尿L-FABP与2型糖尿病患者肾功能变化的纵向研究
2015-04-21祝超瑜高清歌蒋伏松
朱 珍,祝超瑜,高清歌,徐 立,喻 晶,蒋伏松,魏 丽
尿L-FABP与2型糖尿病患者肾功能变化的纵向研究
朱 珍,祝超瑜,高清歌,徐 立,喻 晶,蒋伏松,魏 丽*
(上海市交通大学附属第六人民医院内分泌代谢科,上海 201306)
尿肝脏型脂肪酸结合蛋白(L-FABP)是早期预测急性和慢性肾功能损伤的优良生物学标志物。本项研究旨在前瞻性地探讨尿L-FABP水平预测2型糖尿病患者肾病进展及估算的肾小球滤过率(eGFR)下降率的价值。对2010年1月至2012年6月于我院内分泌科住院的288名2型糖尿病患者进行分组(正常尿白蛋白组、微量尿白蛋白组、大量尿白蛋白组)并随访2年,测定各组随访前后尿L-FABP水平、尿白蛋白排泄率(UAER)和eGFR。随着研究的进展,微量尿白蛋白组和大量尿白蛋白组随访后的L-FABP水平均高于随访前水平(<0.05),而仅大量白蛋白尿组随访后的UAER高于随访前水平(<0.05)。Pearson线性相关分析结果显示:随访前,大量尿白蛋白组和微量尿白蛋白组的尿L-FABP水平与UAER均呈显著正相关(分别为=0.573,=0.219;<0.05);随访后,大量尿白蛋白组和微量尿白蛋白组的尿L-FABP水平亦均与UAER呈显著正相关(分别为=0.689,=0.203;<0.05)。多元逐步回归分析结果提示,随访前和随访后大量尿白蛋白组尿L-FABP水平与eGFR变化率显著相关(分别为=-0.397,=-4.376;=-0.455,=-4.854;<0.05);随访前和随访后微量尿白蛋白组的尿L-FABP水平与eGFR变化率亦显著相关(分别为=-0.327,=-2.987;=-0.378,=-4.298;<0.05)。尿L-FABP水平与糖尿病患者的肾功能相关,动态监测尿L-FABP可早期预测2型糖尿病肾病的进展。尿L-FABP可能早期独立预测2型糖尿病肾病患者的eGFR下降情况。
糖尿病肾病;肝脏型脂肪酸结合蛋白;尿白蛋白排泄率;肾小球滤过率
糖尿病肾病是导致慢性肾脏疾病(chronic kidney disease,CKD)的主要病因之一,也是主要的糖尿病远期微血管并发症之一[1]。近年研究发现,肝脏型脂肪酸结合蛋白(liver-type fatty acid binding protein,L-FABP)与肾损伤关系密切,是早期预测急性肾损伤的优良生物学标志物[2]。然而,L-FABP在临床预测糖尿病患者肾功能变化方面的作用尚不确定[3−5]。本前瞻性研究拟探讨尿L-FABP水平与2型糖尿病患者肾功能进展的关系,以及其预测肾小球滤过率(glomerular filtration rate,GFR)变化情况的价值。
1 对象与方法
1.1 研究对象
收集2010年1月至2012年6月上海市第六人民医院内分泌代谢科住院的2型糖尿病患者。纳入标准:60~80岁之间、且符合1999年世界卫生组织(World Health Organization,WHO)制定的2型糖尿病诊断标准的糖尿病患者。排除标准:糖尿病急性并发症、恶性肿瘤、伴有其他急、慢性肾脏疾病和肝功能异常者;过去3个月内患有心脑血管疾病者(包括冠心病、心肌缺血、脑血管疾病或周围动脉疾病);过去3个月内因感染而住院的患者;血压未控制者。共纳入符合条件者350例,受试者均已签署知情同意书。
1.2 研究方法
对所有研究对象随访2年,在研究初始和结束时分别收集人口学资料和临床数据,包括:研究对象的年龄、性别、身高、体质量、血压和糖尿病病程等,并计算体质量指数。空腹抽取静脉血,测定空腹血糖(fasting blood glucose,FBG)、糖化血红蛋白(glycosylated hemoglobin A1c,HbA1c)、血肌酐(serum creatinine,Scr)、甘油三酯(triglycerides,TC)、总胆固醇(total cholesterol,TC)、高密度脂蛋白胆固醇(high-density lipoprotein cholesterol,HDL-C)等生化指标的水平。血糖、血脂和Scr测定使用酶法,HbA1c用高压液相法。研究初始和研究结束分别收集1次24h尿液样本,采用免疫透射比浊法测定尿白蛋白排泄率(urinary albumin excretion rate,UAER)。L-FABP用免疫酶联法测定(人L-FABP ELISA试剂盒,Hycult Biotech公司,荷兰)。根据试剂盒说明书,每个L-FABP标本测2次,所得的平均值进行进一步的统计分析。
估算肾小球滤过率(estimated glomerular filtration rate,eGFR)根据慢性肾脏病流行病学合作研究(Chronic Kidney Disease Epidemiology collaboration,CKD-EPI)公式[6]通过Scr计算,女性:当Scr≤62μmol/L时,eGFR=144×(Scr/62)-0.329×(0.993)年龄;当Scr>62μmol/L时,eGFR=144×(Scr/62)-1.209×(0.993)年龄;男性:当Scr≤80μmol/L时,eGFR=141×(Scr/80)-0.411×(0.993)年龄;当Scr>80μmol/L时,eGFR=141×(Scr/80)-1.209×(0.993)年龄。GFR变化率通过以下的公式计算:GFR变化率=(研究结束时eGFR−基线eGFR)/(基线eGFR×随访时间)。
本研究的纳入群体根据研究初始的UAER情况分为3组:正常白蛋白尿组(group 1),UAER<30mg/24h;微量白蛋白尿组(group 2),UAER在30~300mg/24h之间;大量白蛋白尿组(group 3),UAER≥300mg/24h。所有入选糖尿病患者根据美国糖尿病联合会(American Diabetes Association,ADA)糖尿病治疗指南的方案进行治疗[7]。
1.4 统计学处理
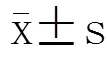
2 结 果
2.1 各组研究对象的临床特征及相关临床指标随访前后的变化
350例研究对象中,有54例未能完成随访且失访,8例在随访期间死亡(其中4例死于冠心病,2例死于脑血管意外,2例死于肾功能衰竭)。因此,最终分析288例的结果(回访率82.3%)。研究初始,研究对象的年龄(66.1±9.2)岁,糖尿病病程为(7.8±6.5)年。在288例2型糖尿病患者中,正常尿白蛋白组有111例,占回访病例总数的38.5%,其中男性57例(51.4%);微量尿白蛋白组有100例(34.7%),男49例(49.0%);大量尿白蛋白组有77例(26.7%),男50例(64.9%)。
研究群体相关临床指标随访前后的变化情况具体见表1。研究初始(随访前),3组患者的年龄、体质量指数、舒张压、血糖、血脂情况差异无统计学意义(>0.05);胰岛素、血管紧张素转换酶抑制剂、血管紧张素受体拮抗剂、贝特类降脂药、他汀类降脂药物使用情况也无明显差异(>0.05);大量尿白蛋白组和微量尿白蛋白组的糖尿病病程均比正常白蛋白尿组延长(<0.05),大量尿白蛋白组的收缩压(systolic blood pressure,SBP)、尿素氮(blood urea nitrogen,BUN)、Scr也高于正常尿白蛋白组(<0.05)。随着研究的进展,随访后各组研究对象的体质量指数、血压、血糖、血脂水平较随访前无明显改变(>0.05),胰岛素、血管紧张素转换酶抑制剂、血管紧张素受体拮抗剂、贝特类降脂药、他汀类降脂药物使用情况较随访前也无明显改变(>0.05),但大量尿白蛋白组的BUN和Scr较随访前有明显升高(<0.05)。
2.2 随访前后eGFR、UAER、L-FABP的变化
随访前和随访后,大量尿白蛋白组的eGFR均低于正常尿白蛋白组、微量尿白蛋白组(<0.05),微量尿白蛋白组的eGFR与正常尿白蛋白组无明显差异(>0.05)。随访后微量尿白蛋白组和大量尿白蛋白组的eGFR水平较随访前降低(<0.05)。
随访前和随访后,大量尿白蛋白组的UAER均显著高于正常尿白蛋白组和微量尿白蛋白组(<0.05),微量尿白蛋白组的UAER也均高于正常尿白蛋白组(<0.05)。而随访后仅大量尿白蛋白组的UAER水平较随访前升高(<0.05)。
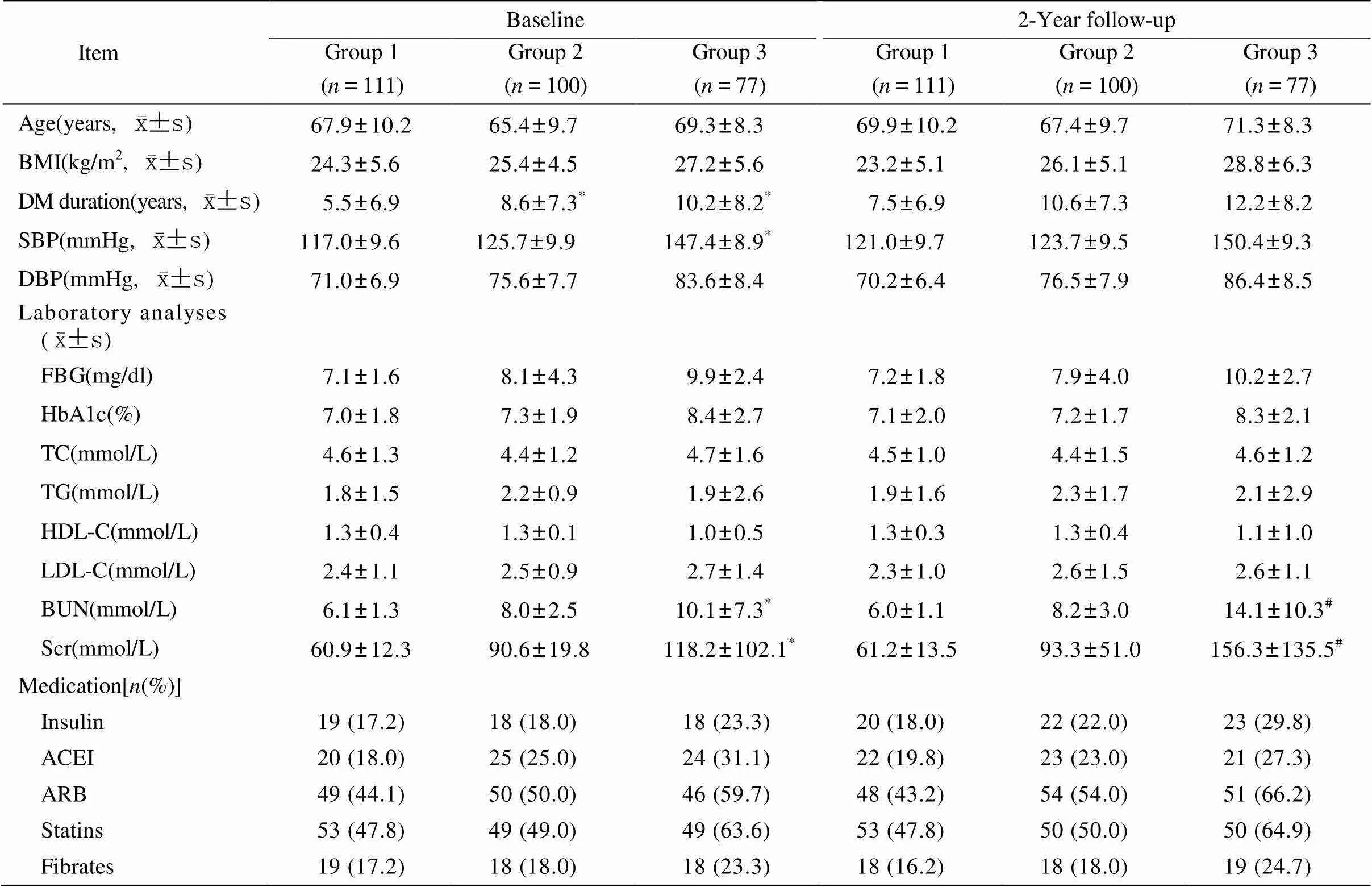
表1 各组患者相关临床指标随访前后的变化情况
BMI: body mass index; DM: diabetes mellitus; SBP: systolic blood pressure; DBP: diastolic blood pressure; FBG: fasting blood glucose; HbA1c: glycosylated hemoglobin A1c; TC: total cholesterol; TG: triglycerides; HDL-C: high-density lipoprotein cholesterol; LDL-C: low-density lipoprotein cholesterol; BUN: blood urea nitrogen; Scr: serum creatinine; ACEI: angiotensin-converting-enzyme inhibitor; ARB: angiotensin receptor blocker. Group 1: normo-albuminuria group; group 2: micro-albuminuria group; group 3: macro-albuminuria group. Compared with group 1,*<0.05; compared with baseline level,#<0.05
随访前和随访后,大量尿白蛋白组的L-FABP均高于正常尿白蛋白组和微量尿白蛋白组(<0.05),微量尿白蛋白组的L-FABP也高于正常尿白蛋白组(<0.05)。随访后微量尿白蛋白组和大量尿白蛋白组的L-FABP水平均较随访前升高(<0.05,表2)。提示L-FABP在糖尿病患者肾脏功能变化早期即出现升高,动态监测尿L-FABP可能是早期预测2型糖尿病肾病的进展的生物学指标。
2.3 尿L-FABP水平和eGFR的相关性分析
Pearson线性相关结果提示:研究初始,大量尿白蛋白组和微量尿白蛋白组的尿L-FABP水平与eGFR呈负相关(分别为=-0.461,=-0.194;<0.05),大量尿白蛋白组和微量尿白蛋白组的尿L-FABP基线水平与UAER呈正相关(=0.573,=0.219;<0.05);其他与尿L-FABP呈正相关的指标有收缩压、HbA1c、BUN和Scr。随访后,尿大量白蛋白组和微量尿白蛋白组的尿L-FABP水平仍与eGFR呈负相关(分别为=-0.379,=-0.199;<0.05),大量尿白蛋白组和微量尿白蛋白组的尿L-FABP水平与UAER显著正相关(=0.689,=0.203;<0.05);其他与尿L-FABP呈正相关的指标有SBP、FBG、HbA1c、BUN和Scr(表3)。
计算各组患者eGFR变化率,并与各项肾损伤指标包括血压、BUN、Scr、UAER、尿F-LABP等进行多元回归分析。结果提示,随访前微量尿白蛋白组和大量尿白蛋白组的尿L-FABP水平与eGFR变化率显著相关(分别为=-0.327,=-2.987;=-0.397,=-4.376;<0.05);其他与eGFR变化率显著相关的因素包括:大量尿白蛋白组的UAER水平和SBP;随访后微量尿白蛋白组和大量尿白蛋白组的尿L-FABP水平与eGFR变化率显著相关(分别为=-0.378,=-4.298;=-0.455,=-4.854;<0.05),其他与eGFR变化率显著相关的因素包括随访后大量尿白蛋白组的BUN、Scr和SBP(表4)。结果提示尿L-FABP可早期独立预测2型糖尿病肾病患者的eGFR下降情况。
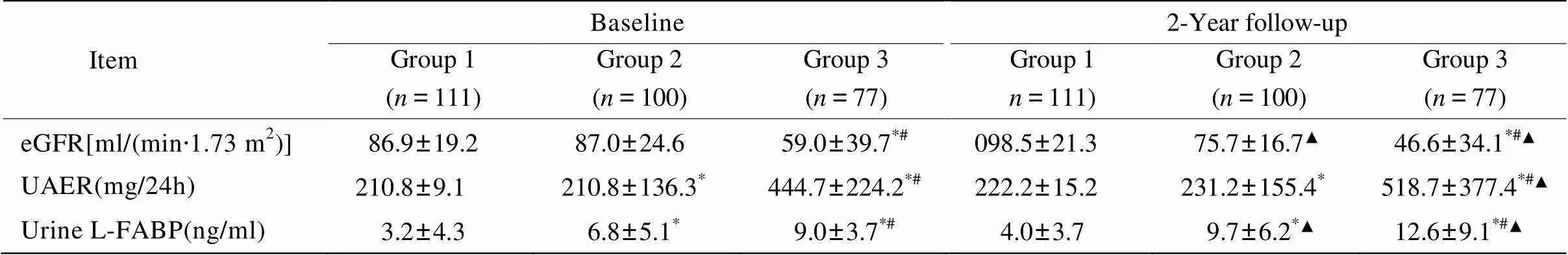
表2 随访前后3组患者eGFR、UAER、尿L-FABP的变化
eGFR: estimated glomerular filtration rate; UAER: urinary albumin excretion rate; L-FABP: liver-type fatty acid binding protein. Group1: normo-albuminuria group; Group 2: micro-albuminuria group; Group 3: macro-albuminuria group. Compared with group 1,*<0.05; compared with group 2,#<0.05; compared with baseline level,▲<0.05
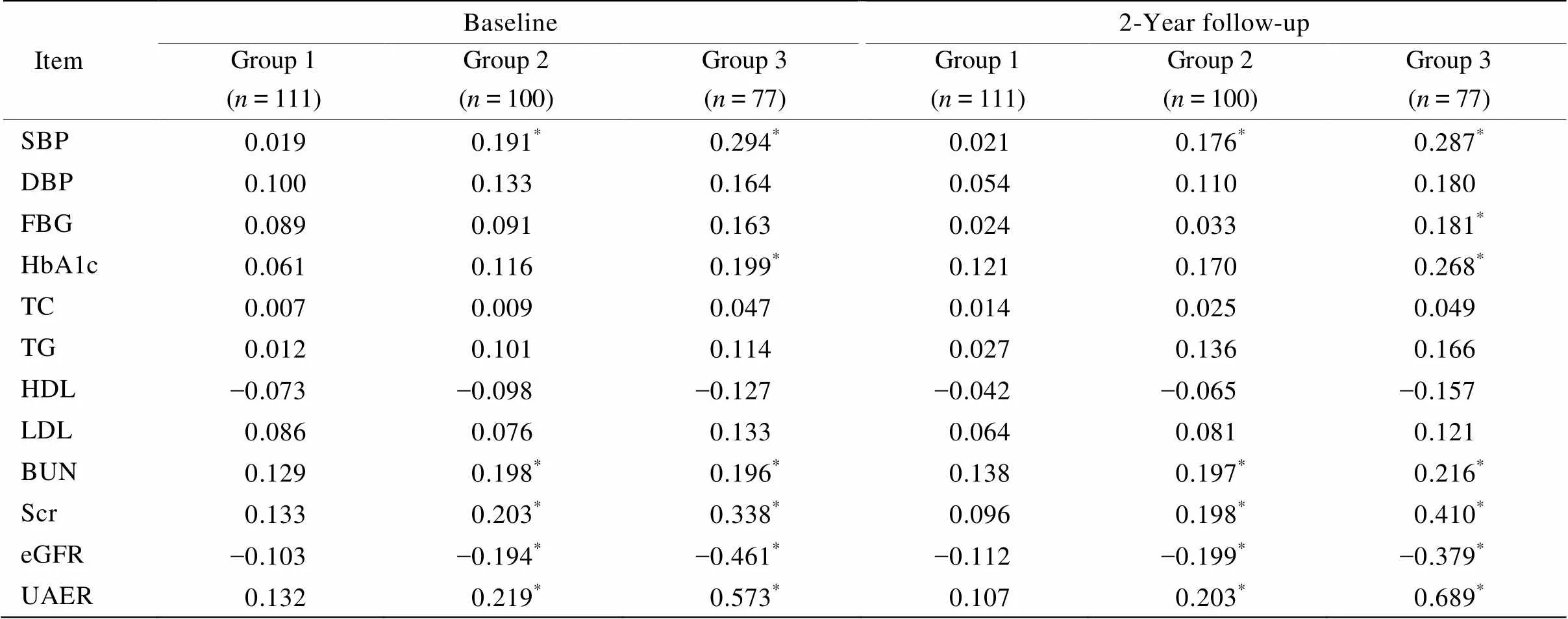
表3 尿L-FABP和各项临床生化指标的线性关系
SBP: systolic blood pressure; DBP: diastolic blood pressure; FBG: fasting blood glucose; HbA1c:glycosylated hemoglobin A1c; TC: total cholesterol; TG: triglycerides; HDL-C: high-density lipoprotein cholesterol; LDL-C: low-density lipoprotein cholesterol; BUN: blood urea nitrogen; Scr: serum creatinine; eGFR: estimated glomerular filtration rate: UAER: urinary albumin excretion rate. Group 1: normo-albuminuria group; group 2: micro-albuminuria group; group 3: macro-albuminuria group. Significantly correlated with urine L-FABP level,*<0.05
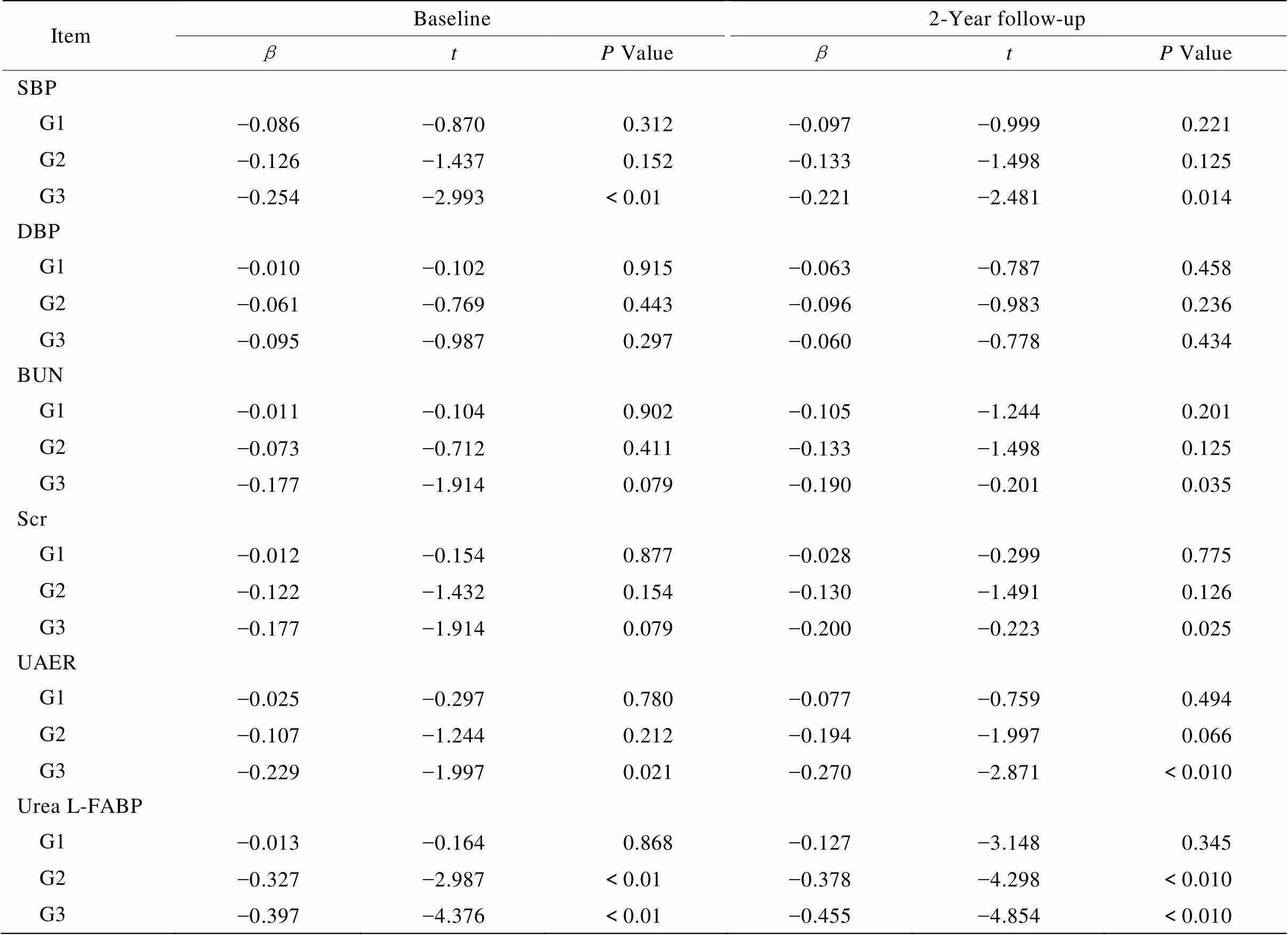
表4 各组患者eGFR变化率与随访前后尿L-FABP的多元逐步回归分析
SBP: systolic blood pressure; DBP: diastolic blood pressure; BUN: blood urea nitrogen; Scr: serum creatinine; UAER: urinary albumin excretion rate; L-FABP: liver-type fatty acid binding protein; G1: group 1, normo-albuminuria group; G2: group 2, micro-albuminuria group; G3: group 3, macro-albuminuria group
3 讨 论
糖尿病肾病是CKD的主要原因[8]。糖尿病肾病进入大量尿白蛋白阶段或CKD阶段时,肾脏损伤常不可逆[9]。早期检测和干预是治疗糖尿病肾病的关键[10,11]。微量尿白蛋白为糖尿病肾病的早期表现,与疾病进展至终末期肾脏疾病和心血管事件相关[12−15]。但多项研究表明,长期患有糖尿病的患者在微量尿白蛋白的发生前即可出现肾脏病理性异常[16],甚至出现严重肾功能受损[17−19]。故寻找一个能早期预测糖尿病患者肾功能变化的生物标志物是国内外学者目前研究的热点。
虽然大多数糖尿病肾病患者由于出现肾小球改变而导致蛋白尿,但是否最终形成终末期肾功能衰竭,还是取决于肾小管间质纤维化改变[20−22],故肾小管损伤的标志物可有助于确定肾功能衰退的程度。最近的几项研究表明,肾小管损伤标志物,如肾损伤分子−1(kidney injury molecule-1,KIM-1)、中性粒细胞明胶酶相关脂质运载蛋白(neutrophil gelatinase associated lipocalin,NGAL)和L-FABP,可能是确定糖尿病患者肾功能减退程度的潜在的临床标志物[23−26]。L-FABP在肾小管上皮细胞中表达,近期一些学者发现肾小管周围毛细血管血流量减少引起缺氧时,L-FABP则脱落到尿液中,进一步研究发现,2型糖尿病患者尿L-FABP水平与eGFR下降率显著相关,尿L-FABP可能成为反映糖尿病患者肾功能减退的临床生物标志物[27−29]。据上,国外专家对尿L-FABP和糖尿病肾病的研究较为热门,但均在初步阶段,研究不够全面,也不够深入,研究病例数也均较少。国内尚无尿L-FABP和糖尿病肾病或糖尿病肾功能受损方面的研究,我们首先进行了这方面的研究,具有创新性。本研究包括288名不同程度的糖尿病肾病患者,使用肾小管损伤的生物标志物尿L-FABP来预测2型糖尿病肾病患者的肾功能变化。通过前瞻性研究,我们发现,尿L-FABP水平与糖尿病患者的肾功能改变相关,动态监测尿L-FABP可早期预测2型糖尿病肾病的进展,进一步研究发现,尿L-FABP可能早期独立预测2型糖尿病肾病患者的eGFR下降情况。
综上,我们的结果显示尿L-FABP水平与2型糖尿病肾病的进展和肾功能下降情况密切相关,尤其尿L-FABP可能是早期独立预测2型糖尿病患者肾功能下降的潜在临床标志物。定期检测尿L-FABP水平和积极治疗,对预防和延缓糖尿病患者肾功能恶化尤其是尿毒症的发生发展可能有一定作用。本研究仍存在一些局限,由于本篇主要为随访观察研究,未将正常糖耐量人群的资料作为对照,同时也缺乏干预,但我们的试验数据提示横断面分析与纵向分析结果一致,而这项研究也正在不断进行和完善中,相信进一步的研究将提供更有力的临床依据。
[1] Gray SP, Cooper ME. Diabetic nephropathy in 2010: alleviating the burden of diabetic nephropathy[J]. Nat Rev Nephrol, 2011, 7(2): 71−73.
[2] McMahon BA, Murray PT. Urinary liver fatty acid-binding protein: another novel biomarker of acute kidney injury[J]. Kidney Int, 2010, 77(8): 657−659.
[3] Fu WJ, Li BL, Wang SB,. Changes of the tubular markers in type 2 diabetes mellitus with glomerular hyperfiltration[J]. Diabetes Res Clin Pract, 2012, 95(1): 105−109.
[4] Fu WJ, Xiong SL, Fang YG,. Urinary tubular biomarkers in short-term type 2 diabetes mellitus patients: a cross-sectional study[J]. Endocrine, 2012, 41(1): 82−88.
[5] Nielsen SE, Andersen S, Zdunek D,. Tubular markers do not predict the decline in glomerular filtration rate in type 1 diabetic patients with overt nephropathy[J]. Kidney Int, 2011, 79(10): 1113−1118.
[6] Wang HB, Xia XK, Wu JH. Assessment of CKD-EPI equation estimating glomerular filtration rate[J]. Int J Lab Med, 2011, 32(9): 936−941. [王宏斌, 夏先考, 吴建华. CKD-EPI方程估算肾小球滤过率的评价[J]. 国际检验医学杂志, 2011, 32(9): 936−941.]
[7] American Diabetes Association. Standards of medical care in diabetes—2012[J]. Diabetes care, 2012, 35 (Suppl 1): S11−S63.
[8] Rossing P. Diabetic nephropathy: worldwide epidemic and effects of current treatment on natural history[J]. Curr Diab Rep, 2006, 6(6): 479−483.
[9] Nielsen SE, Schjoedt KJ, Astrup AS,. Neutrophil gelatinase-associated lipocalin (NGAL) and kidney injury molecule 1 (KIM1) in patients with diabetic nephropathy: a cross-sectional study and the effects of lisinopril[J]. Diabet Med, 2010, 27(10): 1144−1150.
[10] Nickolas TL, Barasch J, Devarajan P. Biomarkers in acute and chronic kidney disease[J]. Curr Opin Nephrol Hypertens, 2008, 17(2): 127−132.
[11] Dronavalli S, Duka I, Bakris GL. The pathogenesis of diabetic nephropathy[J]. Nat Clin Pract Endocrinol Metab, 2008, 4(8): 444−452.
[12] de Zeeuw D, Ramjit D, Zhang Z,. Renal risk and renoprotection among ethnic groups with type 2 diabetic nephropathy: a post hoc analysis of RENAAL[J]. Kidney Int, 2006, 69(9): 1675−1682.
[13] Ruggenenti P, Remuzzi G.. Time to abandon microalbuminuria[J]? Kidney Int, 2006, 70(7): 1214−1222.
[14] Dinneen SF, Gerstein HC. The association of microalbuminuria and mortality in non-insulin- dependent diabetes mellitus. A systematic overview of the literature[J]. Arch Intern Med, 1997, 157(13): 1413−1418.
[15] Ninomiya T, Perkovic V, de Galan BE,. Albuminuria and kidney function independently predict cardiovascular and renal outcomes in diabetes[J]. J Am Soc Nephrol, 2009, 20(8): 1813−1821.
[16] Fioretto P, Steffes MW, Brown DM,. An overview of renal pathology in insulin-dependent diabetes mellitus in relationship to altered glomerular hemodynamics[J]. Am J Kidney Dis, 1992, 20(6): 549−558.
[17] Klausen K, Borch-Johnsen K, Feldt-Rasmussen B,. Very low levels of microalbuminuria are associated with increased risk of coronary heart disease and death independently of renal function, hypertension, and diabetes[J]. Circulation, 2004, 110(1): 32−35.
[18] Adler AI, Stevens RJ, Manley SE,. Development and progression of nephropathy in type 2 diabetes: the United Kingdom Prospective Diabetes Study(UKPDS 64)[J]. Kidney Int, 2003, 63(1): 225−232.
[19] Yamazaki M, Tani N, Igarashi K,. Changes in the glomerular pore size selectivity in patients with typeⅡ diabetes mellitus[J]. J Diabet Complications, 1991, 5(2-3): 138−139.
[20] Mauer SM, Steffes MW, Ellis EN,. Structural-functional relationships in diabetic nephropathy[J]. J Clin Invest, 1984, 74(4): 1143−1155.
[21] Bohle A, Wehrmann M, Bogenschutz O,. The pathogenesis of chronic renal failure in diabetic nephropathy. Investigation of 488 cases of diabetic glomerulosclerosis[J]. Pathol Res Pract, 1991, 187(2−3): 251−259.
[22] Phillips AO, Steadman R. Diabetic nephropathy: the central role of renal proximal tubular cells in tubulointerstitial injury[J]. Histol Histopathol, 2002, 17(1): 247−252.
[23] Najafian B, Alpers CE, Fogo AB. Pathology of human diabetic nephropathy[J]. Contrib Nephrol, 2011, 170: 36−47.
[24] Bolignano D, Lacquaniti A, Coppolino G,. Neutrophil gelatinase-associated lipocalin as an early biomarker of nephropathy in diabetic patients[J]. Kidney Blood Press Res, 2009, 32(2): 91−98.
[25] Nauta FL, Boertien WE, Bakker SJ,. Glomerular and tubular damage markers are elevated in patients with diabetes[J]. Diabetes Care, 2011, 34(4): 975−981.
[26] Araki S, Haneda M, Koya D,. Predictive effects of urinary liver-type fatty acid-binding protein for deteriorating renal function and incidence of cardiovascular disease in type 2 diabetic patients without advanced nephropathy[J]. Diabetes Care, 2013, 36(5): 1248−1253.
[27] Kamijo A, Sugaya T, Hikawa A,. Urinary liver-type fatty acid binding protein as a useful biomarker in chronic kidney disease[J]. Mol Cell Biochem, 2006, 284(1−2): 175−182.
[28] Panduru NM, Forsblom C, Saraheimo M,. Urinary liver-type fatty acid-binding protein and progression of diabetic nephropathy in type 1 diabetes[J]. Diabetes Care, 2013, 36(7): 2077−2083.
[29] Chou KM, Lee CC, Chen CH,. Clinical value of NGAL, L-FABP and albuminuria in predicting GFR decline in type 2 diabetes mellitus patients[J]. PLoS One, 2013, 8(1): e54863.
(编辑: 刘子琪)
Longitudinal study on relationship of urinary L-FABP with renal function in type 2 diabetes mellitus patients
ZHU Zhen, ZHU Chao-Yu, GAO Qing-Ge, XU Li, YU Jing, JIANG Fu-Song, WEI Li*
(Department of Endocrinology, the Sixth People’s Hospital Affiliated to Shanghai Jiao Tong University, Shanghai 201306, China)
Urine liver-type fatty acid binding protein (L-FABP) is emerging as an excellent biomarker for the early prediction of acute and chronic kidney injury. The aim of this prospective study was to determine the value of urine L-FABP in predicting the progression of nephropathy and the decline of estimated glomerular filtration rate (eGFR) in type 2 diabetic patients.A longitudinal cohort study was conducted on 288 type 2 diabetic patients for 2 consecutive years, who were admitted to Department of Endocrinology of our hospital during January 2010 to June 2012. They were divided into normo-, micro- and macro-albuminuria groups according to their 24h-urinary albumin excreting rate (UAER). Urine levels of L-FABP, and UAER were determined.The follow-up levels of urinary L-FABP in both the micro- and macro-albuminuria groups were significantly higher than their baseline levels (<0.05), but only the macro-albuminuria group had the level of UAER higher than its baseline (<0.05). The results of Pearson correlations showed that urine L-FABP was correlated with UAER in the micro- and macro-albuminuria groups at both before and after follow-up (before:=0.573,=0.219,<0.05; after:=0.689,=0.203,<0.05). Multivariate stepwise regression analysis indicated that the urinary L-FABP levels before and after follow-up were significantly correlated with eGFR decline rate in both the macro- and micro-albuminuria groups (macro-albuminuria group:=-0.397,=-4.376,=-0.455,=-4.854,<0.05; micro-albuminuria group:=-0.327,=-2.987,=-0.378,=-4.298,<0.05).Urine L-FABP is correlated with renal function in the patients with type 2 diabetes mellitus, and its dynamic monitoring may predict the progression of diabetic nephropathy. Urine L-FABP may independently predict the early decline of eGFR in type 2 diabetic nephropathy patients.
diabetic nephropathy; liver-type fatty acid binding protein; albuminuria; glomerular filtration rate
(PWZxq2014-07).
R587.1
A
10.11915/j.issn.1671-5403.2015.05.077
2014−12−26;
2015−04−02
上海市浦东新区卫生系统重点学科群建设基金(PWZxq2014-07)
魏 丽, E-mail: 18930173636@189.com
