银杏叶提取物通过Wnt减轻内质网应激诱导的心肌细胞损伤
2015-04-21沈明志李冬云付振虹韩宝石刘海斌
沈明志,李冬云,范 利,付振虹,韩宝石,李 可,胡 鑫,刘海斌,薛 桥*
银杏叶提取物通过Wnt减轻内质网应激诱导的心肌细胞损伤
沈明志1,2,李冬云1,范 利2,付振虹1,韩宝石1,李 可1,胡 鑫1,刘海斌3,薛 桥1*
(1解放军总医院海南分院心内科,三亚 572013;2解放军总医院老年心内科,北京 100853;392474部队医院内科,三亚 572018)
探讨银杏叶提取物(EGB)对内质网应激诱导心肌细胞损伤的影响以及Wnt通路在其中的作用。取1d龄乳鼠心肌细胞培养后,应用衣霉素(Tm)构建心肌细胞损伤模型,随机分为对照组、Tm组、Tm+EGB组、EGB组。噻唑蓝(MTT)比色法检测心肌细胞存活率,双荧光报告系统检测Wnt活性,实时定量PCR检测C-myc、CyclinD1基因水平。Tm降低了心肌细胞存活率,EGB处理改善了心肌细胞存活率;与对照组比较,Tm组Wnt活性显著降低,与Tm组比较,Tm+EGB组Wnt活性、C-myc、CyclinD1水平明显升高,而应用Wnt抑制剂分泌型卷曲相关蛋白(sFRP),Wnt活性显著下调,C-myc、CyclinD1水平下调,EGB的保护作用显著下降。EGB可以抑制Tm诱导的大鼠心肌细胞损伤,其机制可能与改善Wnt信号有关。
银杏叶提取物;衣霉素;内质网应激;心肌细胞;Wnt
内质网参与了膜蛋白与分泌蛋白的折叠过程,在细胞维持功能与存活过程中扮演了重要角色[1,2]。糖剥夺、病毒感染、错误折叠或未折叠蛋白的增加将会干扰内质网的功能,从而诱发内质网应激(endoplasmic reticulum stress,ERS)[3,4]。ERS是机体对外界或自身反应的一种自我保护机制,以维持内质网的稳态,促进细胞生存,但过强或持续时间过长的ERS可以诱导细胞凋亡[5,6]。
研究表明,Wnt信号通路在抗凋亡过程中扮演了重要角色[7−9]。然而,Wnt信号在ERS过程中扮演的作用迄今尚无报道。
银杏叶又名白果叶,是从银杏科植物银杏的干燥叶提取的有效成分,主要化学成分包括银杏内酯及黄酮苷,具有保护心血管、改善微循环的功效[10,11],但机制尚不完全清楚。
本实验拟应用衣霉素(tunicamycin,Tm)诱导大鼠心肌细胞损伤模型[12],观察银杏叶提取物(extracts ofleaf,EGB)对衣霉素诱导心肌细胞损伤的影响,以及对Wnt信号通路表达水平的影响及意义,为EGB防治ERS相关性疾病提供理论依据。
1 材料与方法
1.1 实验动物和试剂
1日龄SD乳鼠(解放军总医院医学动物实验中心);EGB(中国药科大学植化教研室);杜尔贝科改良培养基(Dulbecco’s modified Eagle medium,DMEM,Gibco公司);胎牛血清(四季青公司);胶原酶、噻唑蓝(MTT)双荧光报告系统(Promega公司);二甲基亚砜(dimethyl sulfoxide,DMSO)、5−溴−2−脱氧核苷尿嘧啶(BrdU)(Sigma公司);实时定量聚合酶链反应(polymerase chain reaction,PCR)检测试剂盒(TaKaRa公司)。
1.2 原代培养新生大鼠心肌细胞
方法同文献[13],取1d龄乳鼠心脏,冰磷酸盐缓冲液(phosphate buffered saline,PBS)清洗3次,剔除心房以及大血管等成分,将其剪成小米粒大小组织块,用Ⅰ型胶原酶(1mg/ml)分次消化(3~5min/次)。收集心肌细胞悬液,加入含10%血清DMEM培养基中以终止消化,1000转/min离心5min,弃上清。重悬细胞,用滴管吹打至单细胞悬液,经200目筛网过滤后接种于培养瓶,并在孵箱中差速贴壁1h。加入BrdU母液,使其终浓度为100μmol/L。37℃CO2细胞培养箱孵育24h后,更换含BrdU的DMEM培养液,37℃继续孵育培养48h,备用。
1.3 药物处理和实验分组
实验分对照组、Tm组、Tm+EGB组和EGB组。对照组:单纯培养液,不给予任何药物;Tm组:培养液中加100ng/ml Tm;Tm+EGB组:培养液中加100ng/ml Tm和20、50、100μg/ml EGB;EGB组:培养液中加50μg/ml EGB。
1.4 MTT比色法检测心肌细胞存活率
心肌细胞接种于96孔板,经无血清培养基处理后,随机分组。经过相应处理,每孔加入5mg/ml MTT母液10μl,37℃孵育4 h。吸弃上清,加入150μl DMSO以溶解沉淀,选择490nm波长,振荡3min。检测各孔吸光度()值(值与活细胞数呈正比),实验重复5次。
1.5 实时定量PCR
提取各组细胞总RNA,应用Quanti Tect Reverse Transcription Kit试剂盒进行细胞cDNA合成。使用ABI prism 7500实时定量PCR仪,应用the Power SYBR Green PCR Master Mix进行实时定量PCR荧光检测。β-actin作为内参照。c-myc正义链:5'-CCTCAGTGGTCTTCCC-CTAC-3',反义链:5'-CTGGAGCATTTGCGGTTG-3';cyclinD1正义链:5'-GAGGAGCAGAAGTGCGAAGA-3',反义链:5'- GGAGGGTGGGTTGGAAAT-3';β-actin正义链:5'-AGGGAAATCGTGCGTGAC-3',反义链:5'-CTGGAAGGTGGACAGTGAG-3'。
1.6 H9C2心肌细胞系培养及Topflash活性检测
H9C2心肌细胞系(ATCC公司),应用含有10%胎牛血清的DMEM培养基在5% CO2孵箱中37℃情况下进行培养。取第三代以后细胞进行实验。
检测Topflash活性方法同前[14],应用Lipo2000将Topflash和pRL-TK质粒共转染H9C2细胞中,转染36h后,应用裂解缓冲液裂解细胞,应用双荧光系统报告试剂盒分析Topflash活性。
1.7 统计学处理
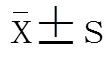
2 结 果
2.1 不同浓度EGB对Tm诱导大鼠心肌细胞损伤的影响
将对照组心肌细胞存活率定义为100%。与对照组比较,Tm组心肌细胞存活率显著下降,差异有统计学意义(<0.05);与Tm组相比,Tm(100ng/ml)+EGB(20μg/ml)组心肌细胞存活率轻度升高,而Tm+EGB(50或100μg/ml)心肌细胞存活率显著升高,呈浓度依赖性升高,差异有统计学意义(<0.05,图1)。本部分实验表明,EGB能够减轻Tm诱导的心肌细胞损伤。
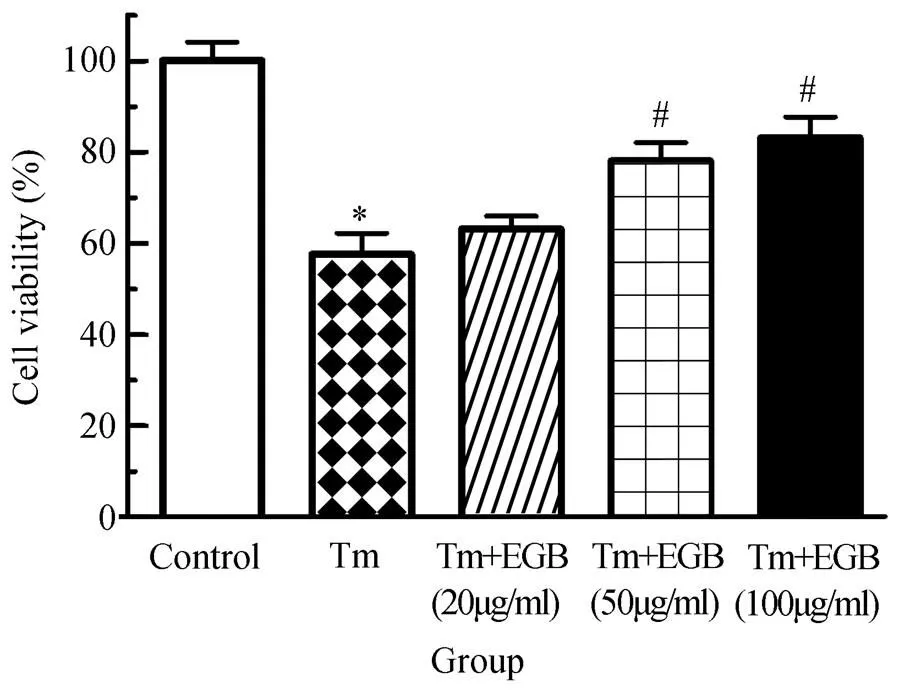
图1 EGB对Tm诱导的心肌细胞存活率的影响
Figure 1 Effects of EGB on Tm-induced cardiomyocytes viability
EGB: extracts ofleaf; Tm: tunicamycin. Compared with control group,*<0.05; compared with Tm group,#<0.05
2.2 EGB对Tm诱导Wnt信号通路的影响
为了研究Wnt信号通路在EGB改善心肌细胞存活率中的作用,我们应用Topflash双荧光报告系统转染H9C2心肌细胞系,检测Wnt活性。结果表明,Tm可以显著降低Topflash活性,差异有统计学意义(<0.05);而加用EGB可以显著改善Topflash活性,与Tm组相比,差异有统计学意义(<0.05)。与对照组相比,单用EGB时Topflash活性没有明显变化,差异无统计学意义(>0.05,图2)。本部分研究结果表明,Tm能够抑制Wnt活性,而加用EGB可以显著改善Wnt活性。
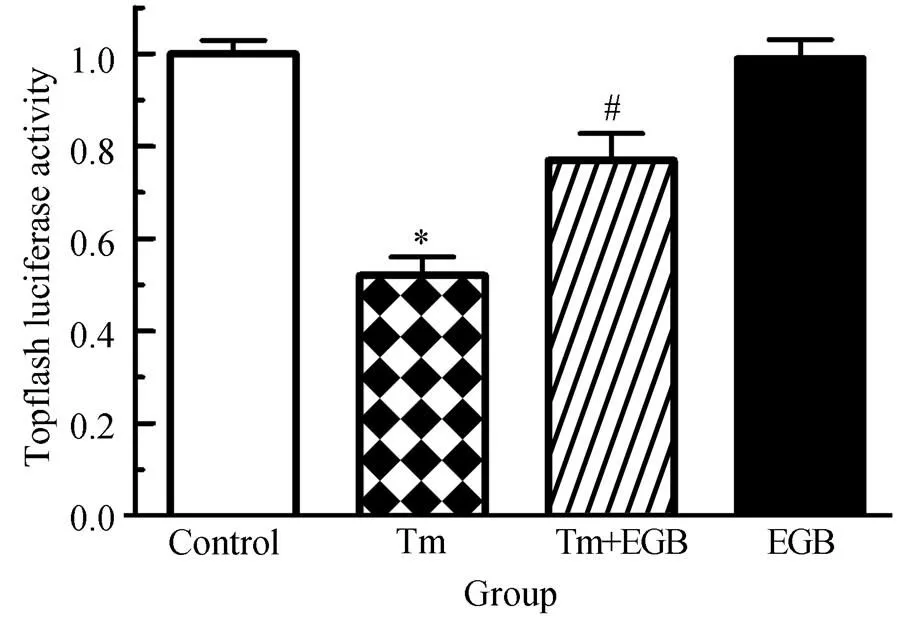
图2 EGB对Tm诱导的心肌细胞Wnt活性的影响
Figure 2 Effects of EGB on Tm-induced Wnt activity in cardiomyocytes
EGB: extracts ofleaf; Tm: tunicamycin. Compared with control group,*<0.05; compared with Tm group,#<0.05
2.3 EGB对Tm诱导C-myc、CyclinD1的影响
为了进一步验证Wnt信号通路在EGB改善心肌细胞存活率中的作用,我们检测了Wnt信号通路下游基因C-myc、CyclinD1的水平。研究结果表明,Tm可以显著下调C-myc、CyclinD1水平,与对照组相比,差异有统计学意义(<0.05);而加用EGB两者表达水平显著上调,并且呈现EGB浓度依赖性上调,与Tm组相比,差异有统计学意义(<0.05)。然而,与对照组相比,单用EGB时两者表达水平并没有显著变化(>0.05,图3和图4)。本部分研究结果进一步表明,Tm抑制了Wnt活性,而EGB能够显著改善Wnt活性。
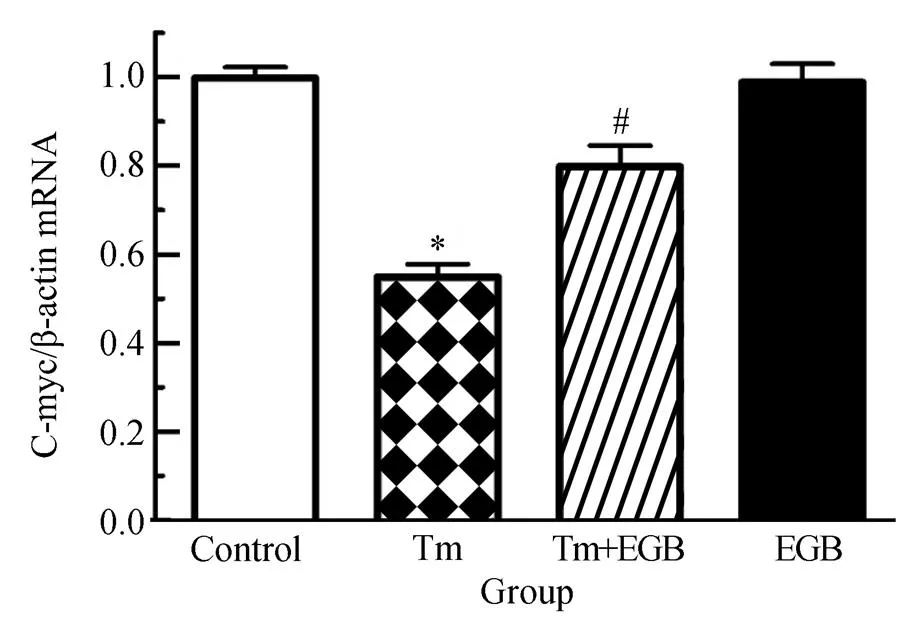
图3 EGB对Tm诱导的心肌细胞C-myc水平的影响
Figure 3 Effects of EGB on Tm-induced C-myc in cardiomyocytes
EGB: extracts ofleaf; Tm: tunicamycin. Compared with control group,*<0.05; compared with Tm group,#<0.05
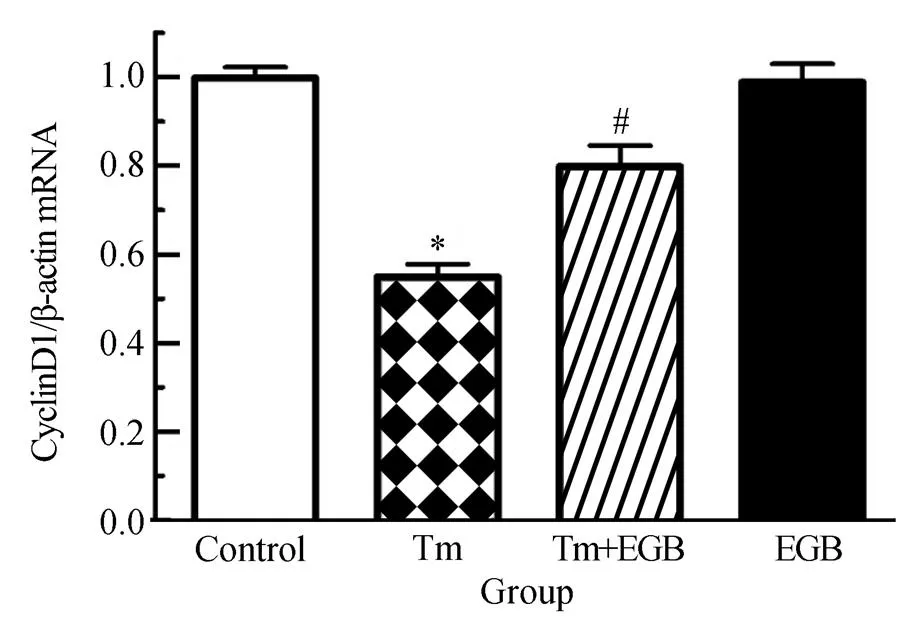
图4 EGB对Tm诱导的心肌细胞CyclinD1水平的影响
Figure 4 Effects of EGB on Tm-induced CyclinD1 in cardiomyocytes
EGB: extracts ofleaf; Tm: tunicamycin. Compared with control group,*<0.05; compared with Tm group,#<0.05
2.4 抑制Wnt信号通路抵消EGB的保护作用
为了进一步验证Wnt信号通路在EGB发挥保护作用中的地位,我们给予Wnt抑制剂分泌型卷曲相关蛋白(secreted frizzled related protein,sFRP,图5)。结果表明,与Tm+EGB组相比,Tm+EGB+sFRP组心肌细胞存活率显著降低,差异具有统计学意义(<0.05)。本部分研究结果表明,EGB主要是通过Wnt信号通路发挥保护作用的。
3 讨 论
心血管疾病的发病率和死亡率居高不下,是威胁人类健康的“第一杀手”。在发达国家,心血管疾病的病死率呈现了下降趋势,而在我国,心血管疾病的病死率还在迅猛增长,防控形势不容乐观。心血管疾病发病过程伴随心肌细胞凋亡,由于心肌细胞属于终末分化细胞,因而导致心肌细胞数量绝对减少,使心肌收缩力下降,从而发展至心功能不全,导致心力衰竭[14]。研究表明,死亡受体和线粒体途径是导致细胞凋亡最主要的途径;近十年来,ERS途径诱导的细胞凋亡越来越受到关注[15],Tm可以抑制内质网内新生蛋白质糖基化从而诱导ERS[16]。
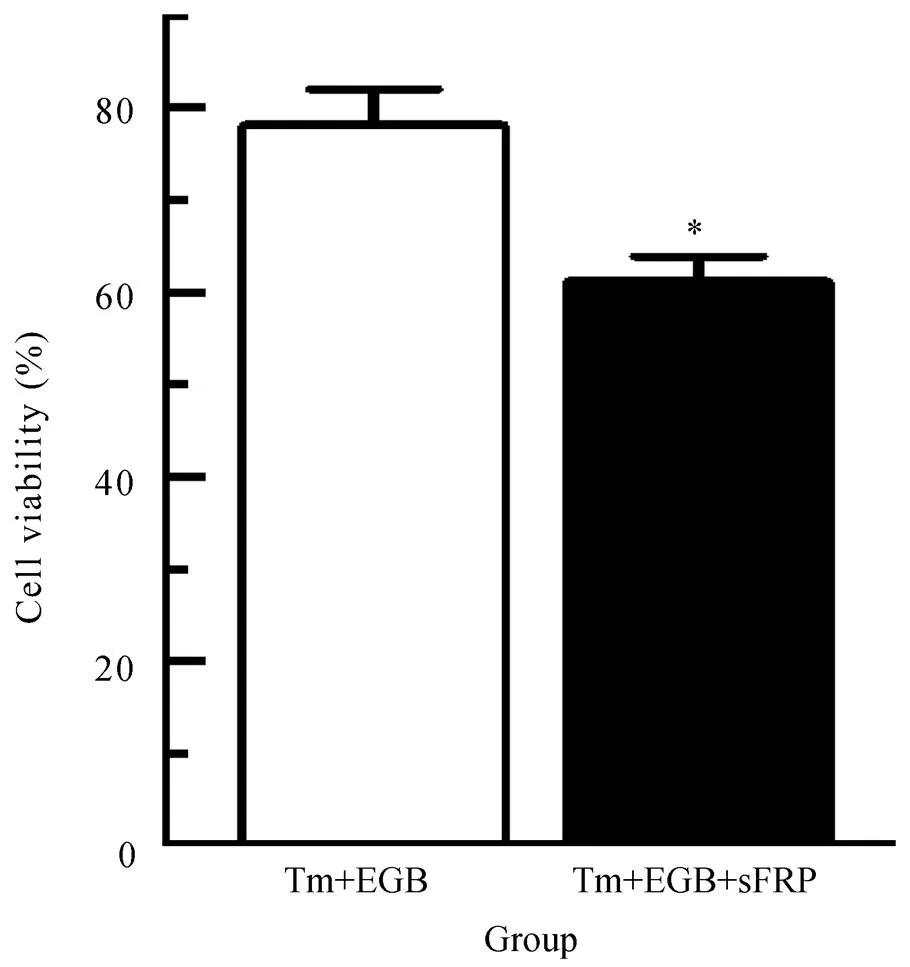
图5 Wnt信号通路抑制剂对EGB保护作用的影响
Figure 5 Effects of Wnt signal pathway inhibitor on EGB protective role in cardiomyocytes
EGB: extracts ofleaf; Tm: tunicamycin; sFRP: secreted frizzled related protein. Compared withTm+EGB group,*<0.05
EGB是银杏叶经过多步骤分离提取后得到的天然活性物质,主要为黄酮类、萜类内酯化合物、多糖类等。大量研究表明,EGB具有拮抗血小板活化因子、清除自由基、抗炎及抗过敏等作用。然而,EGB对Tm诱导的心肌损伤是否有保护作用目前尚未清楚。本实验应用Tm构建ERS诱导的心肌细胞损伤模型,观察EGB对细胞存活状况的影响。
本研究表明,应用Tm处理乳鼠心肌细胞可以导致心肌细胞损伤。而加用EGB的心肌细胞存活率明显升高,并且呈现了实验剂量范围内的浓度依赖性,首次发现了EGB可以减轻Tm诱导的心肌细胞损伤。
为了进一步验证EGB的保护机制,我们将目光转移到具有抗凋亡作用的Wnt信号通路上来。研究发现,Tm可以显著降低Wnt活性,而加用EGB可以显著恢复Wnt活性。为了进一步证明两者的相关性,我们检测了其下游基因C-myc、CyclinD1水平。研究发现,Tm可以降低两者的水平,而加用EGB可以显著恢复它们的水平,进一步表明EGB可以显著影响Wnt信号通路。为了证明EGB的保护作用是通过Wnt信号通路实现的,我们应用了Wnt信号通路抑制剂sFRP,结果表明,加用sFRP抵消了EGB的保护作用,进一步证实了EGB通过Wnt减轻了心肌ERS诱导的心肌细胞损伤,达到保护受损心肌细胞的目的。
综上所述,本实验结果表明Tm激活了心肌细胞ERS,降低了Wnt活性,导致了心肌细胞损伤;而加用EGB,可以恢复Wnt活性,从而达到保护受损心肌细胞的目的。这可能为EGB抑制ERS诱导的心肌损伤提供新的治疗靶点。
[1] Kaufman RJ. Stress signaling from the lumen of the endoplasmic reticulum: coordination of gene transcriptional and translational controls[J]. Genes Dev, 1999, 13(10): 1211−1233.
[2] Hetz C. The unfolded protein response: controlling cell fate decisions under ER stress and beyond[J]. Nat Rev Mol Cell Biol, 2012, 13(2): 89−102.
[3] Sciarretta S, Zhai P, Shao D,. Activation of NADPH oxidase 4 in the endoplasmic reticulum promotes cardiomyocyte autophagy and survival during energy stress through the protein kinase RNA-activated-like endoplasmic reticulum kinase/eukaryotic initiation factor 2α/activating transcription factor 4 pathway[J]. Circ Res, 2013, 113(11): 1253−1264.
[4] He B. Viruses, endoplasmic reticulum stress, and interferon responses[J]. Cell Death Differ, 2006, 13(3): 393−403.
[5] Zou CG, Cao XZ, Zhao YS,. The molecular mechanism of endoplasmic reticulum stress-induced apoptosis in PC-12 neuronal cells: the protective effect of insulin-like growth factorⅠ[J]. Endocrinology, 2009, 150(1): 277−285.
[6] Rao RV, Ellerby HM, Bredesen DE. Coupling endoplasmic reticulum stress to the cell death program[J]. Cell Death Differ, 2004, 11(4): 372−380.
[7] Zhu Y, Chi J, Liu Y,. Knockdown of dishevelled-1 attenuates cyclosporine A-induced apoptosis in H9c2 cardiomyoblast cells[J]. Mol Cell Biochem, 2013, 374(1−2): 113−123.
[8] Zhu P, Chen G, You T,. High FFA-induced proliferation and apoptosis in human umbilical vein endothelial cell partly through Wnt/beta-catenin signal pathway[J]. Mol Cell Biochem, 2010, 338(1−2): 123−131.
[9] Mill C, George SJ. Wnt signalling in smooth muscle cells and its role in cardiovascular disorders[J]. Cardiovasc Res, 2012, 95(2): 233−240.
[10] Liu PP, Pan SH. Advance in study of ginkgolic acid contained inpreparations[J]. China J Chin Mater Med, 2012, 37(3): 274−277. [刘平平, 潘苏华. 银杏叶制剂中银杏酚酸研究进展[J]. 中国中药杂志, 2012, 37(3): 274−277.]
[11] Shen MJ, Xu Y, Li RB,. Effects of EGB on AGEs-induced Endoplasmic Reticulum Stress in Cardiomyocytes[J]. Prog Mod Biomed, 2013, 13(4): 2633−2635, 2793. [沈明志, 徐 勇, 李榕彬, 等. 银杏叶提取物对AGEs诱导心肌细胞内质网应激的影响[J]. 现代生物医学进展, 2013, 13(4): 2633−2635, 2793.]
[12] Shen MJ, Liu JN, Zhai YL,. Construction of Neonatal Rat Cardiomyocyte Apoptosis Model by tunicamycin-induced endoplasmic reticulum stress[J]. Prog Mod Biomed, 2011, 11(5): 801−804. [沈明志, 刘佳妮, 翟雅莉, 等. 衣霉素诱导大鼠心肌细胞内质网应激凋亡模型的构建[J]. 现代生物医学进展, 2011, 11(5): 801−804.]
[13] Shen M, Wang L, Wang B,. Activation of volume-sensitive outwardly rectifying chloride channel by ROS contributes to ER stress and cardiac contractile dysfunction: involvement of CHOP through Wnt[J]. Cell Death Dis, 2014, 5: e1528.
[14] Hodges P. Heart failure: epidemiologic update[J]. Crit Care Nurs Q, 2009, 32(1): 24−32.
[15] Okada K, Minamino T, Tsukamoto Y,. Prolonged endoplasmic reticulum stress in hypertrophic and failing heart after aortic constriction: possible contribution of endoplasmic reticulum stress to cardiac myocyte apoptosis[J]. Circulation, 2004, 110(6): 705−712.
[16] Han C, Nam MK, Park HJ,. Tunicamycin-induced ER stress upregulates the expression of mitochondrial HtrA2 and promotes apoptosis through the cytosolic release of HtrA2[J]. J Microbiol Biotechnol, 2008, 18(6): 1197−1202.
(编辑: 李菁竹)
EGB attenuates endoplasmic reticulum stress-induced injury in cultured rat neonatal cardiomyocytes through Wnt signal pathway
SHEN Ming-Zhi1,2, LI Dong-Yun1, FAN Li2, FU Zhen-Hong1, HAN Bao-Shi1, LI Ke1, HU Xin1, LIU Hai-Bin3, XUE Qiao1*
(1Department of Cardiology, Hainan Branch of Chinese PLA General Hospital, Sanya 572013, China;2Department of Geriatric Cardiology, Chinese PLA General Hospital, Beijing 100853, China;3Department of Internal Medicine, Hospital of Troop 92474, Sanya 572018, China)
To determine the effect of the extracts ofleaf (EGB) on the endoplasmic reticulum stress-induced cardiomyocyte injury and investigate the role of Wnt signal pathway in this process.Tunicamycin(Tm) was used to establish the endoplasmic reticulum stress model in rat cardiomyocytes which were isolated from neonatal rats in 1d after born and then cultured for 48h. There were 4 groups of cardiomyocytes, that is, control, Tm treated, Tm+EGB treated and EGB treated. MTT assay was used to detect cell viability. The dual-luciferase report system was used to measure Wnt activity. C-myc and CyclinD1 were detected by real-time PCR.Compared to control cells, Tm treatment resulted in significantly decreased cell viability, but the presence of EGB markedly attenuated the cell injury. The treatment also decreased the activity of Wnt, whereas co-treatment of Tm and EGB led to not only the increase in Wnt activity, but also recovery of the C-myc and CyclinD1 levels. However, Wnt inhibitor, secreted frizzled-related protein (sFRP) decreased Wnt activity, C-myc and CyclinD1 levels, and inversed EGB-induced protective effect.These findings demonstrate that EGB protects cardiomyocytes against Tm-induced injury through improving Wnt activity.
extracts ofleaf; tunicamycin; endoplasmic reticulum stress; cardio myocytes; Wnt
R284.14; R978.12; R329.24
A
10.11915/j.issn.1671-5403.2015.03.050
(CWS12J122).
2014−12−24;
2015−01−09
全军后勤科研计划(CWS12J122)
薛 桥, E-mail: xueqiao301@sina.com
