RJ,Higuchi N(2001)Tree damage,allometric relationships,and aboveground net primary production in central Amazon forest.For Ecol Manag 152:73–84
Champion HG,Seth SK(1968)Revised survey of the forest types of India.Manager of Publications,New Delhi
Chandrashekara UM(1996)Ecology of Bambusa arundinacea(Retz.) Willd.growing in teak plantations of Kerala,India.For Ecol Manag 87:149–162
Chaturvedi RK,Raghubanshi AS(2013)Aboveground biomass estimation of small diameter woody species of tropical dry forest.New For 44:509–519
Chaturvedi OP,Singh JS(1982)Total biomass and biomass production in Pinus roxburghii tree growing in all aged natural forests.Can J For Res 12:632–640
Chaturvedi OP,Singh JS(1987)The structure and function of pine forest in central Himalaya.I.Dry matter dynamics.Ann Bot 60:237–252
Chaturvedi RK,Raghubanshi AS,Singh JS(2010)Non-destructive estimation of tree biomass by using wood specific gravity in the estimator.Natl Acad Sci Lett 33:133–138
Chaturvedi RK,Raghubanshi AS,Singh JS(2011)Carbon density and accumulation in woody species of tropical dry forest in India.For Ecol Manag 262:1576–1588
Chaturvedi RK,Raghubanshi AS,Singh JS(2012)Biomass estimation ofdry tropicalwoody species atjuvenile stage.SciWorld J. doi:10.1100/2012/790219
Cooper CF(1983)Carbon storage in managed forests.Can JFor Res 13:155–166
Dabas M,Bhatia S(1996)Carbon sequestration through afforestation: role of tropical industrial plantations.Ambio 25:327–330
Dickman D,Pregitzer KS(1992)The structure and dynamics of woody plant root systems.In:Mitchell CP,Ford-Robertson JB, Hinckley T,Sennerby-Forsse L(eds)Ecophysiology of short rotation forest crops.Elsevier Applied Science,London, pp 95–123
Dixon RK,Brown S,Houghton RA,Solomon AM,Trexler MC, Wisniebsky J(1994)Carbon pools and flux of global forest ecosystems.Science 263:185–190
Dudley NS,Fownes JH(1992)Preliminary biomass equations for eight species of fast growing tropical trees.J Trop For Sci 5:68–73
Durkaya B,Durkaya A,Makineci E,Ulkudur M(2013)Estimation of above-ground biomass and sequestered carbon of Taurus Cedar (Cedrus libani L.)in Antalya,Turkey.iForest.doi:10.3832/ ifor0899-006
Eliyani I,Handoko I,Kuhne RF(2005)Process based modeling of growth and carbon sequestration of teak(Tectona grandis L.F.). In:Proceedings of the international conference on information and communication technology.Mercu Buana University, Jakarta,pp 21–33
Faruqui O(1972)Organic and mineral structure and productivity of plantation of Sal(Shorea robusta)and Teak(Tectona grandis). Ph.D.Thesis,Banaras Hindu University
Feldpausch TR,Banin L,Phillips OL,Baker TR,Lewis SL,Quesada CA,Affum-Baffoe K,Arets EJMM,Berry NJ,Bird M, Brondizio ES,de Camargo P,Chave J,Djagbletey G, Domingues TF,Drescher M,Fearnside PM,Franca MB,Fyllas NM,Lopez-Gonzalez G,Hladik A,Higuchi N,Hunter MO,Iida Y,Salim KA,Kassim AR,Keller M,Kemp J,King DA,Lovett JC,Marimon BS,Marimon-Junior BH,Lenza E,Marshall AR, Metcalfe DJ,Mitchard ETA,Moran EF,Nelson BW,Nilus R, Nogueira EM,Palace M,Patino S,Peh KSH,Raventos MT, Reitsma JM,Saiz G,Schrodt F,Sonke B,Taedoumg HE,Tan S, White L,Woll H,Lloyd J(2011)Height-diameter allometry of tropical trees.Biogeosciences 8:1081–1106
Feller MC(1980)Biomass and nutrient distribution in two eucalypt forest ecosystems.Aust J Ecol 5:309–333
Gaucher C,Gougeon S,Mauffette Y,Messier C(2005)Seasonal variation in biomass and carbohydrate partitioning of understorey sugar maple(Acer saccharum)and yellow birch(Betula alleghaniensis)seedlings.Tree Physiol 25:93–100
George M,Verghese G(1992)Nutrient cycling in Tectona grandis plantation.J Trop For 8:127–133
Gower ST,Krankina O,Olson RJ,Apps M,Linder S,Wang C(2001) Netprimary production and carbon allocation patterns of boreal forest ecosystems.Ecol Appl11:1395–1411
Greenwood DJ,Mckee JMT,Fuller DP,Burns IG,Mulholland BJ (2007)A novel method of supplying nutrients permits predictable shoot growth and root:shoot ratio of pretransplant bedding plants.Ann Bot 99:171–182
Hase H,Foelster H(1983)Impact of plantation forestry with teak (Tectona grandis)on the nutrientstatus ofyoung alluvialsoils of West Venezuela.For Ecol Manag 6:33–57
Hertel D,Moser G,Culmsee H,Erasmi S,Horna V,Schuldt B, Leuschner C(2009)Below-and above-ground biomass and net primary production in a paleotropical natural forest(Sulawesi, Indonesia)as compared to neotropical forests.For Ecol Manag 258:1904–1912
Hiratsuka M,Viriyabuncha C,Peawsa-ad K,Janmahasatien S,Sato A, Nakayama Y,Matsunami C,Osumi Y,Morikawa Y(2005)Tree biomass and soil carbon in 17-and 22-year-old stands of teak (Tectona grandis L.f.)in northern Thailand.Tropics 14:377–382
Jansons A,Sisenis L,Neimane U,Risketins JR(2013)Biomass production ofyoung lodgepole pine(Pinus contorta var latifolia) stands in Latvia.iForest 6:10–14
Jha KK(2005)Storage and flux of organic carbon in young Tectona grandis plantations in moist deciduous forest.Indian For 131:647–659
Jha KK(2014)Temporal patterns of storage and flux of N and P in young Teak plantations of tropicalmoistdeciduous forest,India. J For Res 25:75–86
Jha KK,Singh JS(1999)Temporal patterns of bole volume and biomass of young teak plantations raised in moist deciduous forest region,India.Int J Ecol Environ Sci 25:177–184
Johnson FL,Risser PG(1974)Biomass,annual net primary productivity and dynamics of six mineralelements in a postoak–black jack oak forest.Ecology 55:1246–1258
Jordan CF(1971)Productivity of a tropicalrain forestand its relation to a world pattern of energy storage.J Ecol 59:127–142
Kajimoto T,Matsuura Y,Sofronov MA,Volokitina AV,Mori S, Osawa A,Abaimov AP(1999)Above-and belowground biomass and net primary productivity of a Larix gmelinii stand near Tura,central Siberia.Tree Physiol 19:815–822
Karmacharya SB,Singh KP(1992)Biomass and net productivity of teak plantation in dry tropical region of India.For Ecol Manag 55:233–247
Kaul ON,Sharma DC,Sharma VN,Srivastava BPL(1979)Organic matter and plantnutrients in a teak(Tectona grandis)plantation. Indian For 105:573–582
Kaul ON,Sharma DC,Tandon VN(1983)Biomass distribution and productivity in a poplar plantation.Indian For 109:822–828
Keh K(1997)Whither goest Myanmar teak plantation establishment? In:Proceedings of the XIworld forestry congress
Keogh R(2000)The world of teak plantations.Int For Rev 2:123
Ketterings QM,Coe R,van Noordwijk M,Ambagu Y,Palm CA (2001)Reducing uncertainty in use of allometric biomass equations for predicting above-ground biomass in mixed secondary forests.For Ecol Manag 146:199–202
Klinge H,Rodrigues WA(1968)Litter production in an area of Amazonian terra firma forest,1&2.Amazoniana 1:287–302, 303–310
Komiyama A,Havanond J,Srisawatt W,Mochida Y,Fujimoto K, Ohnishi T,Ishihara S,Miyagi T(2000)Top/rootbiomass ratio of a secondary mangrove(Ceriops tagal Perr.C.B.Rob.)Forest. For Ecol Manag 139:127–134
Kraenzel M,Castillo A,Moore T,Potvin C(2003)Carbon storage of harvestage teak(Tectona grandis)plantations,Panama.For Ecol Manag 173:213–225
Kridiborworn P,Chidthaisong A,Yuttitham M,Tripetchkul S(2012) Carbon sequestration by mangrove forestplanted specifically for charcoal production in Yeesarn,Samut Songkram.J Sustain Energy Environ 3:87–92
Krishnapillay B(2000)Silviculture and management of teak plantations.Unasylva 51:14–19
Kumar S,Sharma AK(1990)Numerical classification of some soils of Indian Tarai.Indian Soc Soil Sci 38:265–271
Kumar BM,George SJ,Jamaludheen V,Suresh TK(1998)Comparison of biomass production,tree allometry and nutrient use efficiency of multipurpose trees grown in woodlotand silvopastoralexperiments in Kerala,India.For Ecol Manag 112:145–163
Kuyah S,Dietz J,Muthuri C,Noordwijk MV,Neufeldt H(2013) Allometry and partitioning of above-and below-ground biomass in farmed eucalyptus species dominant in western Kenyan agricultural landscapes.Biomass Bioenergy 55:276–284
Lamlom S,Savidge R(2006)Carbon content variation in boles of mature sugar maple and giantsequoia.Tree Physiol26:459–468
Larsen HS,Carter MC,Gooding JW,Hyink DM(1976)Biomass and nitrogen distribution in four13-year-old loblolly pine plantations in the hilly coastal plain of Alabama.Can J For Res 6:187–194
Lim H,Lee K-H,Lee K-H,Park IH(2013)Biomass expansion factors and allometric equations in an age sequence for Japanese cedar (Cryptomeria japonica)in southern Korea.J For Res 18:316–322
Lima AJN,Suwa R,Ribeiro HPM,Kajimoto T,Santos I,Silva RP, Sonza CAS,Barros PC,Noguchi H,Ishizuka M,Higuchi N (2012)Allometric models of estimating above-and below-ground biomass in Amazonian forest at Sao Gabriel da Cachoeira in the upper Rio Negro,Brazil.For Ecol Manag 277:163–172
Litton CM,Kauffman JB(2008)Allometric models for predicting aboveground biomass in two widespread woody plants in Hawaii.Biotropica 40:313–320
Lodhiyal LS,Singh RP,Singh SP(1995)Structure and function of an age series of poplar plantations in central Himalaya.I.Dry matter dynamics.Ann Bot 76:191–199
Lodhiyal N,Lodhiyal LS,Pangtey YPS(2002)Structure and function of shisham forests in central Himalaya,India.Dry matter dynamics.Ann Bot 89:41–54
Lugo AE,Figueroa J(1985)Performance of Anthocephalus chinensis in Puerto Rico.Can J For Res 15:577–585
Lugo AE,Brown S,Chapman J(1988)An analytical review of production rates and stem wood biomass of tropical forest plantations.For Ecol Manag 23:179–200
Luo Y,Wang X,Zhang X,Booth TH,Lu F(2012)Root:shoot ratio across China’s forests:forest type and climate effects.For Ecol Manag 269:19–25
Masamichi T,Dokrak M,Samreong P,Keizo H(2012)Carbon cycling in teak plantations in comparison with seasonally dry tropical forests in Thailand.In:Juan AB(ed)Forest ecosystems—more than just trees.InTech,pp 209–229.doi:10.5772/ 30196.ISBN:978-953-51-0202-1
Mbaekwe EI,Mackenzie JA(2008)The use of best fit allometric model to estimate above ground biomass accumulation and distribution in an age series of Teak(Tectona grandis L.f.) plantations at Gambari Forest Reserve,Oyo State,Nigeria.Trop Ecol49:259–270
Meunpong P,Wachrinrat C,Thaiutsa B,Kanzaki M,Meekaew K (2010)Carbon pools of indigenous and exotic tree species in a forest plantation,Prachuap Khiri Khan,Thailand.Kasetsart J (Nat Sci)44:1044–1057
Mohdar AH,Zuhaidi AY(2005)Forest plantation development in Malaysia—an overview.In:Lee SS,Lim HF(eds)Conservation of biological diversity through improved forest planning tools. Investment for sustainable heritage and wealth.Proceedings of the conference on forestry and forest products research(CFFPR 2005),22–24 Nov 2005,Kuala Lumpur,pp 337–347
Muller D,Nielson J(1965)Production brute,pertes parrespirations et production nette dans la foret ombrophile tropicale.Forstl Forsogsv Danmark 29:69–160
Murphy PG,Lugo AE(1986a)Structure and biomass in Puerto Rico. Biotropica 18:89–96
Murphy PG,Lugo AE(1986b)Ecology of tropical dry rain forest. Annu Rev Ecol Syst 17:67–88
NegiJDS,Bahuguna VK,Sharma DC(1990)Biomass production and distribution of nutrients in 20 years old teak(Tectona grandis) and gamar(Gmelina arborea)plantation in Tripura.Indian For 116:681–686
Negi MS,Tandon VN,Rawat HS(1995)Biomass and nutrient distribution in young teak(Tectona grandis Linn.F.)plantations in Tarai region of Uttar Pradesh.Indian For 121:455–464
Nelson BW,Mesqita R,Pareira JLG,de Souza SGA,Batista GT, Couto LV(1999)Allometric regressions for improved estimate of secondary forest biomass in the central Amazon.For Ecol Manag 117:149–168
Nihlgard B(1972)Plantbiomass,primary production and distribution of chemical elements in beech and a planted spruce forest in South Sweden.Oikos 23:69–81
Nowak DJ,Crane DE(2002)Carbon storage and sequestration by urban trees in the USA.Environ Pollut 116:381–389
Nwoboshi LC(1984)Growth and nutrient requirements in a teak plantation age series in Nigeria.II.Nutrient accumulation and minimum annual requirements.For Sci30:35–40
Nwoboshi LC(1985)Teak root dimensions and biomass in a thirty year old stand.Niger J For 15:80–84
Oelbermann M,Voroney RP,Gordon AM(2004)Carbon sequestration in tropical and temperate agroforestry systems:a review with examples from Costa Rica and southern Canada.Agric Ecosyst Environ 104:359–377
Ola Adams BA(1983)Effect of spacing on biomass distribution and nutrient content of Tectona grandis Linn.f.(Teak)and Terminalia superba Engl.Diels(Afara)in south western Nigeria. For Ecol Manag 58:299–319
Pande PK(2005)Biomass and productivity in some disturbed tropical dry deciduous teak forests of Satpura plateau Madya Pradesh. Trop Ecol 46:229–239
Prasad R,Mishra GP(1984)Standing biomass of various plantparts in selected tree species of dry deciduous teak forest in M.P. Indian For 110:765–782
Raman SS(1976)Biological productivity of Shorea plantations. Indian For 102:174–184
Rana BS,Singh SP,Singh RP(1989)Biomass and net primary productivity in Central Himalayan forest along an altitudinal gradient.For Ecol Manag 27:199–218
Rana BS,Rao OP,Singh BP(2001)Biomass production in 7 yearold plantations of Casuarina equisitifolia on sodic soil.Trop Ecol 42:207–212
Russo A,Escobedo FJ,Timilsina N,Schmitt AO,Varela S,Zerbe S (2014)Assessing urban tree carbon storage and sequestration in Bolzano,Italy.Int JBiodivers SciEcosyst Serv Manag 10:54–70
Scarascia-Mugnozza GE,Ceulemans R,Heilman PE,Isebrands GJ, Stettler RF,Hinkley TM(1997)Production physiology and morphology of Populus species and their hybrids grown under short rotation.II.Biomass components and harvest index of hybrid and parental species clones.Can J For Res 27:285–294
Segura M,Kanninen M(2005)Allometric models fortree volume and totalaboveground biomass in a Tropical Humid Forests in Costa Rica.Biotropica 37:2–8
Seth SK,Kaul ON(1978)Tropicalforestecosystem of India:the teak forest.In:Tropical forest ecosystem.UNESCO,Paris
Sharma A,Naik ML(1989)Biomass and productivity studies in teak (Tectona grandis Linn.F.)under artificial plantation in Surguja district(M.P.).J Trop For 5:97–104
Sharma G,Sharma E,Sharma R,Singh KK(2002)Performance of an age series of Alnus-Cardamum plantations in Sikkim Himalaya: productivity,energetics and efficiency.Ann Bot 89:261–272
Singh AK,Gupta BN(1993)Biomass production and nutrient distribution in some important tree species on Bhatta soil of Raipur(Madhya Pradesh)India.Ann For 1:47–53
Singh KP,Mishra R(1979)Structure and functioning of natural, modified and silvicultural ecosystems in Eastern Uttar Pradesh. Final Technical Report(1975–1978),MAB Research Project. BHU,Varanasi
Singh L,Singh JS(1991)Species structure,dry matter dynamics and carbon flux of a dry tropicalforestin India.Ann Bot68:263–273
Singh KP,Srivastava SK(1984)Spatialdistribution of fine rootmass in young trees(Tectona grandis)of varying girth sizes. Paedobiologia 27:161–170
Singh V,TewariA,Kushwaha SPS,Dadhwal VK(2011)Formulating allometric equations for estimating biomass and carbon stock in small diameter trees.For Ecol Manag 261:1945–1949
Specht A,West PW(2003)Estimation of biomass and sequestered carbon on farm forestplantations in northern New South Wales, Australia.Biomass Bioenergy 25:363–379
Sreejesh KK,Thomas TP,Rugmini P,Prasanth KM,Kripa PK(2013) Carbon sequestration potential of teak(Tectona grandis)plantations in Kerala.Res J Recent Sci 2:167–170
Swain SL,Bahera N(1997)Biomass and net production of Tectona grandis(Teak)in a regenerating forestfrom Orissa.Indian J For 20:112–117
Tadaki YN,Ogata N,Nagatoma Y(1965)The dry matter productivity in several stands of Cryptomaria japonica in Kyusha.Bull Gov For Exp Stn Tokyo 173:45–66
Takimoto A,Nair PKR,Nair VD(2008)Carbon stock and sequestration potential of traditional and improved agroforestry systems in the West African Sahel.Agric Ecosyst Environ 125:159–166
Terakunpisut J,Gajaseni N,Ruankawe N(2007)Carbon sequestration potential in aboveground biomass of Thong Pha Phum national forest,Thailand.Appl Ecol Environ Res 5:93–102
Thaiutsa B,Puangchit L,Yarwudhi C,Wacharinrat C,Kobayashi S (2004)Coppicing ability of teak(Tectona grandis)after thinning.In:Kobayashi S,TurnbullJW,Toma T,Mori T,Majid NMNA(eds)Rehabilitation of degraded tropical forest ecosystems:workshop proceedings,2–4 Nov 1999,Bogor,pp 151–156
UNFCCC(2008)Kyoto Protocol Reference Manualon accounting of emissions and assigned amount.http://unfccc.int/resource/docs/ publications/08_unfccc_kp_ref_manual.pdf
Wang X,Fang J,Zhu B(2008)Forest biomass and root:shoot allocation in northeast China.For Ecol Manag 255:4007–4020
Watanabe Y,Masunaga T,Owusu-Sekyere E,Buri MM,Oladele OI, Wakatsuki T(2009)Evaluation of growth and carbon storage as influenced by soil chemical properties and moisture on teak (Tectona grandis)in Ashanti region,Ghana.J Food Agric Environ 7:640–645
Whittaker RH(1966)Forest dimension and production in the Great Smoky Mountains.Ecology 47:103–121
Whittaker RH,Woodwell GM(1968)Dimension and production relations of trees and shrubs in Brookhaven forest,New York. J Ecol 56:1–25
Whittaker RH,Woodwell GM(1969)Structure,production and diversity of the oak pine forestat Brookhaven,New York.J Ecol 57:155–174
Whittaker RH,Woodwell GM(1971)Measurement of net primary production in forests.In:Productivity of forest ecosystems. UNESCO,Paris,pp 159–175
Zech W,Drechsel P(1991)Relationship between growth,nutrition and site factors of teak(Tectona grandis)plantations in the rainforest zone of Liberia.For Ecol Manag 41:221–235
16 October 2013/Accepted:22 September 2014/Published online:28 April 2015
©Northeast Forestry University and Springer-Verlag Berlin Heidelberg 2015
The online version is available at http://www.springerlink.com
Corresponding editor:Hu Yanbo
✉Kaushalendra Kumar Jha jhakk1959@gmail.com
1Technical Forestry,Indian Institute of Forest Management, PO Box No.357,Nehru Nagar,Bhopal 462 003,MP,India
杂志排行
Journal of Forestry Research的其它文章
- Management of pests and diseases of tropical sericultural plants by using plant-derived products:a review
- Gamma generalized linear model to investigate the effects of climate variables on the area burned by forest fire in northeast China
- Diversity,abundance,and structure of tree communities in the Uluguru forests in the Morogoro region,Tanzania
- Brazilian savanna re-establishment in a monoculture forest: diversity and environmental relations of native regenerating understory in Pinus caribaea Morelet.stands
- Use of infrared thermal imaging to diagnose health of Ammopiptanthus mongolicus in northwestern China
- Analysis of three types of triterpenoids in tetraploid white birches (Betula platyphylla Suk.)and selection of plus trees
