蟾毒灵对TGF-β1诱导的HCT-116细胞上皮间质转化作用的影响
2015-05-16赵汝楠石晓静邱艳艳钱雅新殷佩浩
赵汝楠,于 卉,石晓静,邱艳艳,钱雅新,殷佩浩
上海中医药大学附属普陀医院普外科,上海 200062
蟾毒灵对TGF-β1诱导的HCT-116细胞上皮间质转化作用的影响
赵汝楠,于 卉,石晓静,邱艳艳,钱雅新,殷佩浩
上海中医药大学附属普陀医院普外科,上海 200062
目的 观察蟾毒灵(Bufalin)对大肠癌HCT-116细胞上皮间质转化作用的影响及其可能的机制。方法使用TGF-β1诱导大肠癌HCT-116细胞发生上皮间质转化(epithelial-mesenchymal transition,EMT),制备EMT模型。实验分为空白对照组、模型组、蟾毒灵组。72 h后,应用光学显微镜、侵袭实验、迁移实验观察蟾毒灵对HCT-116细胞形态学、侵袭与迁移能力的影响,Western blot检测EMT相关蛋白E-cadherin、Vimentin及β-catenin表达,免疫荧光法观察β-catenin蛋白分布。结果 模型组较空白组细胞明显伸长变窄且细胞间连接相对疏松。与模型组相比,蟾毒灵组部分细胞呈不典型鹅卵石样上皮细胞改变,部分细胞拉长变窄,细胞间连接较紧密;72 h穿膜侵袭细胞数较模型组明显减少(110.00±26.46vs.413.33±41.63,P<0.05),72 h迁移细胞数亦较模型组明显减少(507±38.16 vs.898±49.49,P<0.05),两者与空白对照组均无差异。Western blot检测显示:模型组较空白对照组上皮标志物E-cadhrin下降,间质标志物Vmentin上升;蟾毒灵组则较模型组E-cadhrin上调,Vimentin下调,β-catenin表达无差异。免疫荧光观察示,模型组β-catenin较空白对照组从细胞膜、胞质移至胞核内,蟾毒灵组β-catenin则较模型组集中于细胞质,胞核表达较少。结论 TGF-β1可诱导结肠癌HCT-116细胞EMT;蟾毒灵可能通过抑制β-catenin核移位、阻止HCT-116细胞EMT发挥抑制HCT-116细胞侵袭、迁移的作用。
大肠癌;蟾毒灵;上皮间质转化;转化生长因子-β1;侵袭;迁移
大肠癌是一种发病率居高不下的常见恶性肿瘤[1]。蟾毒灵(Bufalin)是一种有效的天然、中药抗肿瘤药物,具有抑制肿瘤细胞增殖、诱导凋亡、抗耐药等多种功效。研究表明,蟾毒灵在抑制肿瘤侵袭、转移方面具有较好疗效[2],上皮间质转化(epithelialmesenchymal transition,EMT)被认为是其抗侵袭转移的重要机制之一。EMT是指上皮细胞失去上皮特征,转化为长梭形的间质细胞现象;发生EMT的细胞由于其细胞间连接变得疏松,细胞运动能力增强,因而具有很强的侵袭转移能力。本实验应用TGF-β1诱导剂诱导大肠癌HCT-116细胞EMT,初步探讨蟾毒灵对HCT-116细胞EMT的作用及其与侵袭性的关系和可能机制。
1 材料与方法
1.1 细胞培养
HCT-116细胞购自中科院上海细胞中心;接种于含10%胎牛血清(fetal bovine serum,FBS)、100 U/ml青、链霉素的RP-1640培养液中,置于37℃,5%CO2饱和湿度的细胞培养箱内培养,0.25%胰酶消化、传代,取对数生长期细胞进行实验。
1.2 试剂与抗体
蟾毒灵购自成都瑞芬思生物科技有限公司;TGF-β1 购 自 Peprotech 公 司 ,产 品 编 号 100-21。RPMI-1640培养基购自北京海克隆公司;FBS购自BI公司;Transwell小室购自Corning公司;Matrigel基质胶购自BD Biosciences公司;结晶紫购自Biosharp公司;WB用GAPDH购自康为公司;E-cadherin、Vimentin、β-catenin抗体购自 abcam 公司;山羊抗小鼠二抗,山羊抗兔二抗,山羊抗兔荧光二抗:康为世纪公司。
1.3 实验方法与分组
选择对数生长期的HCT-116细胞按2×105/孔接种于6孔板内,每组3个复孔,每孔2 ml;置于37℃,5%CO2细胞培养箱内常规培养过夜。次日,更换新鲜培养基。实验分为空白对照组(1640培养基2 ml,即HCT-116+PBS)、模型组(含TGF-β1 10 ng/ml的1640培养基2 ml,即 HCT-116+TGF-β1 10 ng/ml)、蟾毒灵组(含TGF-β1 10 ng/ml+蟾毒灵1 nmol/L的1640培养基 2 ml,即 HCT-116+TGF-β1 10 ng/ml+蟾毒灵1 nmol/L)。37℃,5%CO2细胞培养箱内常规培养72 h。
1.4 细胞形态学观察
各组在37℃,5%CO2细胞培养箱内培养72 h后,倒置显微镜下观察,拍照。
1.5 侵袭、迁移实验
应用移液枪,枪头,Transwell小室(小室孔径8 μm),将Matrigel胶从4℃冰箱取出后,移入超净工作台的冰盒上备用。在24孔板的小室上铺胶,每孔50 μl。37℃温箱过夜。分别预处理各组细胞,37℃常规培养24 h后,收集离心,磷酸盐缓冲液(PBS)洗涤3次。空白组、模型组上室的相应细胞仅用单RPMI-1640培养基调整细胞浓度至1×106/ml,每孔200 μl接种,实验组上室细胞用含1 nmol/L的蟾毒灵(1 nmol/L)单RPMI-1640培养基接种。空白组小室下室加入含10%FBS的 RPMI-1640 500 μl,模型组、实验组则均加入含10%FBS及TGF-β1 10 ng/ml(作为诱导剂)的RPMI-1640 500 μl作为诱导剂。细胞在37℃,5%CO2条件下常规培养48 h后,取出小室,洗涤后甲醇固定20 min,风干,0.1%结晶紫染液染色20 min,显微镜下观察穿过小室的细胞数,拍照,计数。迁移实验除不在小室铺胶外,其余步骤同上述侵袭实验。
1.6 Western blot实验
各组细胞分别培养72 h后,使用碧云天蛋白裂解液(强)提总蛋白,二喹啉甲酸(BCA)法测定蛋白质浓度。取40 μg/孔蛋白进行10%SDS-PAGE分离,湿转法将蛋白质转移到聚偏二氟乙烯膜(PVDF)膜上,5%BSA室温封闭2 h,分别加入适量抗 体 E-cadherin(1∶10 000)、Vimentin(1∶1 000)、β-catenin(1∶10 000)、GAPDH(内参,1∶10 000),4℃孵育过夜;土温与三乙醇胺缓冲盐水溶液(TBST)洗涤3次,分别加入相应二抗,山羊抗兔(1∶2 000)、山羊抗小鼠(1∶10 000),室温孵育1 h,TBST洗涤3次,加增强化学发光法(ECL)发光试剂,暗室内曝光显影,图像分析。
1.7 细胞免疫荧光检测
用各组对应的培养基调整细胞浓度至1×105/ml,将细胞铺至无菌玻片上,37℃,5%CO2细胞培养箱内培养72 h后,取出玻片,PBS洗涤3次,4%多聚甲醛固定15 min,风干,5%BSA封闭1 h,β-catenin 1∶250稀释4℃孵育过夜,次日取出,PBS洗涤3次,加山羊抗兔荧光二抗(1∶400)孵育1 h后,PBS洗涤3次,4'6-二脒基-2-苯基吲哚(DAPI)核染2 min,PBS洗涤3次,甘油封片,倒置显微镜拍照。
1.8 统计学分析
采用SPSS19.0软件。实验数据以x±s表示。多组间比较采用单因素方差分析,组间比较采用LSDt法。以P<0.05为差异有统计学意义。
2 实验结果
2.1 形态学改变
空白组HCT-116细胞呈典型的上皮细胞形态结构,细胞多数呈鹅卵石样贴壁聚集生长,细胞间连接紧密,少量漂浮生长。模型组细胞经TGF-β1 72 h诱导,细胞明显拉长变窄,细胞间连接疏松,提示EMT模型制备成功。蟾毒灵组与模型组相比,细胞拉伸明显减弱,细胞间连接相对紧密,表明蟾毒灵在一定程度上抑制TGF-β1诱导的HCT-116细胞EMT(图1)。
2.2 侵袭、迁移能力
如图2,蟾毒灵组能穿透基质胶至小室膜下表面即穿膜细胞数明显较模型组减少(110.00±26.46vs.413.33±41.63,P<0.05),而空白组(86.67±11.72)与蟾毒灵组无差异。如图3,蟾毒灵组穿透小室膜即迁移细胞数亦较模型组明显减少(507±38.16vs.898±49.49,P<0.05),空白组(475±69.41)与药物组无差异。
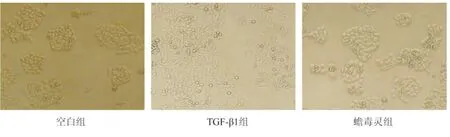
图1 各组细胞的形态(×100)Fig.1 Cell morphology in each group(×100)
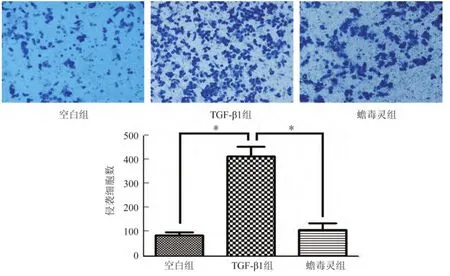
图2 各组细胞侵袭能力(×200)Fig.2 The invasion ability of cells in each group(×200)

图3 各组细胞的迁移能力(×200)Fig.3 The migration ability of cells in each group(×200)
2.3 EMT相关蛋白表达
如图4所示,上皮标志E-cadherin表达在模型组降低,蟾毒灵组上调;间质标志Vimentin表达在模型组上调,蟾毒灵组下调;β-catenin蛋白无变化。提示蟾毒灵上调E-cadherin、下调Vimentin表达,对β-catenin无影响,表明蟾毒灵的作用可能与EMT有关。

图4 各组细胞EMT相关蛋白表达Fig.4 The expression of EMT-related markers in each group
2.4 免疫荧光检测
空白组β-catenin主要集中于细胞膜与细胞质内;TGF-β1(模型)组β-catenin除胞膜与胞质有较强表达外,胞核内也有部分表达;蟾毒灵组较模型组β-catenin核内表达减少,胞质、胞膜表达较强(图5)。提示,与空白组相比,模型组β-catenin发生了核易位;而蟾毒灵在一定程度上抑制了模型组的核易位。
3 讨论
蟾毒灵是传统中药蟾酥的有效成分之一,现代药理学证明具有抑制肿瘤细胞增殖[3]、诱导凋亡[4]、抑制血管新生[5]和肿瘤侵袭转移[6]、抗多药耐药[7]等功效。EMT是指细胞失去上皮特性向间质细胞转化的现象,是肿瘤发生侵袭转移的重要机制之一。发生EMT的细胞逐渐失去其上皮细胞的形态与功能,细胞伸长变窄,细胞间连接变疏松,上皮标志E-cadherin表达降低,间质标志Vimentin表达升高;使得上皮细胞间粘附性与极性显著降低,细胞运动能力增强,最终使肿瘤细胞脱离原发肿瘤组织并向周围组织侵袭转移[8-9]。已有研究表明,蟾毒灵能抑制肿瘤侵袭转移,但在EMT中的作用知之甚少。
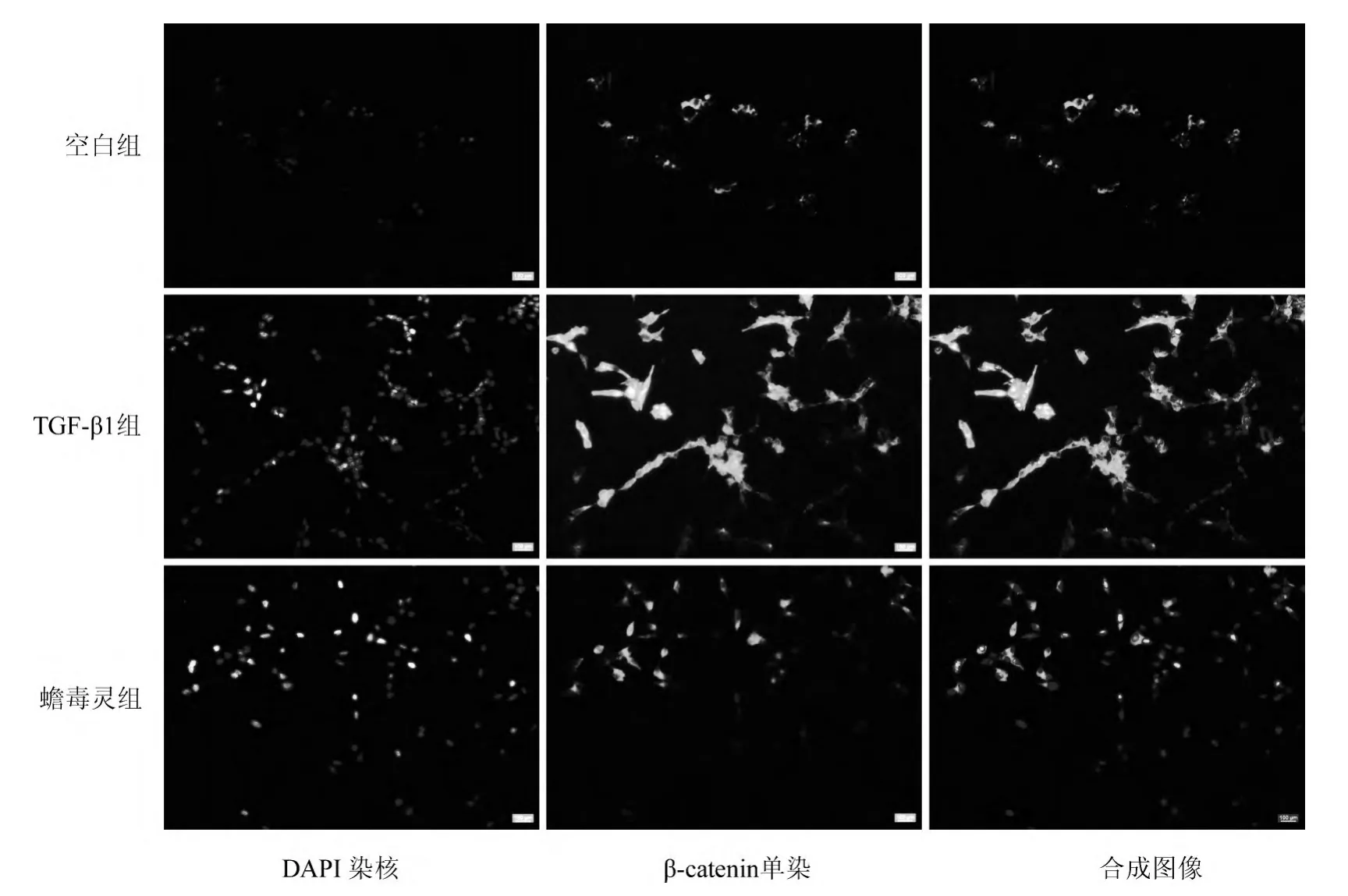
图5 各组细胞β-catenin表达(×500)Fig.5 The expression of β-catenin in each group(×500)
TGF-β1是TGF-β家族的重要因子之一,在多种肿瘤的发生发展中被证实为EMT的主要诱导剂[10-13],另外还是实体肿瘤转移侵袭的重要因子之一[14]。本实验应用TGF-β1作为EMT诱导剂诱导HCT-116细胞EMT以制备EMT模型。由于蟾毒灵具有很强的细胞毒性,故选取其无细胞毒性浓度[15]以排除细胞死亡造成的实验误差,即在EMT模型组基础上添加1 nmol/L的蟾毒灵单体进行细胞实验。作用72 h后经细胞形态学观察发现,与模型组相比,蟾毒灵组细胞具有不典型的上皮细胞特征,提示蟾毒灵在一定程度上抑制HCT-116细胞EMT;且侵袭、迁移实验和Western blot检测提示,蟾毒灵组较模型组细胞侵袭、迁移能力明显下降,蟾毒灵能逆转EMT标志蛋白表达。这些结果表明,蟾毒灵通过抑制TGF-β1诱导的HCT-116细胞EMT抑制其侵袭转移能力。
为进一步证明上述现象,本实验还检测了β-catenin蛋白表达及其分布情况。Western blot虽提示β-catenin总蛋白基本一致,免疫荧光检测则表明,模型组细胞中β-catenin核移位现象显著;而蟾毒灵在一定程度上抑制β-catenin向细胞核内移位,从而抑制其对相应靶基因的激活、最终抑制肿瘤侵袭转移。β-catenin属连环蛋白的一种,是调节细胞生长繁殖的重要因子之一;不但是Wnt/β-catenin经典信号通路的关键分子[16];还能与细胞膜上E-cadherin结合成复合体,参与细胞黏附、转移及细胞上皮极性[17]的调节。研究表明,E-cadherin对β-catenin的调节非常重要,该复合体调节细胞内β-catenin含量,一旦其含量变化可能会导致Wnt/β-catenin通路激活,最终影响肿瘤发展及预后[18]。另外,Wnt/β-catenin通路主要通过Wnt配体与相应受体结合,激活细胞内蛋白Dishevelled、抑制丝/苏氨酸激酶GSK3β活性,使其不能磷酸化β-catenin,去磷酸化的β-catenin在胞浆内累积、进入胞核与转录调节因子LEF/TCF共同作用,从而激活靶基因转录。诸多研究已证实Wnt/β-catenin通路在结肠癌中的作用。Qi等[19]报道,Wnt/β-catenin通路的激活能促进肿瘤血管生成拟态形成。Gao等[20]则认为,β-catenin表达水平及分布与结肠癌的预后、TNM分期、淋巴结转移等密切相关,并指出β-catenin核移位是结肠癌的临床病理分期及预后的重要评估指标。本实验结果提示,蟾毒灵通过抑制TGF-β1诱导的大肠癌HCT-116细胞EMT最终抑制其侵袭、转移,可能与抑制β-catenin核移位有关。但蟾毒灵对EMT的详细作用机制和信号通路相关性,还需作进一步的探讨。
[1] Rebecca L,Kimberly D,Ahmedin J.Cancer statistics[J].CA Cancer J Clin,2015,65(1):5-29.
[2] Chueh FS,Chen YY,Huang AC,et al.Bufalin-inhibited migration and invasion in human osteosarcoma u-2 os cells is carried out by suppression of the matrix metalloproteinase-2,ERK,and JNK signaling pathways[J].Environ Toxicol,2014,29(1):21-29.
[3] Yin P,Wang Y,Qiu Y,et al.Bufalin-loaded mPEG-PLGAPLL-Crgd nanoparticles:preparation,cellular uptake,tissue distribution,and anticancer activity[J].Int J Nanomedicine,2012,7(1):3961-3969.
[4] Zhu ZT,Sun HZ,Ma GY,et al.Bufalin induces lung cancer cell apoptosis via the inhibition of PI3K/AKT pathway[J].Int J Mol Sci,2012,13(2):2025-2035.
[5] Lee DY,Yasuda M,Yamamoto T,et al.Bufalin inhibits endouthelial cell proliferation and angiogenesis invitro[J].Life Sci,1997,60(2):127-134.
[6] Qiu YY,Hu Q,Tang QF,et al.MicroRNA-497 and bufalin act synergistically to inhibit colorectal cancer metastasis[J].Tumor Biol,2014,35(3):2599-2606.
[7] Zhai X,Lu J,Wang Y,et al.Reversal effect of bufalin on multidrug resistance in K562/VCR vincristine-resistant leukemia cell line[J].J Tradit Chin Med,2014,34(6):678-683.
[8] Grünert S,Jechlinger M,Beug H.Diverse cellular and molecular mechanisms contribute to epithelial plasticity and metastasis[J].Nat Rev Mol Cell Biol,2003,4(8):657-665.
[9] Hugo H,Ackland ML,Blick T,et al.Epithelial-mesenchymal and mesenchymal-epithelialtransitionsin carcinoma progression[J].J Cell Physiol,2007,213(2):374-383.
[10] Fan JM,Ng YY,Hill PA,et al.Transforming growth factor-beta regulates tubular epithelial myo Fibroblast transdifferentiation in vitro[J].Kidney Int,1999,56(4):1455-1467.
[11] Kasai H,Allen JT,Mason RM,et al.TGF-BETA1 induces human alveolarepithelialto mesenchymalcelltransition(EMT)[J].Respir Res,2005,6(1):56.
[12] Ellenrieder V,Handler SF,Boeck V,et al.Tranforming growth factor beta1 treatment leads to an epithelial-mesenchymal transdifferentiation of pancreatic cancer cells requiring extracelluar signal-regulated kinase 2 activatoin[J].Cancer Res,2001,61(10):4222-4228.
[13] Rees JR,Onwuegbusi BA,Save VE,et al.In vivo and in vitro evidence for transforming growth factor-beta1-mediated epithelialto mesenchymaltransiton in esophagealsdenocarcinoma[J].Cancer Res,2006,66(19):9583-9590.
[14] Zhu QC,Gao RY,Wu W,et al.Epithelial-mesenchymal transition and its role in the pathogenesis of colorectal cancer[J].Asian Pac J Cancer Prev,2013,14(5):2689-2698.
[15] 王旭,陈腾,奉典旭,等.蟾毒灵对人大肠癌HCT116细胞增殖的影响[J].时珍国医国药,2011,22(6):1513-1514.
[16] Brabletz T,Jung A,Hermann K,et al.Nuclear overexpression of the oncoprotein beta-catenin in colorectal cancer is localized predominantly at the invasion front[J].Pathol Res Pract,1998,194(10):701-704.
[17] Guarino M,Rubino B,Ballabio G.The role of epithelialmesenchymal transition in cancer pathology[J].Pathology,2007,39(3):305-318.
[18] Ghanhari NM,Babashah S.Interplay between microRNAs and Wnt/β-catenin signaling pathway regulates epithelialmesenchymal transition in cancer[J].Eur J Cancer,2015,51(12):1638-1649.
[19] Qi L,Song W,Liu Z,et al.Wnt3a promotes the vasculogenic mimicry formation of colon cancer via Wnt/β-catenin signaling[J].Int J Mol Sci,2015,16(8):18564-18579.
[20] Gao ZH,Lu C,Wang MX,et al.Differential β-catenin expression levels are associated with morphological features and prognosis of colorectal cancer[J].Oncol Left,2014,8(5):2069-2076.
The influence of Bufalin on TGF-β1-induced epithelial mesenchymal transition in HCT-116 cells
ZHAO Runan,YU Hui,SHI Xiaojing,QIU Yanyan,QIAN Yaxin,YIN Peihao
Department of General Surgery,Putuo Hospital,Shanghai University of Traditional Chinese Medicine,Shanghai 200062,China
ObjectiveTo explore the influence of Bufalin on epithelial mesenchymal transition(EMT)in colorectal cancer HCT-116 cells and its possible mechanism.MethodsEMT models of colorectal cancer HCT-116 cells were prepared by TGF-β1.Blank control group,model group and bufalin group were divided,and interventions were conducted.After 72 h of intervention,the morphological changes,invasion and migration capabilities,expression of EMT-related proteins including E-cadherin,Vimentin and β-catenin,and β-catenin protein distribution of HCT-116 cells in each group were determined by using light microscope,Transwell-invasion and migration tests,Western blotting and immunofluorescence method,respectively.ResultsCells in model group became elongated and spindle,with relatively lax connections between cells when compared with blank control group,and some cells in Bufalin group were elongated and narrow,with close cell connections when compared with model group.The number of cell permeation in Bufalin group(110.00±26.46)was significantly smaller than that in model group(413.33±41.63)(P<0.05),and the number of cell migration in Bufalin group was significantly decreased when compared with model group(507±38.16vs.898±49.49,P<0.05),whereas there was no significant difference in the numbers of cell permeation and migration between blank control group and Bufalin group.Compared with model group,the expression of E-cadherin was increased,and the expression of Vimentin was decreased in Bufalin group.Compared with blank control group,the expression of E-cadherin was decreased,and the expression of Vimentin was increased in model group.However,there was no significant difference in β-catenin expression among groups.The β-catenin expression moved from cell membrane and cytoplasm to nucleus in model group when compared with blank control group,and there was more β-catenin expression in cytoplasm than nucleus in Bufalin group when compared with model group.Conclusions TGF-β1 induces EMT of colorectal cancer HCT-116 cells.Bufalin inhibits invasion and migration of HCT-116 cells by inhibiting EMT and β-catenin nuclear translocation.
Colorectal cancer;Bufalin;Epithelial mesenchymal transition;Transforming growth factor-β1;Invasion;Migration
R656.9
A
2095-378X(2015)04-0217-06
10.3969/j.issn.2095-378X.2015.04.001
上海中医药大学“杏林学者”(B-X-72)和中西医结合一流学科创新基金项目(B-X-73);上海市普陀区卫计委“315”工程人才培养计划学科带头人后备人才(14Q-RC-08)
赵汝楠(1989—),女,硕士,研究肿瘤分子病理
殷佩浩,yinpeihao1975@hotmail.com
