Ultrastructural study on route of gut bacterial translocation in a rat after spinal cord injury
2015-05-22ChunhongBAIXinlongMA
Chun-hong BAI, Xin-long MA
Tianjin Hospital, the Clinical orthopedics of Tianjin Medical University, Tianjin, 300211, China
Ultrastructural study on route of gut bacterial translocation in a rat after spinal cord injury
Chun-hong BAI, Xin-long MA
Tianjin Hospital, the Clinical orthopedics of Tianjin Medical University, Tianjin, 300211, China
doi 10.13459/j.cnki.cjap.2015.06.012
Objective: To observe the ultrastructural change of the route of gut bacterial translocaon in a rat with spinal cord injury (SCI).Methods: Forty Wistar rats were divided into the following groups: control group and 3 SCI groups (10 in each group). The rats in the SCI groups were established SCI model at 24 h, 48 h, and 72 h aer SCI. Small intesne mucous membranessue was idenf i ed and assayed by transmission electron microscope, scanning electron microscope and immunofluorescence microscopy. Results: Small intesne mucous membranessue in control group was not damaged signif i cantly, but those in SCI groups were damaged significantly. Proliferation bacteria in gut lumen attached on microvilli. The extracellular bacteria torn the intesnal barrier and perforated into the small intesnal mucosal epithelial cell. The bacteria and a lot of parcles of the seriously damaged region penetrated into the lymphac system and the blood system directly. Some bacteria were internalized into the goblet cell through the apical granule. Some bacteria and parcles perforated into the submucosa of the M cell running the long axis of M cells through the tight junctions. In the microcirculation of mucosa, the bacteria that had already broken through the microvilli into blood circulaon swim accompanying with erythrocytes. Conclusion: The routes of bacterial translocaon interact and format a vicious circle. At early step, the transcellular pathway of bacterial translocaon is major. Following with the destroyed small intesne mucous, the routes of bacterial translocaon through the lymphac system and the blood system become direct pathways. The goblet cell-dendric cell and M cell pathway also play an important role in the bacterial translocaon.
spinal cord injury; ultrastructural study; bacterial translocaon
Introduction
Gastrointestinal dysfunction aer spinal cord injury (SCI) are extremely difficult problems. The gut barrier function can be damaged likeing the skin. Bai et.al[1] had showed that rats aer spinal cord injury had endotoxemia and bacterial translocation (BT) caused by insuf fi cient gut motility, overgrowth of gut bacteria, and the injured gut barrier function. Studies demonstrated that an ongoing BT may lead to the multiple organ failure (MOF) liking a persistent inflammatory response in a patient. Nevertheless, the ultrastructural change of bacterial translocation of the gut in a rat after spinal cord injury had not been reported systematically.is study aims were to observe the procedure and the exact mechanism of BT of the gut.
Materials and Methods
Animals
Fourty adult male Wistar rats weighing 250 to 300 g were divided into two groups: control group and 3 experimental groups (10 in each group).e rats in the SCI groups were established SCI model at 24 h, 48 h, and 72 h aer SCI. Before the experiment, rats were feed with normal food in caves for one week at 22-25°C . Rats were deprived of food and water for 12 hours before SCI.
The model of paraplegia
Bacterial label and intestinal inoculation
E. coli which were transfected by the pSELECTGFPzeo-mcs was pretreated by 100 ml LB medium supplemented with zeocin (50 μg/ml) overnight at 37 °C for 24 h. The rats without zeocin-resistant bacteria drank sterile water containing zeocin (50 μg/ml) for 7 days to facilitate the transformed E. coli colonized in the enteric lumen; then the transformed E. coli were gavaged to the rats once daily on 4 and 5 two consecutive days (10 ml/kg). On 5 to 7 three consecutive days, the stool samples were collected and then dissolved in LB medium supplemented with zeocin (50 μg/ml) and incubated overnight at 37°C until the E. coli labeled with GFP had colonized in the enteric lumen.
Immunohistochemistry
Frozen section specimens were fixated with 10% formaldehyde for 10 min. The slides were then treated with DAPI dyeing liquid for 5 min following with block with calf serum at 37°C at room temperature for 30 min. Images were obtained using a ff uorescence microscope.
Scanning electron microscopy
Fresh small intestine samples were fixed in 2.5% glutaraldehyde, dehydrated through graded concentrations of acetone (30%, 50%, 70%, 95%, and 100%), critical point dried, mounted on aluminum stubs, sputter coated with gold, and examined with a scanning electron microscopy.
Transmission electron microscopy
Fresh small intestine samples for electron microscopy were cut into small (2 mm3) cubes, fi xed with 2.5% glutaraldehyde and phosphate buffer for 2 h, postfi xed with 1% osmium tetroxide, then dehydrated by passage through graded concentrations of alcohol and acetone, and saturated, and embedded in epoxide resin. Ultra-thin sections were prepared, stained with uranyl acetate and lead citrate, and examined with a transmission electron microscopy.
Results
Fluorescence microscope observations
With the development of the time, the small intestinal mucous destoryed in different degree after SCI. A lot of E. coli labeled with GFP penetrated into the small intestine mucous leading to the leakage of intestinal villus. In contrast, less E. coli labeled with GFP could penetrate into the small intestine mucous with structural integrity (Fig. 1).
Scanning electron microscopy observations
Intestinal villi in control group smooth, complete and neat rows (Fig. 2A).e epithelia in SCI group were atrophic with disordered arrangement and widened spaces of the villi. The Intestinal mucosal epithelial cells of the seriously damaged region defects along with evident atrophy and exfoliated microvilli in SCI group. Cellular leaks were formed extensively in intestinal mucosa (Fig. 2B).
Transmission electron microscopy observations
Orderly mucosal villi, integrated tight junctions, and intact organelles with regular nuclei were observed in control group (Fig. 3A).
The microvilli had varying degrees of stripped, incomplete following with widened cellular spaces in SCI group. Swelling swollen endoplasmic reticulum and mitochondria as well as a small number of lanthanum granules were observed. Intestinal lumen destruction was getting severe. The epithelial cells exhibited severe edema, and some areas of necrosis were observed, and interspaces became very wide. Edema, break, and exfoliation were detected in the microvilli. Bacteria in gut lumen and putative attaching bacteria to gut microvilli were observed (Fig. 3B).
In SCI group, the extracellular bacteria was torning on the intestinal barrier and perforating into the small intestinal mucosal epithelial cell.e bacteria and a lot of particles of the seriously damaged region penetrated into the lymphatic system and the blood system directly (Fig. 4A, B).
The goblet cell secreted much mucus and the bacteria was internalizing into the goblet cell breakthrough the apical granule (Fig. 5).
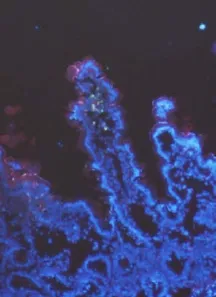
Fig. 1 FM study: After SCI, the mucous membrane of small intestine destoried and a lot of E. coli labeled with GFP penetrated the mucous membrane of small intestine form the leakage of intestinal villus. (×200) The arrow: E. coli Labeled with Green fluorescent protein.
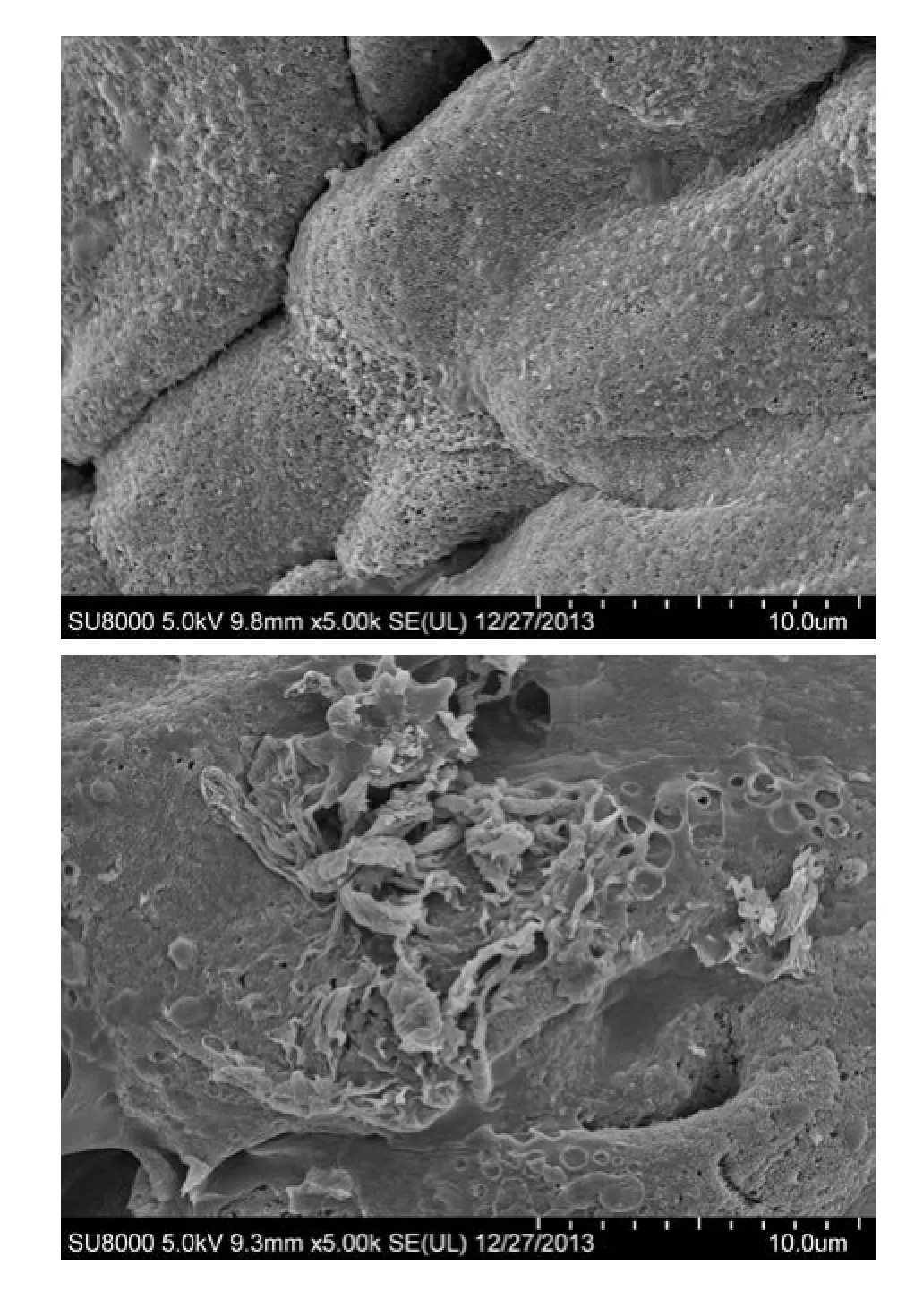
Fig. 2 SEM study A: Smooth surface of intestinal mucosa with a clear structure as well as complete and orderly villi in control group. B: Severely injured intestinal mucosa in mucosal defects along with evident atrophy and disordered villi with widened villous spaces and exfoliated microvilli in group SCI 72h. Scale bars=10 μm. (A and B).
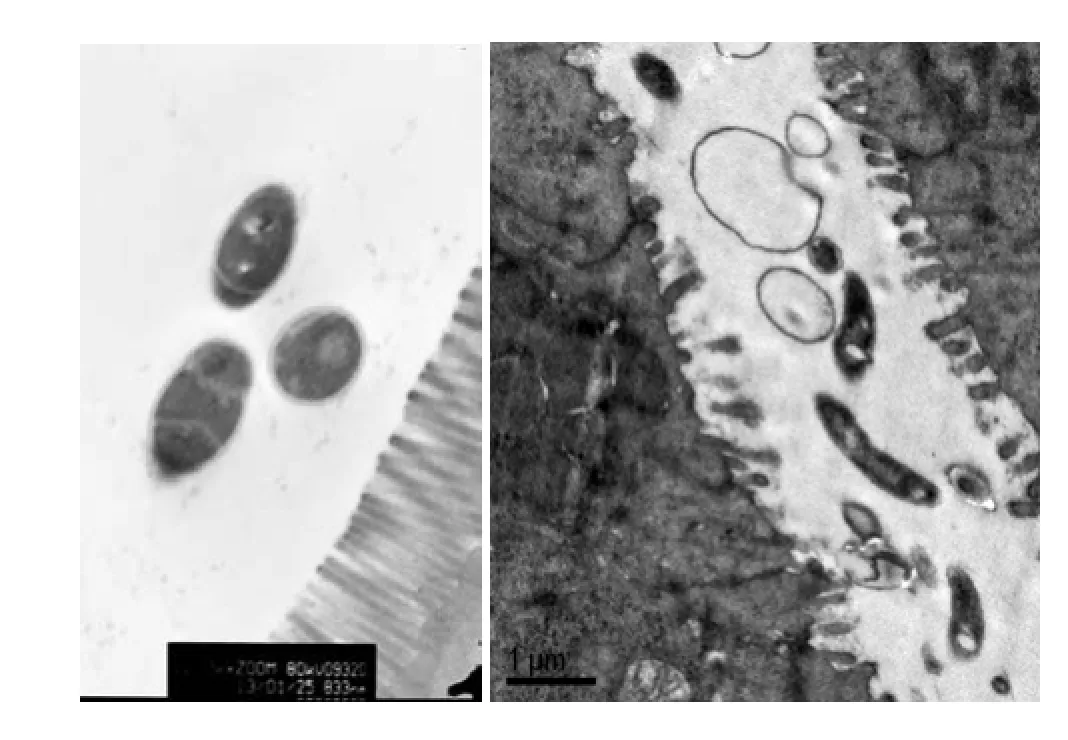
Fig. 3 TEM study A: Orderly mucosal villi and integrated tight junctions as well as intact organelles with regular nuclei were observed with no edge aggregation in chromatin and no lanthanum granules in tissue space and cells in NG. Free form of E. coli as found in the intestinal lumen.(control group) B: The microvilli were injured in different degree, which got exfoliated and incomplete with widened cellular spaces, swollen endoplasmic reticulum and mitochondria as well as a small number of lanthanum granules in SCI. Bacteria in gut lumen, putative attaching bacteria to gut microvilli. (SCI group). Scale bars= 833 nm(A)and 10 μm( B).
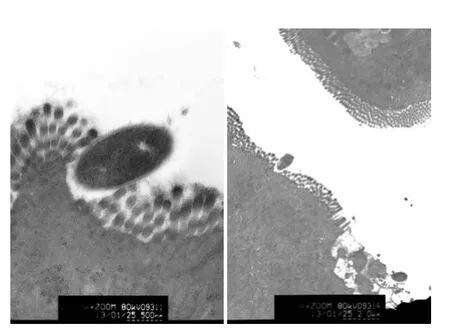
Fig. 4 Ultrastructure study of bacteria transcellular perforation in small intestinal mucosa epithelial cells. A: Extracellular bacteria torn on the terminal web and perforating the small intestinal Intestinal mucosal epithelial cell (SCI group) B: Extracellular bacteria perforating the small intestinal Intestinal mucosal epithelial cell and entrap particles penetrating into the destroyed cell(SCI group). Scale bars= 500 nm(A) and 20 μm( B).
M cells lack an apical rigid brush border and elaborate micro-projections on the membrane surface. We observed extracellular bacteria perforating into the M cell and penetrating particles that run parallel to long axis of M cells through the tight junctions (Fig. 6).
In SCI group, we found bacteria swimming with erythrocytes in the submucosal blood capillary ( Fig. 7).
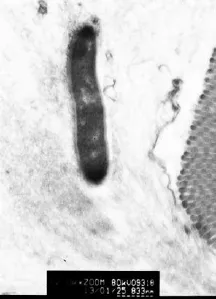
Fig. 5 Ultrastructure study of bacteria internalizated into the GC. Internalization of bacteria into the apical granule mass of the GC(SCI group). Scale bars= 833 nm.
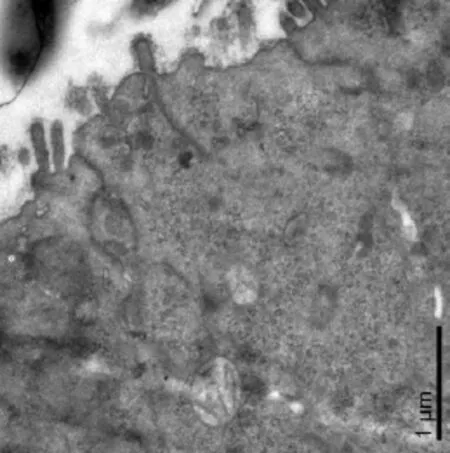
Fig. 6 Ultrastructure study of bacteria internalizated into the M cell. Extracellular bacteria perforating into the M cell and particles penetrating through the tight junctions and running parallel to long axis of cell (SCI group). Scale bars= 1 μm.
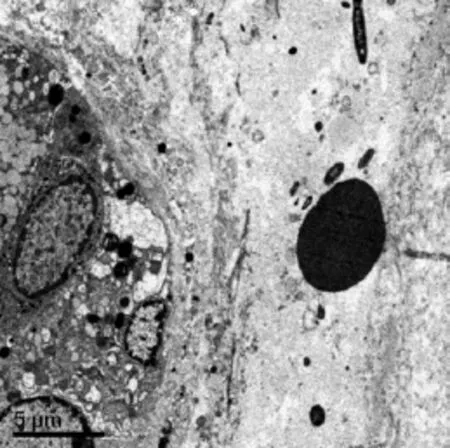
Fig. 7 Ultrastructure study of bacteria translocated into the blood. Bacteria swimming with erythrocytes in the submucosal blood capillary(SCI group). Scale bars= 5 μm.
Discussion
The gastrointestinal tract is a vital barrier against intraluminal pathogens entering the circulation and maintaining the immune homeostasis. The penetration of viable bacteria or bacterial compounds from the gastrointestinal tract to extraintestinal sites is defined as BT. The main promoting factors include: changes of the intestinal microf f ora, mucosal barrier failure, and defect of the host immunity. BT has been associated with systemic inf f ammatory response syndrome (SIRS) and MOF, and mortality rates, therefore it should be taken into account in the therapeutic strategies[3]. The intestinal epithelium associated with immune system have important barrier functions against luminal pathogens or microorganisms[4]. SCI evoked an immediate increase of gut permeability to macromolecules, enhancing the passage of viable bacteria to systemic organs[5].e mechanism of bacterial translocation is wide[6].To overcome this clinical problem, more study are needed, especially the mechanism of bacterial translocation[7].
The pathway of MLNs or/and bloodstream
Transcellular or/and paracellular pathway
BT mostly occurs directly through transcellular pathway even via morphological intact enterocytes, rather than paracellular pathway between enterocytes.e bacteria are transported through the individual epithelial cells the gastrointestinal tract to lymphatics. BT should not be considered as an ‘on-off’ or an ‘all-or-nothing’ phenomenon leading to clinical obvious changes under any circumstances. Babteria translocation by the intercellular route can reach the blood through the lymph. Increase translocation of nonpathogenic bacteria via the transcellular routes has been documented in epithelial cells under inff ammatory situations and metabolic stresses. Recent reports also showed that commensal bacteria may be engulfed into intestinal epithelial cells in the presence of pathogenic invasive bacterial strains[10]. Using a polarized human intestinal epithelial cell model system, it was demonstrated that Campylobacter jejuni not only penetrates into epithelial cells itself but also promotes the internalization and translocation of noninvasive, nonpathogenic E. coli via a lipid dependent mechanism[11].
Structurally, the intestine comprises a single-layer columnar epithelium, the cell-cell junctional complexes with selective paracellular permeability. In summary, intestinal permeability, a feature of intestinal barrier function, is increasingly recognized as relevance with health and disease of being.erefore, this topic arrants more attention[12]. A large number of evidence showed that abnormal passage of bacteria across the epithelial layer may occur via the paracellular routes in disease states.e paracellular route is taken by the bacteria entering the epithelium through the tight and adherens junctions between two neighboring cells. Opening of the cell-to-cell junctions may be a temporal process and potentially close again passed bacteria [13].
Paracellular leak became a direct pathway of the lymphatics lately. Most of the tight junctions shut and only allow small molecules less than 4 angstroms to pass through pores in the claudin proteins. However, paracellular leak can occur when larger pores has formed in the tight junctions due to the expression of dif f erent claudin proteins, allowing macromolecules like proteins and carbohydrates to traverse the epithelium[14]. In our study, the major route of BT aer SCI was transcellular pathway at early time. A lot of E. coli labeled with GFP penetrated into the mucous membrane of small intestine from the leakage of intestinal villus following with the destoried small intestine mucous.
Goblet cells-dendritic cells pathway
M cells pathway
To overcome the primary line of host defense, many microbes have evolved strategies to avert this initial attack and avoid the basolateral epithelial domain in which the immune cells reside. Microfold cells (M cells) are associated with the transepithelial vesicular trafficking of antigens and microorganisms, including pathogens and indigenous microbes, from the intestinal lumen into the underlying gut-associated lymphoid tissue.e structure of this specialized epithelial cell is quite distinct compared with the rest of the cells in the epithelium.e membrane surface of M cells have elaborates microprojections that facilitate ef fi cient endocytosis and transcytosis of microorganisms instead of an apical rigid brush border.e basolateral M cells invaginate and form a large intra epithelial “pocket” with immune cells such as T and B lymphocytes and macrophages[21]. In this study, we found bacteria and particles perforating into the M cell through the tight junctions running parallel to long axis of cell. It suggested that M cells are one of the best antigen delivery pathways of endepidermis.
In conclusion, we demonstrated that the routes of BT through MLNs and bloodstream interact and format a vicious circle. At early step, the transcellular pathway of BT is major. Following with the destoried small intestine mucous, paracellular leak became a direct pathway of the lymphatics lately. Besides transcellular and paracellular pathway, the goblet cells-dendritic cells and M cells pathway also play an important role in BT. We need an in-depth study for preventing BT and related complications in the future.
Conf l ict of interest
Acknowledgements
Supported by National Natural Science Foundation of China (NSFC) program (81101441) and Tianjin city enterprise postdoctoral innovation projects merit aid (First rate).
1. Bai C, An H, Wang S,et al. Treatment and prevention of bacterial translocation and endotoxemia with stimulation of the sacral nerve root in a rabbit model of spinal cord injury[J]. Spine (Phila Pa 1976), 2011, 36(5): 363-371.
3. Rosero O, Kovacs T, Onody P,et al. Bacterial translocation: gap in the shield[J]. Orv Hetil, 2014, 155(8): 304-312.
可靠性监测与检测蕴含着一个巨大的可利用网络服务体系,能认识到它的作用才能走进真正的汽车维修行业网络服务时代。汽修“互联网+”未能成功的原因,就是很多人并不了解维修企业与客户之间的关系。汽车维修企业需要真正解决的问题是有效地解决客户问题,让客户走进企业并留住客户。注意,这里说的客户走进企业是指同一个客户先期认可性;而留住客户指的是该客户成为忠诚性客户。
4. Turner JR. Intestinal mucosal barrier function in health and disease[J]. Nat Rev Immunol, 2009, 9(11): 799-809.
5. Ilan Y. Leaky gut and the liver: a role for bacterial translocation in nonalcoholic steatohepatitis[J]. World J Gastroenterol, 2012, 18(21): 2609-2618.
6. Martinez C, Gonzalez-Castro A, Vicario M,et al. Cellular and molecular basis of intestinal barrier dysfunction in the irritable bowel syndrome[J]. Gut Liver, 2012, 6(3): 305-315.
7. Wiest R, Rath HC. Gastrointestinal disorders of the critically ill. Bacterial translocation in the gut[J]. Best Pract Res Clin Gastroenterol, 2003, 17(3): 397-425.
8. Shifflett DE, Clayburgh DR, Koutsouris A,et al. Enteropathogenic E. coli disrupts tight junction barrier function and structure in vivo[J]. Lab Invest, 2005, 85(10): 1308-1324.
9. Amar J, Chabo C, Waget A,et al. Intestinal mucosal adherence and translocation of commensal bacteria at the early onset of type 2 diabetes: molecular mechanisms and probiotic treatment[J]. EMBO Mol Med, 2011, 3(9): 559-572.
10. Yu LC, Wang JT, Wei SC,et al. Host-microbial interactions and regulation of intestinal epithelial barrier function: From physiology to pathology[J]. World J Gastrointest Pathophysiol, 2012, 3(1): 27-43.
11. Kalischuk LD, Inglis GD, Buret AG. Campylobacter jejuni induces transcellular translocation of commensal bacteria via lipid ras[J]. Gut Pathog, 2009, 1(1): 2.
12. Bischoff SC, Barbara G, Buurman W,et al. Intestinal permeability--a new target for disease prevention and therapy[J]. BMC Gastroenterol, 2014, 14: 189.
13. Backert S, Boehm M, Wessler S,et al. Transmigration route of Campylobacter jejuni across polarized intestinal epithelial cells: paracellular, transcellular or both[J]? Cell Commun Signal, 2013, 11: 72.
14. Knoop KA, Miller MJ, Newberry RD. Transepithelial antigen delivery in the small intestine: dif f erent paths, dif f erent outcomes[J]. Curr Opin Gastroenterol, 2013, 29(2): 112-118.
15. McDole JR, Wheeler LW, McDonald KG,et al. Goblet cells deliver luminal antigen to CD103+dendritic cells in the small intestine[J]. Nature, 2012, 483(7389): 345-349.
16. Rescigno M, Urbano M, Valzasina B, et al. Dendritic cells express tight junction proteins and penetrate gut epithelial monolayers to sample bacteria[J]. Nat Immunol, 2001, 2(4): 361-367.
17. Contreras-Ruiz L, Masli S. Immunomodulatory cross-talk between conjunctival goblet cells and dendritic cells[J]. PLoS One, 2015, 10(3): e0120284.
18. Iwasaki M, Koyanagi S, Suzuki N,et al. Circadian modulation in the intestinal absorption of p-glycoprotein substrates in monkeys[J]. Mol Pharmacol, 2015, 88(1): 29-37.
19. Kim JJ, Khan WI. Goblet cells and mucins: role in innate defense in enteric infections[J]. Pathogens, 2013, 2(1): 55-70.
20. Iwasaki A. Mucosal dendritic cells[J]. Annu Rev Immunol, 2007, 25: 381-418.
21. Maldonado-Contreras AL, McCormick BA. Intestinal epithelial cells and their role in innate mucosal immunity[J]. Cell Tissue Res, 2011, 343(1): 5-12.
Xin-long MA, Orthopedics Professor, Tianjin Hospital, the Clinical Orthopedics of Tianjin Medical University, Tianjin 300211, China. E-mail: mjx969@163.com.
Received 2015-07-01; accepted 2015-10-18
猜你喜欢
杂志排行
中国应用生理学杂志的其它文章
- Wnt/β-catenin pathway might underlie the MET in transdifferentiation from MSC to MSC-derived neuron
- Impacts of exposure to 900 MHz mobile phone radiation on liver function in rats
- Establishment and evaluation of a mouse model of bronchial asthma with Yin deficiency syndrome
- Leptin receptor of the hind brain nuclei is involved in the conditioned taste preference of rats
- Effects of curcumin on sodium currents of dorsal root ganglion neurons in type 2 diabetic neuropathic pain rats
- Synergisms of cardiovascular effects between iptakalim and amlodipine, hydrochlorothiazide or propranolol in anesthetized rats
