Analysis of three types of triterpenoids in tetraploid white birches (Betula platyphylla Suk.)and selection of plus trees
2015-06-05SuiWangHuiZhaoJingJiangGuifengLiuChuanpingYang
Sui Wang•Hui Zhao•Jing Jiang•Guifeng Liu•Chuanping Yang
Analysis of three types of triterpenoids in tetraploid white birches (Betula platyphylla Suk.)and selection of plus trees
Sui Wang1•Hui Zhao1•Jing Jiang1•Guifeng Liu1•Chuanping Yang1
Betulin,oleanolic acid,and betulinic acid are naturally occurring pentacyclic triterpenoids that have significantmedicinalvalue.Considerable amounts of these triterpenoids are available in the outer bark of white birch. In this study,we used ultrasound-assisted extraction(UAE) to extract triterpenoids from birch bark rapidly and with high efficiency.Using high performance liquid chromatography(HPLC),three types of triterpenoids were separated and detected.We examined the differences among triterpenoids extracted from diploid versus tetraploid white birch.Then,we used factor analysis to screen out tetraploid white birches with comprehensively excellent performance.The results indicate that the optimum conditions for extraction include the use of ethanol as an extraction solvent,a solid-to-liquid ratio of 0.1 g/10 ml, ultrasonic power setat100 W,a temperature of 60°C and an extraction time of 15 min.A reversed-phase C18 column(4.6 mm×250 mm×5μm)with a column temperature of 30°C and the mobile phase composed of A (acetonitrile)and B(0.1%aqueous phosphoric acid,v/v) at a flow rate of 0.5 ml/min were used,and the detection wavelength was 195 nm.No significant difference wasobserved between diploid and tetraploid white birch in terms of the content of three types of triterpenoids(at a confidence level of 0.05).As triterpenoid content,height, and DBH(diameter at breast height)are strongly interrelated,we used factor analysis to evaluate all individuals, and we screened out six plus trees with excellent comprehensive characters.
Betula platyphylla Suk·Triterpenoids·High performance liquid chromatography(HPLC)·Tetraploid· Diploid·Factor analysis
Introduction
Betula platyphylla Suk.,or Asian white birch,is a broadleaved deciduous hardwood tree species belonging to the genus Betula,in the family Betulaceae.Asian white birch can be found in temperate or subarctic regions of Asia including Japan,China,Korea,and Siberia.The hoary bark of this tree is marked with long,horizontallenticels,and it often separates into thin,papery plates,which is the most typical characteristic of Asian white birch(MobileReference 2008;Zhang 2008).
Over the course ofhuman history,the bark of birch was found to have high pharmaceutical value,and people in many countries and regions have used this bark to treat diseases(Shizhen 2011;Trivedi2006;Wood 2008).Recent studies have indicated that white birch bark has a complex chemicalcomposition and contains numerous triterpenoids, such as betulin,betulinic acid,lupeol,oleanolic acid,and ursolic acid(Krasutsky 2006).
Triterpenoids are the most ubiquitous class of natural secondary metabolites.These terpenes consist of six isoprene units and have the molecular formula C30H48(Patocˇka 2003;Xu et al.2004).Betulin(C30H50O2)is a lupine-type pentacyclic triterpenoid compound.Recent studies have shown that betulin and its derivatives can serve as inhibitors with great potential for preventing and treating HIV and cancers.Moreover,betulin has anti-inflammatory,antiviral,and antioxidantactivities,and can be used in the food,cosmetic,and medical industries.
Furthermore,betulin can be used(in its naturalform or after chemical modification)as a starting compound for other useful materials and compounds(Alakurtti et al. 2006;Chen etal.2009;Salin etal.2010;Tang etal.2011). Betulinic acid(C30H48O3),a derivative of betulin,is one of the most important lupine-type pentacyclic triterpenoid compounds.This compound is a selective inhibitor of human melanoma and HIV(Fujioka etal.1994;Pisha etal. 1995).More recently,betulinic acid has been shown to have antibacterial,antimalarial,anti-inflammatory,anthelmintic,and antioxidant properties(Fu et al.2011; Yogeeswariand Sriram 2005).
Oleanolic acid(C30H48O3)is another oleanane-type pentacyclic triterpenoid compound.Oleanolic acid possesses several promising pharmacological activities,such as hepatoprotective effects and anti-inflammatory,antioxidant,and anticancer activities.Ithas also,and ithas been used to treat hepatopathy and hyperglycemia(Liu 1995; Pollier and Goossens 2012).The chemical structures of these three triterpenoids are shown in Fig.1.
Polyploids are organisms whose genomes consist of more than two complete sets of chromosomes.There are three major types of polyploids,including autopolyploids, allopolyploids,and segmental allopolyploids.Polyploidization is widespread in organisms,especially plants. Compared with standard diploids,polyploids often have three major advantages.First,the increased number of alleles of a given gene in a polyploid can enable masking of deleterious recessive mutations.Second,allopolyploids and heterozygous autopolyploids exhibit heterosis.Third, duplicated gene copies can evolve to assume new or slightly varied functions.
However,the increased number of chromosomes may have adverse effects on plants.For example,polyploidy sometimes leads to reduced vigor and a reduced adaptive capacity.Moreover,the greater complexity of pairing and segregation interactions in polyploids can cause abnormalities during meiosis and mitosis(Madlung 2013).A number of early studies have revealed thatsome polyploids contain many more secondary metabolites compared to diploids(Dhawan and Lavania 1996;Kim et al.2004). Growing evidence suggests thatto provide valuable species required for the practical production of metabolites, autopolyploids should first be induced,followed by hybridization with other advantageous species to generate allopolyploids,which can have advantageous phenotypes (such as enhanced biomass,disease resistance and increased secondary metabolite content)(Ranney 2006).
It is worth noting that studies of the accumulation of secondary metabolites in polyploid plants have primarily been limited to a single cell or a few herbs,and there are few reports about secondary metabolite accumulation in woody perennial plants(De Jesus-Gonzalez and Weathers 2003;Kim etal.2004;Ng etal.2011;Lavania etal.2012). In this study,given the important medicinal value of birch triterpenoids,and since polyploids may accumulate more secondary metabolites than standard diploids,we analyzed the different contents of the three major triterpenoids(betulin,betulinic acid,and oleanolic acid)in Asian white birch bark using high performance liquid chromatography (HPLC)and selected some superior individualplants with high levels of triterpene accumulation.These plants may provide valuable materials for actual production and genetic breeding of white birch with high triterpenoid contents.
Materials and methods
Equipments and reagents
The analysis was performed using a HPLC system(Waters, Milford,MA,USA)consisting of a Waters 600 Multisovent Delivery System,a Waters 2707 Autosampler,a Waters Analytical Column Oven,and a Waters 2996 PDADetector.The data were gathered and processed using Empower 3 Chromatography Data Software(Waters, Milford,MA,USA).The samples were crushed in a TissueLyser II Bead Mill equipped with Grinding Jar Sets (Qiagen,Hilden,Germany).A KQ3200DE ultrasonic bath (Shumei,Kunshan,China)was used for ultrasonic extraction.The samples were dried in a WG71 electric thermostatic drying oven(Taisite,Tianjin,China).

Fig.1 Chemical structures of three triterpenoids in Betula platyphylla Suk
Betulinic acid and oleanolic acid standard substances were purchased from Sigma–Aldrich Chemical Corporation(Fluka,Saint-Quentin Fallavier,France),and betulin standard was obtained from Sigma–Aldrich Chemical Corporation(Sigma,St.Louis,MO,USA).The chemical structures of these reference compounds are shown in Fig.1.HPLC-grade acetonitrile was employed(J.T.Barker,Philipsburg,NJ,USA).The waterused throughoutthis study was Wahaha purified water(Wahaha,Hangzhou, China).Allother reagents used throughoutthe study were of analytical grade and were obtained from Shanghai Chemical Reagent Corporation(Shanghai,China).
Plant material
White birch(Betula platyphylla suk.)bark samples were collected in April 2013 from the enhanced greenhouse birch seed orchard of Northeast Forestry University(Harbin,China).The selected individual plants were healthy and withoutinsectpests;these plantsincluded fourdiploids and twenty nine tetraploids.Relevantinformation is shown in Table 1.
Preparation of standard solutions
Betulinic acid,oleanolic acid,and betulin standard substances were dissolved respectively in anhydrous ethanol (HPLC-grade)as three kinds of mother liquors(betulinic acid:1 mg/ml,oleanolic acid:1 mg/ml,betulin:2.5 mg/ ml).They were then filtered through 0.45-μm organic liquid membranes respectively and mixed as standard stock solution containing 300μg/ml betulinic acid,1200μg/ml oleanolic acid,and 3000μg/ml betulin.Working standard solutions were prepared by diluting the mixed standard stock solution with anhydrous ethanol(HPLC-grade)to obtain seven differentconcentrations,as shown in Table 2. All solutions were stored in a refrigerator at 4°C before analysis.
Preparation of sample solutions
White birch bark samples were placed into a TissueLyser II bead milland ground for2 min at28 Hz in the presence of liquid nitrogen.The resulting powdered white birch bark was passed through 40-mesh screens,transferred to volumetric flasks and incubated in a ventilated drying oven at 80°C(samples were weighed every 12 h until a weight change of<±0.001 g was recorded on two consecutive weighings).The dried bark powder was weighed,transferred to a 10-ml stoppered conical flask,and subjected to ultrasonic extraction(40 Hz)with 10 mlanhydrous ethanol (HPLC-grade).Then,the extract was dissolved with anhydrous ethanol(HPLC-grade)and diluted to 10 ml. Finally,the extract was filtered through 0.45-μm organic liquid membranes and stored in a refrigerator at 4°C.An orthogonaldesign was employed to optimize technological parameters(such as temperature,duration,and power)of ultrasonic extraction using SPSS software(Tables 3,4).
Chromatographic conditions
Chromatographic analysis was carried out using a Symmetry C18 column(250 mm×4.6 mm,5μm;Waters, Milford,MA,USA).The detection wavelength was set at 195 nm.The mobile phase consisted of A(acetonitrile)and B(0.1%aqueous phosphoric acid,v/v)with linear gradient elution,as shown in Table 5.The flow rate of the mobile phase was 0.5 ml/minute,and the column temperature was maintained at 30°C.Using an autosampler, 10μlvolume of the sample was injected successively.The re-equilibration duration was 10 min between individual runs.
Standard curves and quantitative calculations
Using the chromatographic conditions described above, each of the working standard solutions and sample solutions were injected into the chromatograph.The chromatographic peaks oftargetcompounds in differentsolvent extracts were confirmed by comparing their retention times and UV spectra with those of betulinic acid,oleanolic acid, and betulin standard substances.According to the working standard solution concentration and peak area,a standard curve equation was established.Quantification was carried out by integration of the peaks using the external standard method.The correlation coefficients(r2),limitofdetection (LOD)and limit of quantity(LOQ)were then calculated.
Method validation
To measure resolution(Rs),the half-height method was used.(Dolan 2002;Kromidas and Kuss 2009)To assess the reproducibility of the method,working standard solution④was injected six times.One of the sample solutions was analyzed after0,6,12,24 and 48 h atroom temperature to assess its stability.To evaluate the accuracy ofthe method, the recovery was determined by adding known amounts of working standard solution⑥into a known concentration ofsample solution at low(0.8 ml),medium(1 ml)and high (1.2 ml)levels.The spiked samples were then extracted, processed and quantified according to the methods described above.
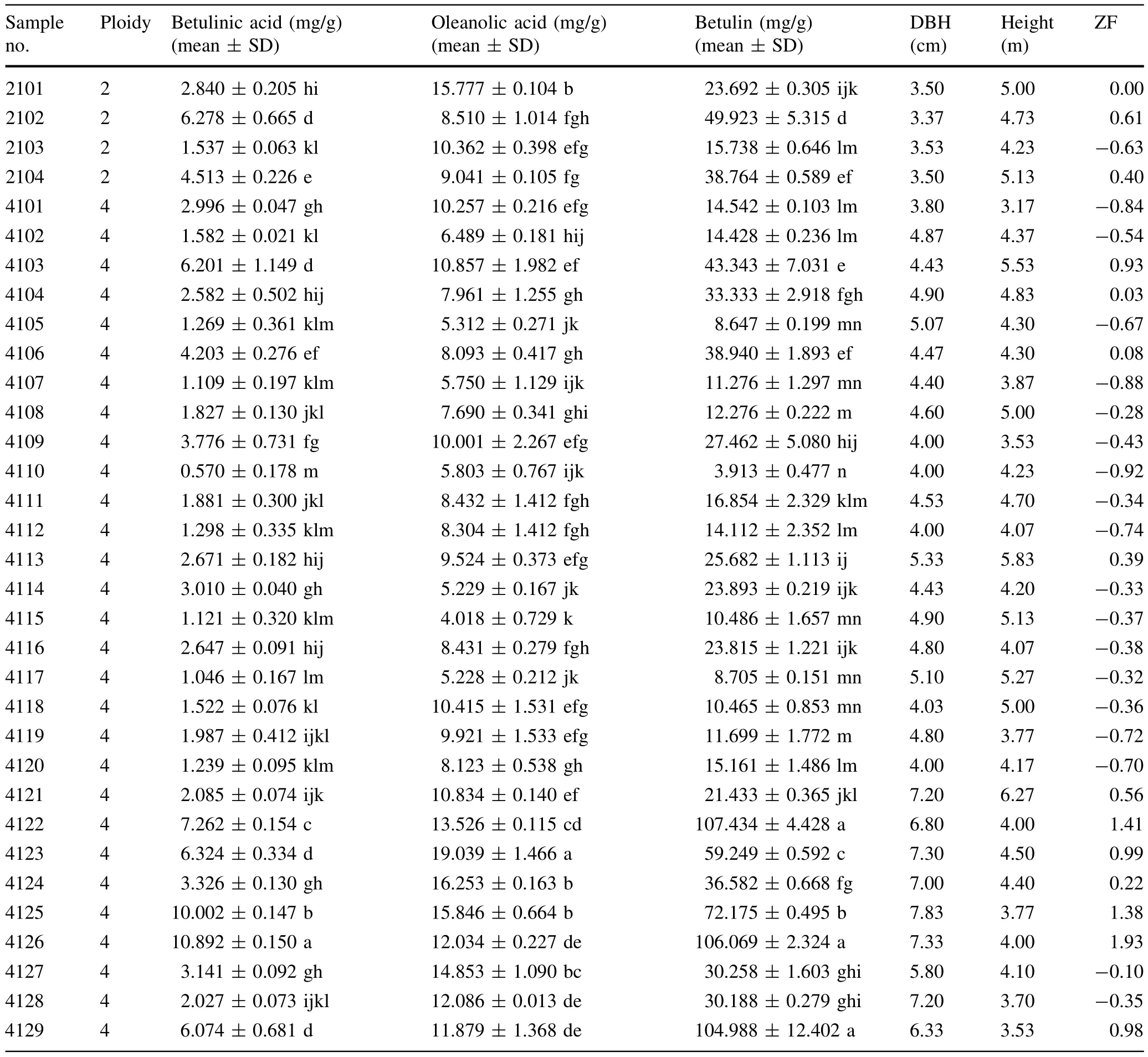
Table 1 The contents of three investigated compounds and other indicators in samples of white birch

Table 2 Seven different working standard solutions

Table 3 Orthogonal experimental factors and levels
Statistical analysis
Allof the data were analyzed using Excel2010(Microsoft, USA)and SPSS 20(IBM,USA)software.The results were expressed as mean±SD.Statistical analysis was performed by ANOVA followed by a multiple comparison test for multiple experimental groups.In some cases,a student’s t test was used to compare two groups.The level of statistical significance was set at p<0.05.
Results
Optimization of chromatographic conditions
To confirm the absorbance peaks of the three standard substances,the PDA detectorwas setto a scanning range of192–400 nm.The results showed thatallof the absorbance peaks of the three standard substances were approximately 195 nm.To select the best mobile phase,we tested both methanol–water and acetonitrile–water mixtures.The results demonstrated that the mobile phase of the methanol–water mixture produced more severe baseline noise than the acetonitrile–water mixture mobile phase.
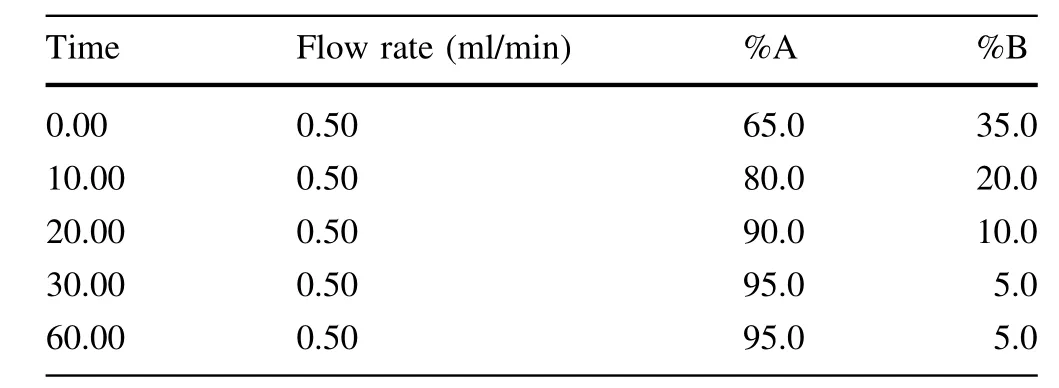
Table 5 Gradient elution program
We compared the performance of acetic acid and phosphoric acid for adjusting the pH of the mobile phase and found thata buffer containing 0.1%phosphoric acid(v/v) was better than buffer containing acetic acid,as the phosphoric acid-containing samples produced chromatograms exhibiting very sharp peaks without leading or trailing edges.We also observed the best separation when the column temperature was maintained at 30°C rather than 20, 25,35,40°C,or 45°C.Under allowable laboratory conditions,resolutions of the sample solutions were tested and compared with different reversed-phase conditions using several analytical columns,including:a Symmetry C18 Column(4.6 mm×250 mm×5μm);Symmetry C18Column(4.6 mm×150 mm×5μm);and an Atlantis T3 Column(4.6 mm×150 mm×5μm).We found that the first Symmetry C18 Column(4.6 mm×250 mm×5μm) provided the best peak shape.
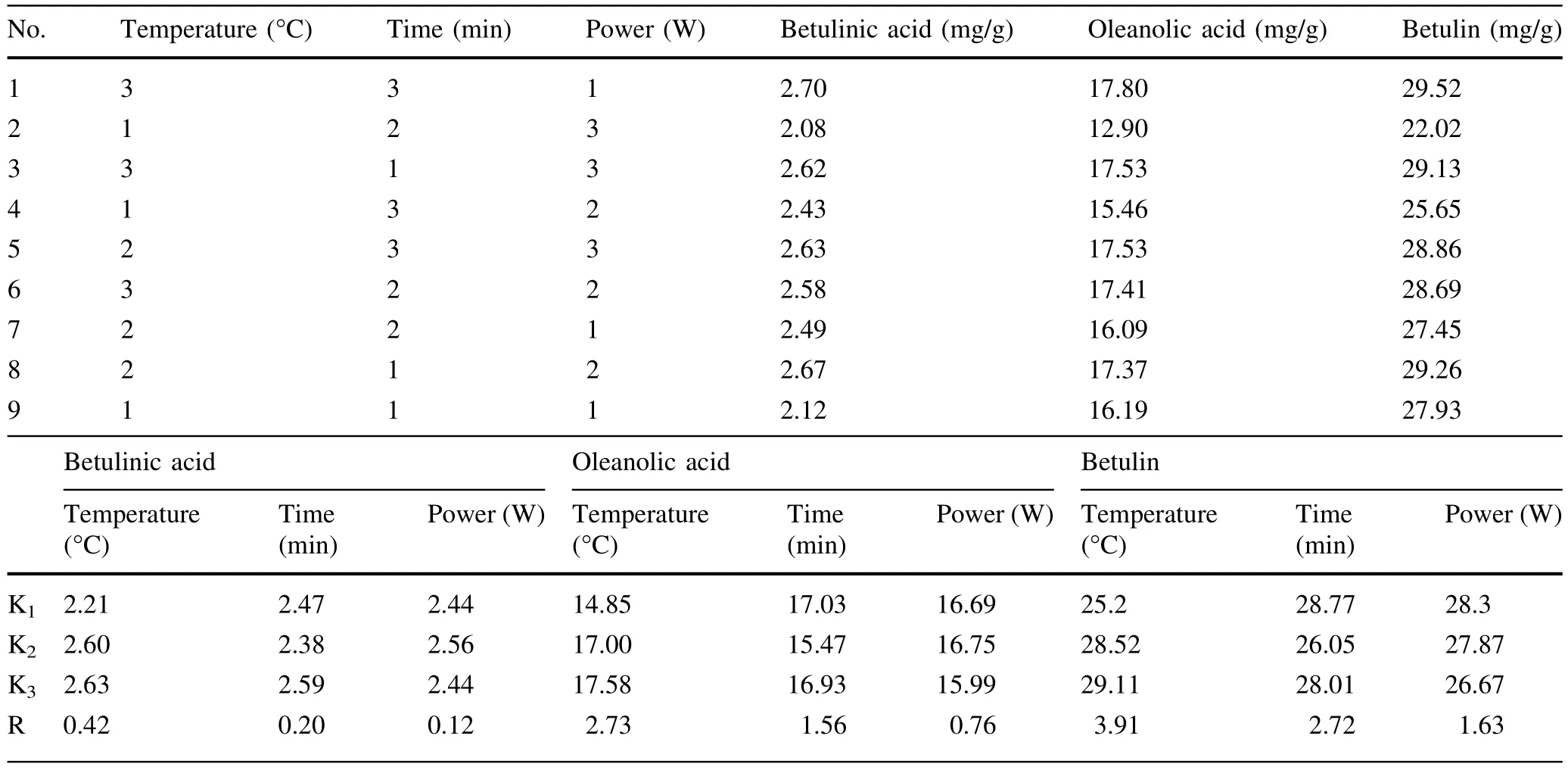
Table 4 Orthogonal experiment table
We tested both the isocratic and gradient elution programs.The results revealed thatboth isocratic and gradient elution can effectively separate three types of triterpenoids in the working standard solutions.However,only gradient elution can effectively separate triterpenoids in sample solutions within an appropriate duration and can get narrower and taller peaks to improve the peak shape for tailed peaks.Table 5 shows the final specific gradient elution program.Representative chromatograms for the working standard solutions and a sample are shown in Fig.2.
Optimization of extraction procedure
We examined the solubility of the triterpenoids in methanol,ethanol,and chloroform to ensure thatthese substances could be thoroughly extracted from white birch bark.The differences observed among the triterpenoid contents of the extracts were notsignificant.Therefore,we used anhydrous ethanol as a solvent to extract the triterpenoids.This solventis less toxic than the others and can be employed with a suitable solid-to-liquid ratio of 0.1 g/10 ml.
To optimize ultrasound-assisted extraction(UAE),an orthogonal design(three factors and three levels,no interaction consideration)was used to find the optimal combination of extraction temperature,exposure time and ultrasonic power level.According to the intuitive analysis presented in Table 4,the three factors that affected the extraction efficiency from most to least important in order were temperature,time,and power. The best extraction conditions for betulinic acid were a power level of 100 W,a temperature of 60°C,and an exposure time of 60 min.However,the best extraction conditions for oleanolic acid were a power level of 100 W,a temperature of 60°C,an exposure time of 15 min;for betulin,a power level of 60 W,a temperature of 60°C,an exposure time of 15 min.Analysis of variance indicated thatonly temperature had a significant effect on the extraction process for betulinic acid;the P value for temperature was less than 0.05(i.e.,0.021; Table 6).The other factors had no significantly different effect on the extraction process.Multiple comparisons revealed a significant difference in the extraction rates of betulinic acid between 40°C and other temperatures. Ultimately,we adopted the following extraction condition:ultrasonic power=100 W,exposure time=15 min,extraction temperature=60°C.
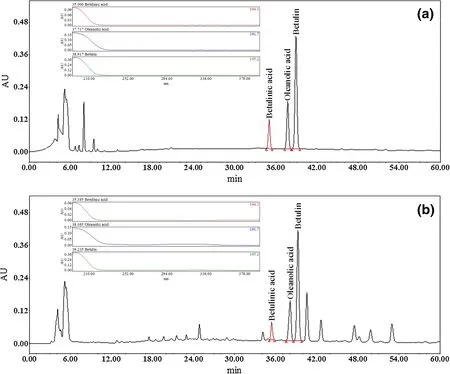
Fig.2 Typical HPLC chromatograms.a Working standard solutions and b tetraploid Betula platyphylla Suk
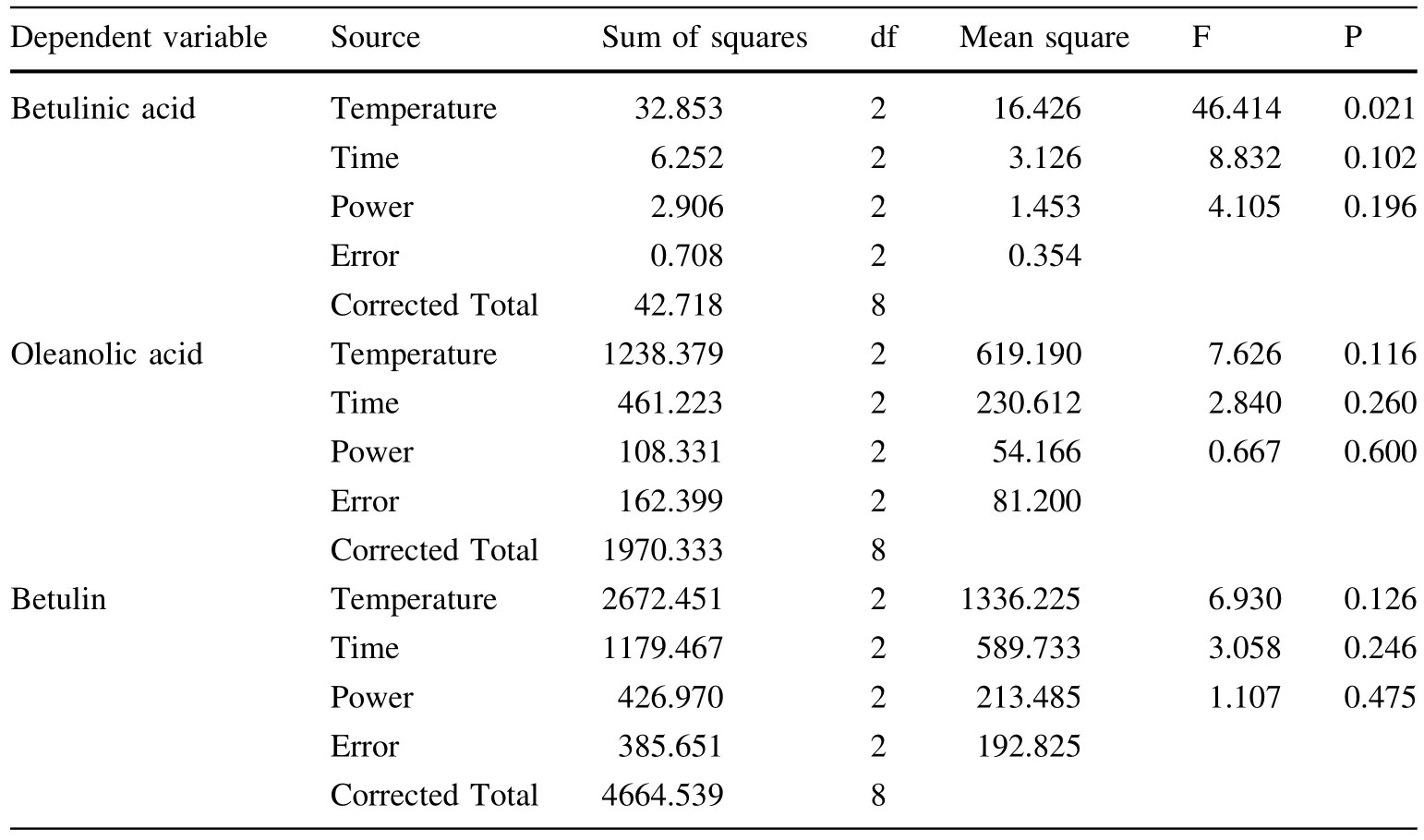
Table 6 Results of analysis of variance(ANOVA)
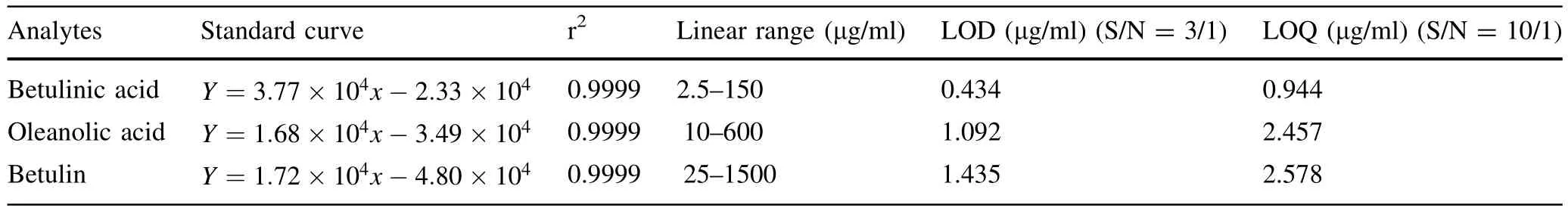
Table 7 Linear regression data,LOD and LOQ of 3 analytes
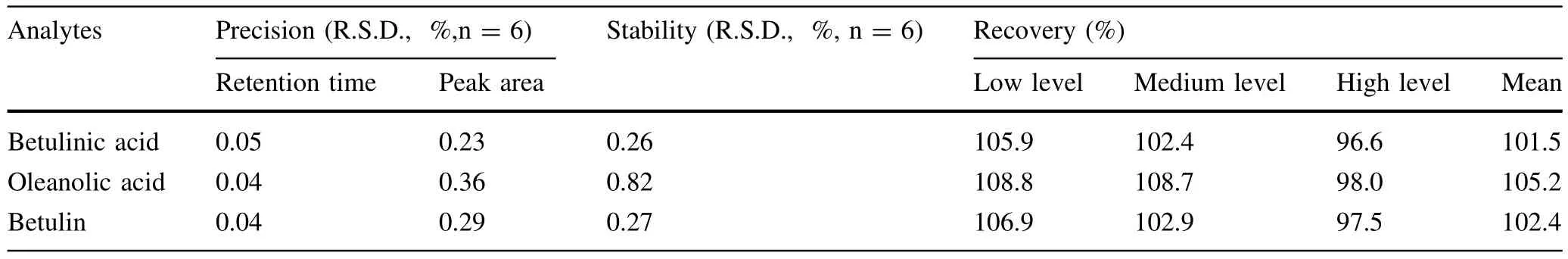
Table 8 Precision,stability and recovery of three analytes
Validation of HPLC method
The results of measuring resolution showed that all Rsof target peaks were bigger than 1.5 and were acceptable for analysis(e.g.,the Rsbetween oleanolic acid and betulin which are very close is 2.3 in Fig.2a and 1.8 in Fig.2b). The calibration graphs were plotted after linear regression of the peak areas versus the corresponding concentrations. The standard curves,linear ranges and other indicators are shown in Table 7 and 8.All three standard substances showed good linearity(r2≥0.9999)over a relatively wide range ofconcentrations.The method had good repeatability and stability expressed by the R.S.D.%(relative standard deviation)which were less than 1.0%in all cases.In addition,the recovery of all standard substances were between 96.6 and 108.8%in three different concentration levels.In conclusion,the method established in the current study was precise enough to enable the determination of the contents of the three triterpenoids.
Sample analysis
The established analytical method was subsequently applied to simultaneously determine the contents of three triterpenoids(betulinic acid,oleanolic acid and betulin)in 33 samples.The results demonstrated that the distribution of all triterpenoids data no longer differed from normality (Kolmogorov–Smirnov,p>0.05).As shown in Table 9,the differences between diploid and tetraploid white birch were compared with an independent sample t-test;the samples were not significantly different(t-test p>0.05, Levene’s test for homogeneity of variance p>0.05). Linear correlation analysis was used to analyze the correlation among ploidy,triterpenoid levels,DBH and height; the results are shown in Table 10.The results indicated that there was a significantly positive correlation between the three triterpenoid contents,and the DBH was significantly positively correlated with ploidy and triterpene contents. For each sample of the same size,a Student–Newman–Keuls(SNK)multiple-comparison test was used to determine specific differences between samples(Tables 1,11). The data indicated that there was marked interindividual variation in the contents of triterpenoids for each sample. The contents of triterpenoids in sample No.4122,4123 and 4125 were significantly higher than that of most other samples,while the triterpenoid contents in sample No. 4105,4107,4110,4115,and 4117 were significantly lower than that of mostof the others.
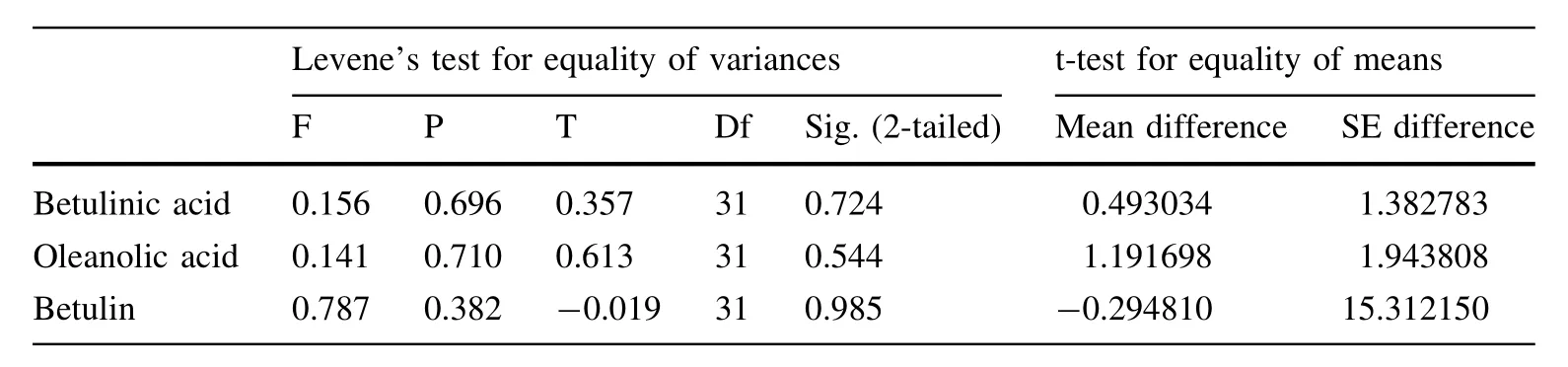
Table 9 Independentsamples test
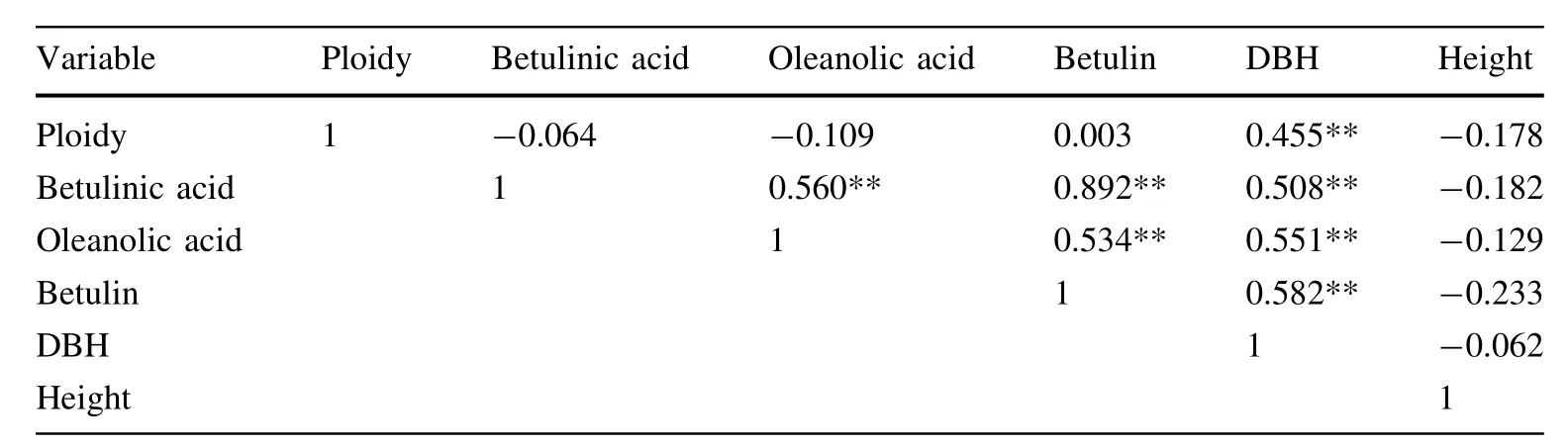
Table 10 Pearson Correlation
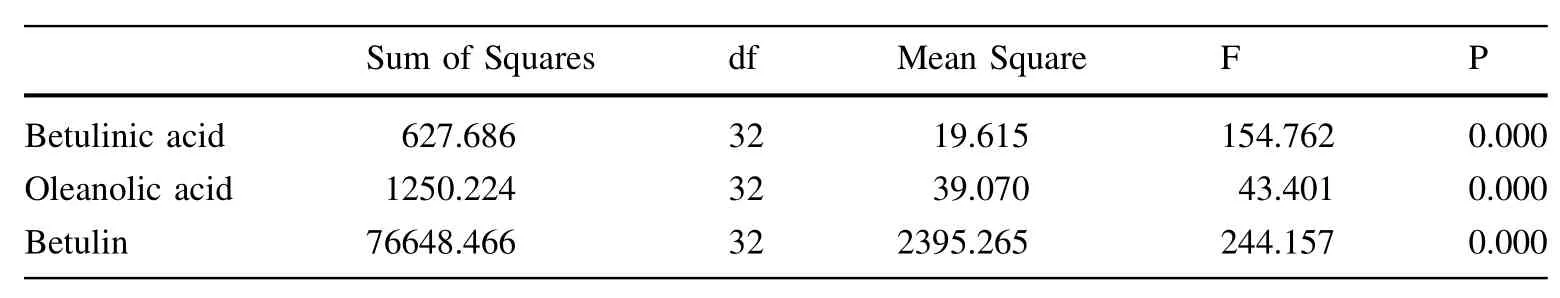
Table 11 Results of analysis of variance(ANOVA)
Comprehensive evaluation and plus trees selection
To selectplus trees with the highesttriterpenoid contents—taking into accountboth DBH and height,which are closely related to lumber yield—we utilized factor analysis to comprehensively evaluate 33 individualwhite birch plants. To ensure proper application of this technique,we inspected severalindices.The results ofthe Kaiser–Meyer–Olkin(KMO)measure ofsampling adequacy and Bartlett’s test of sphericity showed that there was a significant correlation between the variables(KMO=0.691,p<0.01). To simplify the calculation and to better explain the original variance,we set the extraction factor to three.
The results showed thatthe communality value for each variable was 0.75 orhigherand the three extraction factors explained 78.8%of total variance,indicating that the factoranalysis was appropriate.The firstfactorcan explain the contentof betulinic acid and betulin;the second factor, the content of oleanolic acid and the DBH at breast height of trees;and the third factor,the tree height.Table 12 shows the component-score coefficient matrix.The factor formulas are as follows:
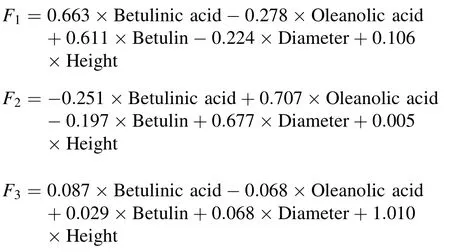
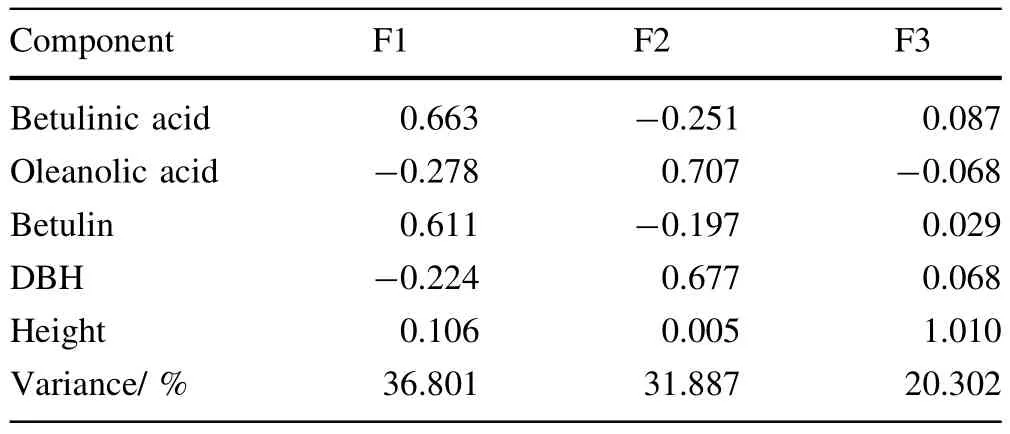
Table 12 Component score coefficient matrix
Finally,we utilized the variance contribution of each component as the weight and obtained the following comprehensive evaluation formula for individual white birch plants:

The six top-scoring individual plants were 4126,4122, 4125,4123,4129 and 4103,with zF scores greater than 0.9;the lowest-scoring individuals were 4110,4107,4101, 4112,4119,and 4120,with zF scores≤-0.70.
Discussion
The UV detector is the most widely used tool for triterpenoid detection(Guo et al.2009;Pai et al.2011;Yu 2008).The chemical structures of betulinic acid,oleanolic acid,and betulin revealthatallof these compounds contain only an isolated double bond,and therefore only absorb shorter wavelengths of ultraviolet light.However,the cutoff wavelength of methanol is 205 nm,and the methanol–water mobile phase may produce a high levels of noise at195 nm.To reduce background noise and to improve the detection limit,acetonitrile was chosen for the mobile phase rather than methanol.To avoid ionization of triterpenoids and to maintain high column efficiency,the mobile phase must be under acidic conditions.For the same reason,lower detection wavelength,phosphoric acid was selected to adjust the pH.
In terms of analyticalcolumns,the Atlantis T3 Column, in theory,provides paramount retention for polar compounds,which should have a betterseparation effectthan the Symmetry C18 Column which has the same length.In actual use,as shown in Table 13,the Symmetry C18 Column (4.6 mm×250 mm×5μm)was the best for it is longer than the Atlantis T3 Column and can produce high column efficiency.Therefore,we ultimately decided to use the Symmetry C18 Column(4.6 mm×250 mm×5μm)in this study,although the Symmetry C18 Column is not necessarily always better than the Atlantis T3 Column.
We first attempted isocratic elution when examined elution modes.This method has many advantages, including simplicity,easy operation,and short retention time.Also,the three types of triterpenoids in standard solutions can be well separated.However,as white birch bark extract is relatively complex,the same elution conditions cannot be used for effective separation of target peaks and non-target peaks.Finally,in reference to previous studies,we decided to use gradient elution to achieve better separation(Claude et al.2004;Guo et al.2009).
Most triterpenoids are not highly polar,and therefore should be extracted with organic solvents such as chloroform,methylene chloride,ethanol,and acetone.Since the purpose of this study was to compare the differences in the contents ofthree types oftriterpenoids between diploid and tetraploid white birch,we selected ethanolas the extraction solution because it is less toxic than the other solvents examined.
Compared with traditionalmethods,UAE can be used to perform extraction in a relatively short period of time and ata relatively low temperature while maintaining maximal bioactivity of the extract.UAE has been widely used for the extraction of plant polysaccharides,cellulose,terpenoids,flavonoids,alcohols,and esters(Schinor et al. 2004).Orthogonal analysis(using three factors and three levels)revealed thattemperature plays an importantrole in the process of triterpenoid extraction.In our preliminary experiments,we found that the rate of extraction of triterpenoids significantly improved with increasing temperature.However,as the boiling point of ethanol under normal pressure conditions is 78.5°C,ethanol seriouslyvolatilizes oreven boils athighertemperatures.Ultimately, we chose an extraction temperature of 60°C.
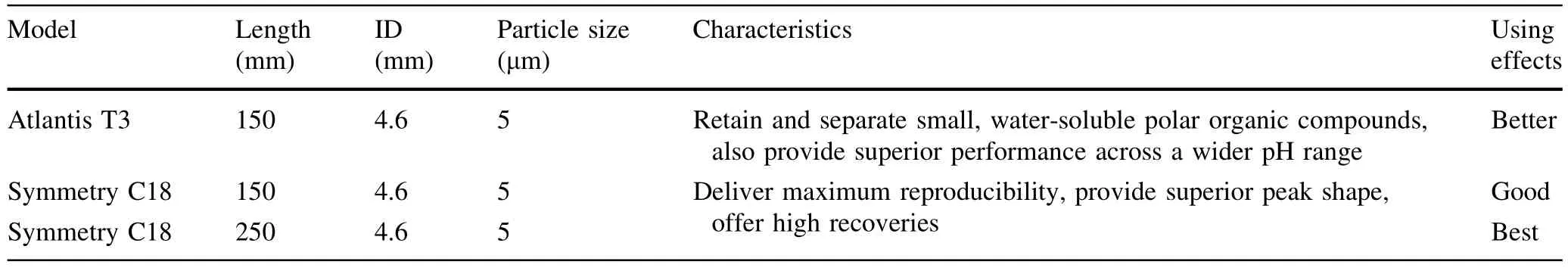
Table 13 Performance comparison of different columns
According to the results of the t-test,there were no significant differences in triterpenoid content between diploid and tetraploid white birch.Increased ploidy levels did not significantly alter the triterpenoid contents.This result was quite unexpected.However,previous studies have also demonstrated that not all secondary metabolites are present in higher levels in polyploid plants than in diploids(Dhawan and Lavania 1996).This observation may be due to the complex pathway of secondary metabolite biosynthesis,which is influenced by many factors,including the relationship between gene dose,gene silencing,and gene expression levels(Ranney 2006).
Moreover,genetic background and environmental conditions are also important factors that cannot be ignored. Triterpenoid content analysis revealed that there were insignificant differences between individuals.The ratio between the maximum and minimum levels of betulinic acid,oleanolic acid,and betulin were 19.1,4.7,and 27.5, respectively.Other studies have also revealed that differences in the contents of secondary metabolites between differentpolyploids are quite large(Caruso etal.2011;De Jesus-Gonzalez and Weathers 2003).
Our results also reflect the complexity of plant secondary-metabolite biosynthesis pathways.Correlation analysis revealed that the correlation coefficient between betulinic acid and betulin was the highest(r=0.892)and the correlation coefficient between oleanolic acid and betulin was the lowest(r=0.534).This resultmay be due to the fact that in the biosynthetic pathways of these pentacyclic triterpenoids,betulinic acid,and betulin are catalyzed and stepwise-synthesized from 2,3-oxidosqualene (2,3-OSC)by lupeol synthases(LUS).The relationship between these two types oftriterpenoids is close,as betulin is a precursor of betulinic acid.While oleanolic acid is catalyzed and synthesized from 2,3-OSC byβ-amyrin synthase(β-AS),this compound has a different synthetic pathway from that of the other compounds and its relationship to these compounds is relatively weak(Huang et al.2012).
Since there were insignificantdifferences in triterpenoid content between individual white birch plants,the screening of high-yielding plus trees is economically important. In this study,we utilized factor analysis.When selecting plus trees,we considered triterpenoid content as well as other important indicators,that is,DBH and height.In the future,these plus trees may be asexually propagated and released directly for production,or heterosis may be used to hybridize the plus tetraploids trees with other plus trees (diploid or tetraploid)to obtain more high-yielding varieties ofwhite birch,which would have greateconomic and social benefits.
AcknowledgmentsThe authors thank ELIXIGEN(SHANGHAI) CO.,LTD.for copyediting this manuscript to eliminate grammatical and spelling errors.
Alakurtti S,Ma¨kela¨T,Koskimies S,Yli-Kauhaluoma J(2006) Pharmacological properties of the ubiquitous natural product betulin.Eur J Pharm Sci 29(1):1–13
Caruso I,Lepore L,De Tommasi N,Dal Piaz F,Frusciante L, Aversano R,Garramone R,Carputo D(2011)Secondary metabolite profile in induced tetraploids of wild Solanum commersonii Dun.Chem Biodivers 8(12):2226–2237
Chen Q,Liu J,Zhang H,He G,Fu M(2009)The betulinic acid production from betulin through biotransformation by fungi. Enzyme Microb Tech 45(3):175–180
Claude B,Morin P,Lafosse M,Andre P(2004)Evaluation of apparent formation constants of pentacyclic triterpene acids complexes with derivatizedβ-andγ-cyclodextrins by reversed phase liquid chromatography.J Chromatogr A 1049(1–2):37–42
De Jesus-Gonzalez L,Weathers PJ(2003)Tetraploid Artemisia annua hairy roots produce more artemisinin than diploids.Plant Cell Rep 21(8):809–813
Dhawan OP,Lavania UC(1996)Enhancing the productivity of secondary metabolites via induced polyploidy:a review. Euphytica 87(2):81–89
Dolan JW(2002)Peak tailing and resolution.LCGC North Am 20(5):430–436
Fu JY,Qian LB,Zhu LG,Liang HT,Tan YN,Lu HT,Lu JF,Wang HP,Xia Q(2011)Betulinic acid ameliorates endotheliumdependent relaxation in L-NAME-induced hypertensive rats by reducing oxidative stress.Eur J Pharm Sci 44(3):385–391
Fujioka T,Kashiwada Y,Kilkuskie RE,Cosentino LM,Ballas LM, Jiang JB,Janzen WP,Chen IS,Lee KH(1994)Anti-AIDS agents,11.Betulinic acid and platanic acid as anti-HIV principles from Syzigium claviflorum,and the anti-HIV activity of structurally related triterpenoids.J Nat Prod 57(2):243–247
Guo S,Duan J,Tang Y,Su S,Shang E,Ni S,Qian D(2009)Highperformance liquid chromatography—two wavelength detection oftriterpenoid acids from the fruits of Ziziphus jujuba containing various cultivars in different regions and classification using chemometric analysis.J Pharmaceut Biomed 49(5):1296–1302
Huang L,Li J,Ye H,Li C,Wang H,Liu B,Zhang Y(2012) Molecular characterization of the pentacyclic triterpenoid biosynthetic pathway in Catharanthus roseus.Planta 236(5):1571–1581
Kim Y,Hahn E,Murthy HN,Paek K(2004)Effect of polyploidy induction on biomass and ginsenoside accumulations in adventitious roots of ginseng.Journalof Plant Biology 47(4):356–360
Krasutsky PA(2006)Birch bark research and development.Nat Prod Rep 23(6):919–942
Kromidas S,Kuss HJ(2009)Quantification in LC and GC:a practical guide to good chromatographic data.Wiley-VCH,Weinheim, p 8
Lavania UC,Srivastava S,Lavania S,Basu S,Misra NK,Mukai Y (2012)Autopolyploidy differentially influences body size in plants,but facilitates enhanced accumulation of secondary metabolites,causing increased cytosine methylation.Plant J 71(4):539–549
Liu J(1995)Pharmacology of oleanolic acid and ursolic acid. J Ethnopharmacol 49(2):57–68
Madlung A(2013)Polyploidy and its effect on evolutionary success: old questions revisited with new tools.Heredity(Edinb) 110(2):99–104
MobileReference(ed)(2008)The illustrated encyclopedia of trees and shrubs:an essential guide to trees and shrubs of the world. Boston,MobileReference
Ng DW,Zhang C,Miller M,Palmer G,Whiteley M,Tholl D,Chen ZJ(2011)cis-and trans-Regulation of miR163 and targetgenes confers natural variation of secondary metabolites in two Arabidopsis species and their allopolyploids.Plant Cell 23(5):1729–1740
Pai SR,Nimbalkar MS,Pawar NV,Dixit GB(2011)Optimization of extraction techniques and quantification of betulinic acid(BA) by RP-HPLC method from Ancistrocladus heyneanus Wall.Ex Grah.Ind Crop Prod 34(3):1458–1464
Patocˇka J(2003)Biologically active pentacyclic triterpenes and their current medicine signification.J Appl Biomed 1:7–12
Pisha E,Chai H,Lee IS,Chagwedera TE,Farnsworth NR,Cordell GA,Beecher CW,Fong HH,Kinghorn AD,Brown DM,Et A (1995)Discovery of betulinic acid as a selective inhibitor of human melanoma that functions by induction of apoptosis.Nat Med 1(10):1046–1051
Pollier J,Goossens A(2012)Oleanolic acid.Phytochemistry 77:10–15
Ranney TG(2006)Polyploidy:from evolution to new plant development.Combined proceedings international plant propagators’society,vol 56,pp 137–142
Salin O,Alakurtti S,Pohjala L,Siiskonen A,Maass V,Maass M,Yli-Kauhaluoma J,Vuorela P(2010)Inhibitory effect of the natural product betulin and its derivatives against the intracellular bacterium Chlamydia pneumoniae.Biochem Pharmacol 80(8):1141–1151
Schinor EC,Salvador MJ,Turatti IC,Zucchi OL,Dias DA(2004) Comparison of classical and ultrasound-assisted extractions of steroids and triterpenoids from three Chresta spp.Ultrason Sonochem 11(6):415–421
Shizhen L(2011)Compendium of materia medica.Jiangsu People’s Publishing House,Nanjing,pp 519–520(in Chinese)
Tang J,Li J,Qi W,Qiu W,Li P,Li B,Song B(2011)Inhibition of SREBP by a small molecule,Betulin,improves hyperlipidemia and insulin resistance and reduces atherosclerotic plaques.Cell Metab 13(1):44–56
Trivedi PC(2006)Medicinal plants:traditional knowledge.I.K.International Publishing House Pvt,New Delhi,p 240
Wood M(2008)The earthwise herbal:a complete guide to old world medicinal plants.North Atlantic Books,Berkeley,pp 139–142
Xu R,Fazio GC,Matsuda SPT(2004)On the origins of triterpenoid skeletal diversity.Phytochemistry 65(3):261–291
Yogeeswari P,Sriram D(2005)Betulinic acid and its derivatives:a review on their biological properties.Curr Med Chem 12(6):657–666
Yu S(2008)Triterpenoid chemistry.Chemical Industry Press, Beijing,pp 1–3,119–128,(in Chinese)
Zhang Z(2008)Dendrology.China Forestry Publishing House, Beijing,pp 194–196(in Chinese)
10 July 2014/Accepted:9 September 2014/Published online:7 July 2015
©Northeast Forestry University and Springer-Verlag Berlin Heidelberg 2015
Project funding:This work was financially supported by National Forestry Department Public Benefit Research Foundation of China (201204302).
The online version is available at http://www.springerlink.com
Corresponding editor:Hu Yanbo
✉Chuanping Yang yangchuanping_ycp@163.com
1State Key Laboratory of Tree Genetics and Breeding, Northeast Forestry University,Harbin 150040,China
杂志排行
Journal of Forestry Research的其它文章
- Management of pests and diseases of tropical sericultural plants by using plant-derived products:a review
- Gamma generalized linear model to investigate the effects of climate variables on the area burned by forest fire in northeast China
- Diversity,abundance,and structure of tree communities in the Uluguru forests in the Morogoro region,Tanzania
- Brazilian savanna re-establishment in a monoculture forest: diversity and environmental relations of native regenerating understory in Pinus caribaea Morelet.stands
- Carbon storage and sequestration rate assessment and allometric model development in young teak plantations of tropical moist deciduous forest,India
- Use of infrared thermal imaging to diagnose health of Ammopiptanthus mongolicus in northwestern China
