Species Composition and Diversity of Macrobenthos in the Intertidal Zone of Xiangshan Bay, China
2015-04-05JIAOHaifengZHENGDanYOUZhongjieXUNianjunLOUDanandHUANGChengwei
JIAO Haifeng, ZHENG Dan YOU Zhongjie XU Nianjun LOU Dan and HUANG Chengwei
1)School of Marine Science,Ningbo University,Ningbo315211,P.R.China
2)Ningbo Academy of Oceanology and Fishery,Ningbo315010,P.R.China
Species Composition and Diversity of Macrobenthos in the Intertidal Zone of Xiangshan Bay, China
JIAO Haifeng1),2),*, ZHENG Dan1),2), YOU Zhongjie1),2), XU Nianjun1), LOU Dan2), and HUANG Chengwei2)
1)School of Marine Science,Ningbo University,Ningbo315211,P.R.China
2)Ningbo Academy of Oceanology and Fishery,Ningbo315010,P.R.China
Xiangshan bay is a narrow semi-closed bay and situated on the northwestern coast of the East China Sea. Over past decades, it has become to a major bay with intensive human activities, dense urbanized area, and poor water quality. The aim of this paper was to reveal the ecological status through the elucidation of the species composition, abundance, biomass and diversity of macrobenthos in this bay. Six intertidal sections were surveyed from January 2007 to November 2008 quarterly. Sections TG, HD and XH are located in the three inner bays, sections QJ and WS are located near the thermal power plants, and section XX is located at the outer part of Xiangshan Bay. Great variations in macrobenthos community were indentified, and the species composition of the community in the present study showed the dominance in the order of molluscs (bivalves and gastropods), crustaceans and others, and only few Polychaeta were recorded. Only three dominant species,Littorina brevicula,Ilyplax tansuiensis, andCerithidea cingulatawere collected in all the sections, and a total of 19 dominant species were recorded only in one section. Two-way ANOVA analyses of abundance indicated that there were significant differences among sections or seasons. Shannon-Wiener diversity index (H') had its maximum (2.45) in section QJ, and minimum (1.76) in section TG. Multiple irregulark-dominance plots clearly showed that the study area was polluted and the macrobenthos community was under stress. We conclude that the macrobenthos of Xiangshan Bay have been disturbed by human activities, especially at the interior bay.
macrobenthos; abundance; biomass; community structure; species diversity; intertidal zone
1 Introduction
Intertidal zone is crisscross zone with high biodiversity, floristic composition and complex community types between land ecosystem and marine ecosystem, and is one of the areas easily disturbed by human activities (Sheng and Shi, 2008). Among the marine biological entities, macrobenthos (body size 〈 1 mm) is an important part of the intertidal zone and plays a significant role in the aquatic community considering its involvement in mineralization, mixing of sediments, flux of oxygen into sediments, and cycling of organic matter (Snelgrove, 1998). The macrobenthos community structure can change as a continuum on various spatial and temporal scales in relation to both natural and anthropogenic gradients (Pearson and Rosenberg, 1978), thus macrobenthos communities represent the best tool to investigate the ecological condition of an aquatic ecosystem (Sergy and Evans, 1975). Due to their reduced mobility and short life cycles, benthic communities are often used as indicators in biomonitoring studies (Gray and Elliot, 2010). The variance incommunity composition, abundance and diversity of benthic fauna can affect the function of the entire ecosystem (Bylyard, 1987). Moreover, macrobenthos distribution and composition vary considerably in response to perturbations, and the macrobenthos fauna is disturbed, impoverished and even generally dominated by stress- tolerant opportunistic species in areas of high level pollution (Estacioet al., 1997; Ingoleet al., 2009). Therefore benthic monitoring programs collect several variables, such as taxonomic composition, relative abundance and biomass distribution among organisms, whose magnitude of change in time represents the main basis for assessing disturbance effects on the macrobenthos communities (Rosenberg, 1973). Limited benthic studies have been conducted in intertidal zone compared to shallow waters.
In recent decades, serious pollution has taken place along the coast of Xiangshan Bay, probably due to the rapid development of aquaculture, urbanization and industry (e.g., thermal power plant, reclamation and dockyard) in this region. As we all know, pollution due to various anthropogenic, industrial, and maritime discharges renders the environment hostile for native species and opens a window for the proliferation of opportunistic native and exotic species (Galil, 2000). Substantial atten-tion has been paid to identify the biota present in mandisturbed areas for proper management, from the marine bio-invasion risk perspective, and this strategic move needs to be preceded by a thorough and synergic study of the biological components of the ecosystem (Sumitet al., 2013). Without the baseline dataset on native biota, it is virtually impossible to imply management protocol stringently and identify alien species (Sumitet al., 2013).
Studies on macrobenthos along the intertidal region of Xiangshan Bay are limited till now, only Yanget al. (2004) studied the benthic macrofauna in the intertidal zone near the Zhejiang Ninghai Power Station and Liuet al. (2008) and Yang (2008) studied the marine macrofauna near Wushashan power plant and Qiangjiao power plant, respectively. Other studies in Xiangshan Bay include those of the macrobenthos community (Gaoet al., 2003; Guet al., 2010), faunal diversity (Guet al., 2010), functional feeding group (Youet al., 2011), and comparative studies on macrobenthos between aquacultured and non-aquacultured areas (Liaoet al., 2010). But these study areas are all in shallow water environment, not in intertidal zone (Ninget al., 1999; Gaoet al., 2005; Wanget al., 2006). In brief, few work has been done to elucidate the macrobenthos community structure of the intertidal zone of Xiangshan Bay on the west coast of the East China Sea. Neither have studies of benthic diversity been conducted around this Bay, information being limited to individual or confined areas of the shelf.
The main objective of the present study is to provide a description (abundance, biomass, diversity) of intertidal macrobenthos organisms and their spatial occurrence along the bay, and to investigate the disturbance of human activities, such as thermal power plant and urbanization, to the distribution of macrobenthos. With this approach, we analyzed the spatial occurrence of the macrobenthos along Xiangshan Bay using to the data 2007-2008.
2 Materials and Methods
2.1 Study Sites
Xiangshan Bay (29°24´-29°48´N, 121°25´-122°03´E) is a narrow semi-closed bay, including around 59 islands and 3 inner bays. The total length of this bay exceeds 60 km, while the average width is only from 3 km to 8 km. The intertidal area is about 171.5 km2. Since past decades, it has become to a major bay with intensive human activities, dense urbanized area, and poor water quality. Extensive aquaculture, urban, and industrial development around the bay have caused substantial changes to the surrounding environment.
2.2 Sampling
Six intertidal sections were surveyed from January 2007 to November 2008 quarterly, and totally 432 samples were taken (triple samples were collected from each sations - 6 sections × 3 intertidal flats× 4 seasons × 2 years × 3). Section TG, HD and XH are located in the three inner bays, section QJ and WS are located near the thermal power plants, and the last section XX is located at the outer part of the bay (Fig.1).
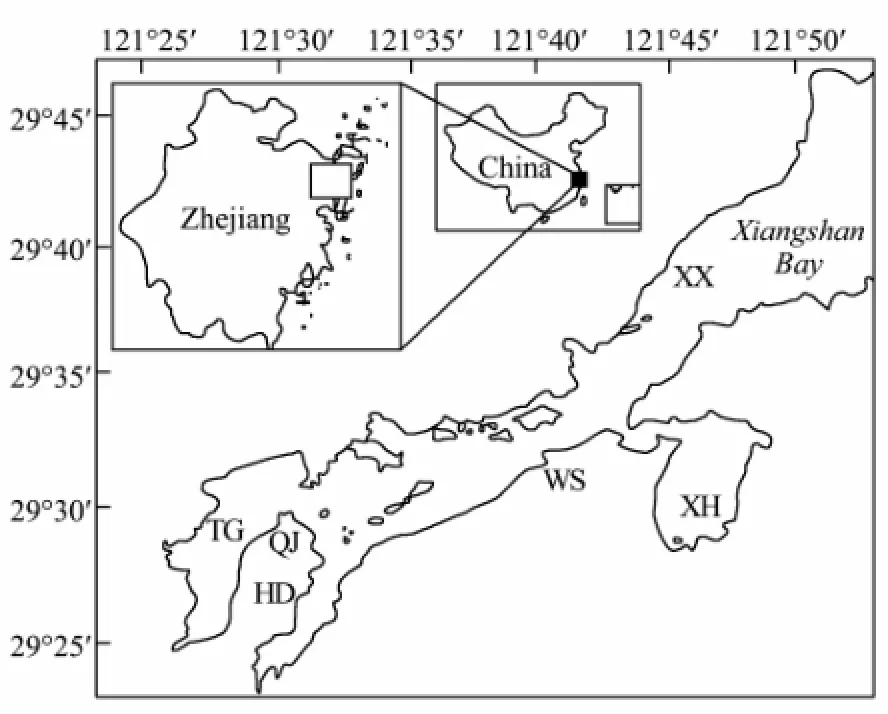
Fig.1 Sampling sections of the intertidal zone in the Xiangshan Bay, China. The first letter indicates the location of the sampling station. XX, Xianxiang; TG, Tiegang inner bay; QJ, Qiangjiao inner bay; HD, Huangdungang inner bay; WS, Wu shashan; XH, Xihugang inner bay.
Macrobenthos samples were collected in triplicate with stainless sieve (50 cm × 50 cm; 20 cm deep) and were in situ washed separately through 1.0 mm mesh size sieves. The retained materials were transferred to plastic bottles and preserved in 5% formalin in seawater containing Rose Bengal stain. Macrobenthos samples were labeled and transported to laboratory for further examination. Prior to identification, biomass (wet weight) was determined by an electronic balance (Mettler Toledo MS304S). Organisms were identified at the lowest possible taxonomic level using a microscope with the help of available taxonomic literature. All the organisms were counted with stereoscopic microscope and abundance was expressed by using the surface area of the sampling cores (0.25 m2).
2.3 Data Analysis
We calculated species richness and four different measures of species diversity: Shannon-Wiener diversity index using log2(H'), Hurlbert index expressed with the estimated number of species per 100 individuals (ES100), Margalef’s species richness (d), and Pielou eveness (J'). Univariate and multivariate analysis were performed using the PRIMER software version 6 (Plymouth Routines in Multivariate Ecological Research) package (Clarke and Gorley, 2006; Clarkeet al., 2008). The graphical tools likek-dominance curve and Ellipse plots and multivariate tools such as Bray-Curtis similarity based on square root transformed abundance were adopted. The hierarchical agglomerative clustering using group-average linking and multidimensional scaling (MDS) both based on macrobenthos abundance after square-root transformation were used.
Two-way ANOVA in SPSS 16.0 software package was used to indicate differences of abundance and diversity in different observation sections or seasons. The study area map was drawn with the help of SURFER 8.0. All theaverage data were represente with mean ± SD. The marks of classification and MDS plot represent section (First number), sampling year (Second number), and sampling month (Last two numbers), respectively.
3 Results
3.1 Abundance and Biomass
A total of 152 species macrobenthos were identified, of which molluscs and crustaceans were the most important groups. Molluscs dominated the macrobenthos (65 species) and contributed numerically up to 41% of the population. Crustaceans consisted of 52 species and contributed 33% of total infauna production. Meanwhile, 11 species were fishes, 6 species were polychaetes, 6 species were echinoderms, 6 species were coelenterates and 4 species belonged to other groups.
The abundance and biomass of the macrobenthos in every season in different sections were calculated, and information on the macrobenthos community structure at the six sections are summarized in Figs.2 and 3. The lowest abundance recorded in all the samples was from section XX in July 2007 (8.27 ± 1.30 ind m-2), and the highest abundance recorded was from section HD in January 2007 (86.81 ± 39.71 ind m-2). In particular,Ruditapes philippinarumwas the most abundant bivalve at section TG and section HD, where it represented 68.52% and 61.35% of total abundance. Two-way ANOVA (Factors: section and season) analysis of abundance indicated that there were significant differences among sections (F= 14.82,P〈0.01) or seasons (F= 4.99,P〈0.01). The abundance for different sections and seasons also showed significant differences (F= 5.32,P〈0.01) by the interactive analysis. In different sections, the abundance in section HD and TG was greater than that in section XH, and the latter was greater than that in section WS. The lowest abundance was in autumn, being lower than in other three seasons by analysis of Turkey HSD.
Two-way ANOVA analysis’s result showed that there are no significant differences in biomass (F= 0.98,P= 0.420) among seasons, but the differences among sections are significant (F= 7.99,P〈0.001). The biomass would be separated into three groups: the first for sections WS, XX and QJ, the second for sections TG and HD, the third for section XH. Among them, the lowest was from section WS (5.84 ± 1.46 g m-2) and the highest was from section XH (14.22 ± 3.47 g m-2). In contrast to abundance, no differences were detected for biomass among seasons, and the biomass of section XH with high abundance but lower than Section TG and HD. For evenness, the pattern was the same as Shannon-Wiener diversity; the lowest evenness was found at section TG and the highest was at section WS.
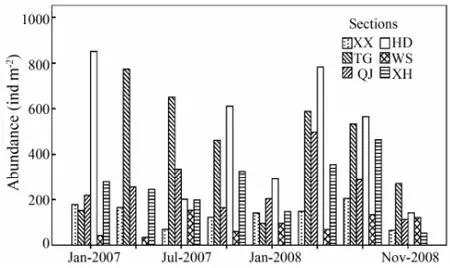
Fig.2 The macrobenthos abundance of Xiangshan Bay in different sections and seasons.
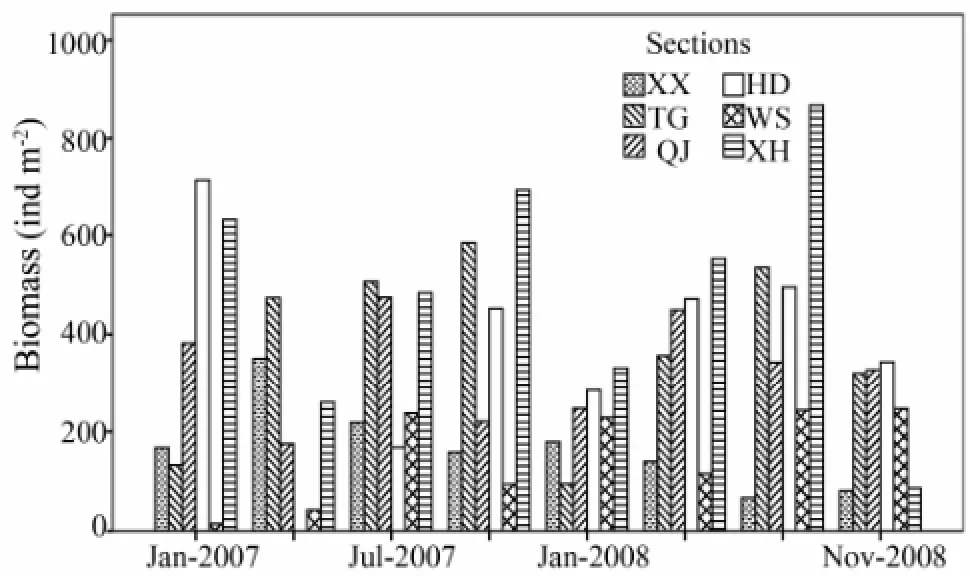
Fig.3 The macrobenthos biomass of Xiangshan Bay in different sections and seasons.
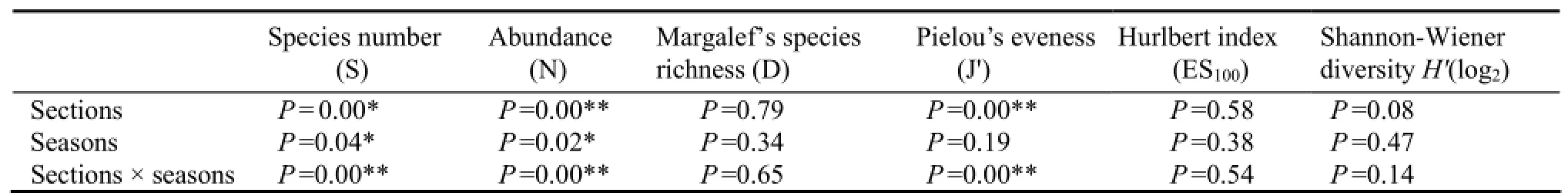
Table 1 Two-way ANOVA on macrobenthos community at six sections.
3.2 Diversity
The recorded species numbers in the six sections varied from 8.7 ± 1.1 to 12.4 ± 1.6 with the average 10.77 ± 0.57. The Shannon-Wiener diversity index (H') of the six sections ranged from 1.76 ± 1.16 to 2.45 ± 0.18, with maximum in section QJ and minimum in section TG, while Shannon-Wiener diversity index (H') of different seasons varied from 2.10 ± 0.14 (Autumn) to 2.34 ± 0.15 (Winter). In section TG,Ruditapes philippinarumwas the dominant species, which contributed 68.52% of abundance; in contrast, Shannon-Wiener diversity was lower than those in other sections with the largest number species. As to the seasons, the maximum value of 379 ± 81 ind m-2was recorded in Spring and the minimum value of 215 ± 44 ind m-2was recorded in Autumn. Significant differences of the macrobenthos abundance was found among the sections (P〈0.01), which varied from 101 ± 18 to 533 ± 101. The Pielou’s evenness (J') ranged from 0.57 ± 0.06 to 0.84 ± 0.03, while the former was in section TG and the latter in section WS. For other index, Margalef’s species rich-ness (d) ranged from 1.70 ± 0.20 to 1.99 ± 0.27, and Hurlbert index (estimated number of species per 100 individuals, ES100) ranged from 8.51 ± 1.05 to 10.17 ± 1.16.
Two-way ANOVA analysis indicated that no significant differences among all sections were detected excepted for abundance and Pilou’s evenness (J': bothP〈0.01), but significant differences among section × season were detected for all the community measures excepted for Margalef’s species richness (d), Hurlbert index (ES100), and Shannon-Wiener diversity (H'). All of them showed no significant seasonal differences (P〉0.05).
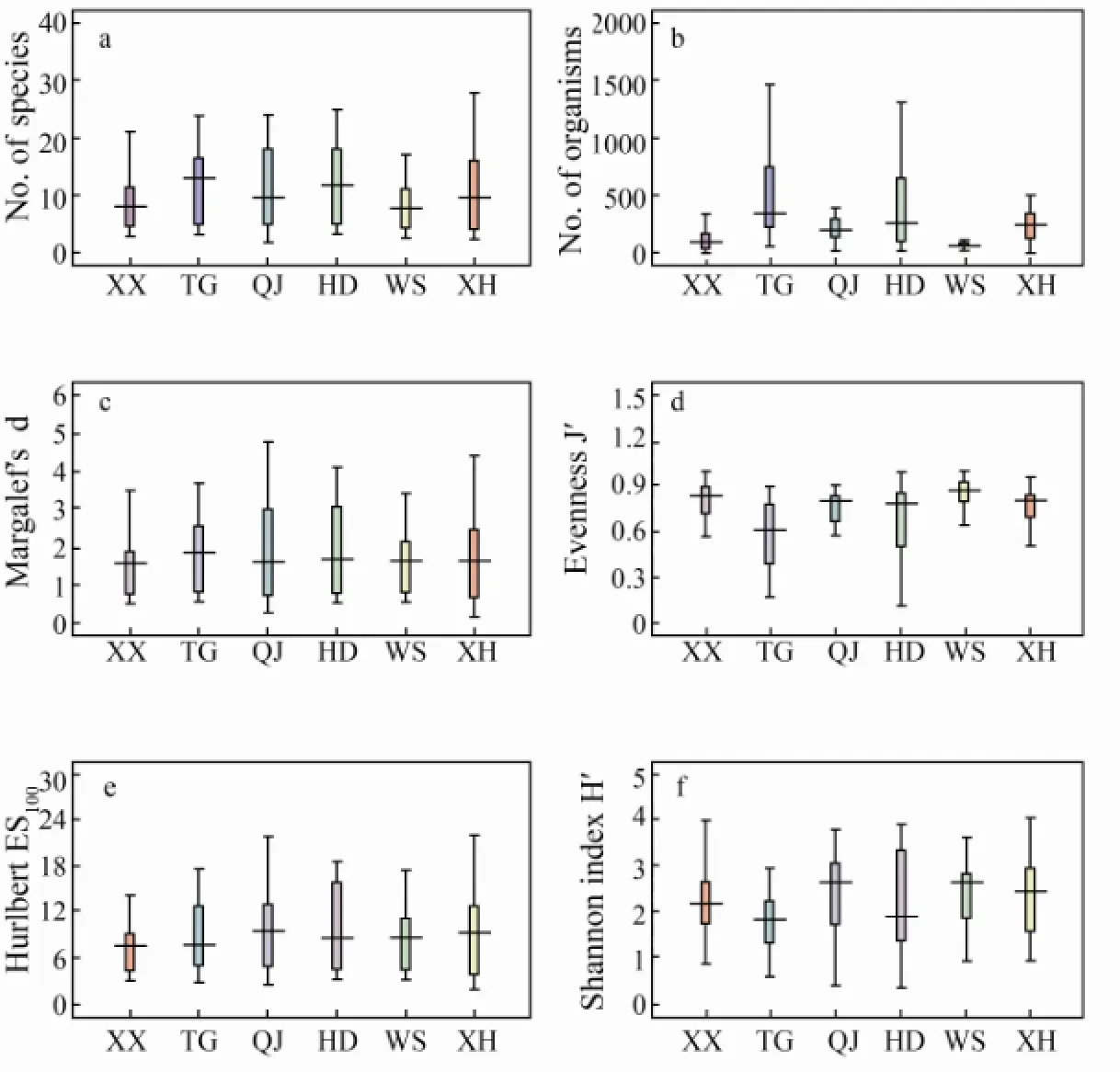
Fig.4 Univariate measures for macrobenthos infauna of Xiangshan Bay (section-wise). a, Number of species; b, abundance; c, Margalef’s species richness; d, Pielou’s evennessJ'; e, Hurlbert index (the estimated number of species per 100 individuals, ES100); f, Shannon-Wiener indexH'. Data presented as mean (lines) ±SE (boxes) ±SD (whiskers).
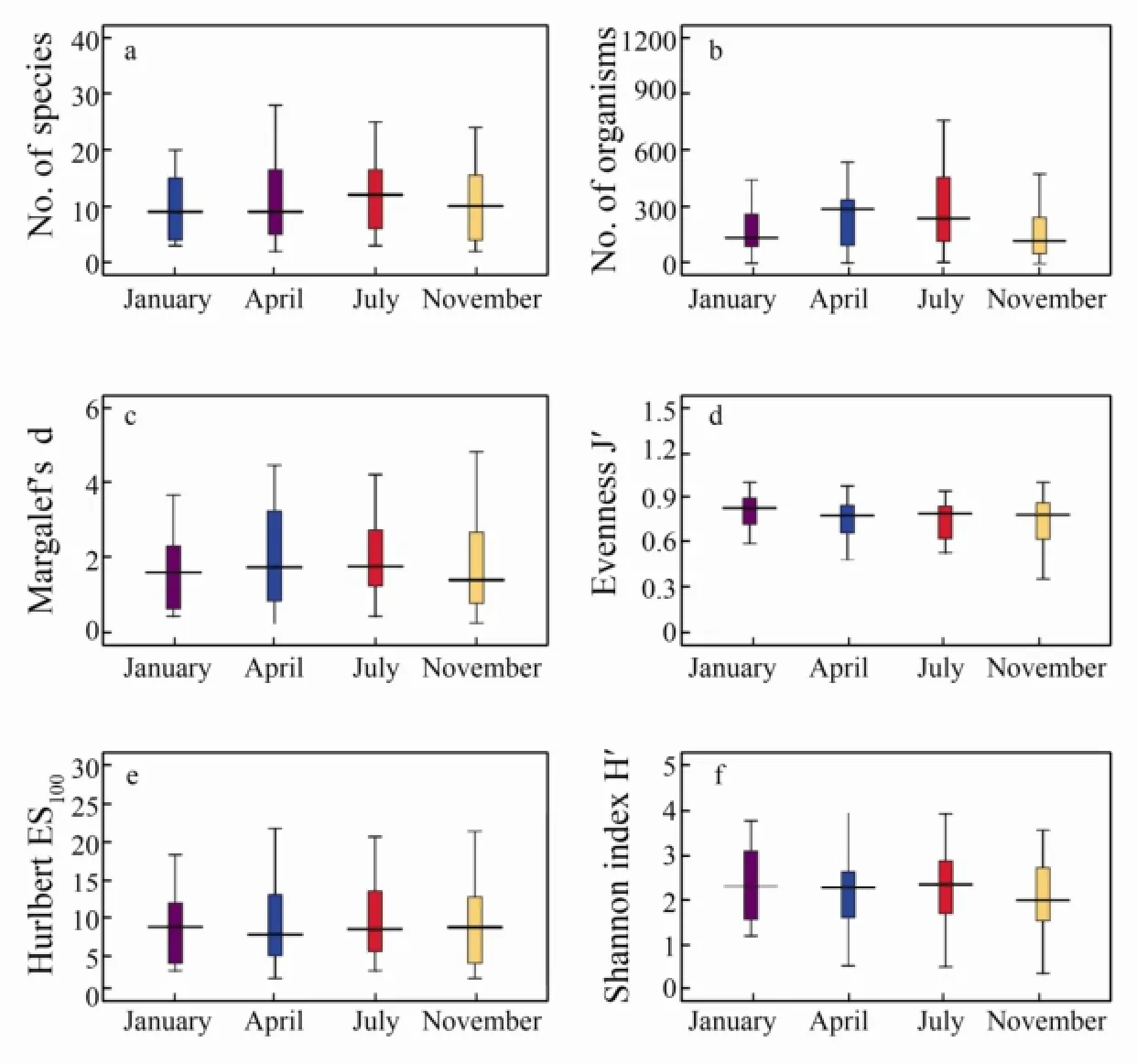
Fig.5 Univariate measures for macrobenthos infauna of Xiangshan Bay (season-wise). a, Number of species; b, abundance; c, Margalef’s species richness; d, Pielou’s evennessJ'; e, Hurlbert index (the estimated number of species per 100 individuals, ES100); f, Shannon-Wiener indexH'. Data presented as mean (lines) ±SE(boxes) ±SD(whiskers).
3.3 Spatial Variation
The classification analyses (using Bray-Curtis similarity), followed by an ordination through MDS on macrobenhos abundance data (number m-2) independently for infauna (152 species), were undertaken. Figs.6 and 7 display the results of hierarchical clustering and MDS ordination, respectively, based on species abundance data representing 6 sections during eight seasons (during 2007 to 2008). From the resulting dendrogram, all samples were classified into three groups: the first group included 7 samples which were form 4 sections (section XX, TG, WS and XH) and 3 seasons (three in autumn, three in winter, only one in summer), the second group included 13 samples which were form 6 sections mainly in spring and autumn, and the other samples were classified into the third group. In the MDS plot, it was found that all the samples of the first group were separated from other samples, which conforms to the dendogram. The other samples could be separated into three groups: one group mainly covered sections XX and HD, the second group mainly covered sections QJ, XH, and WS, and other few samples were separated into the third group.
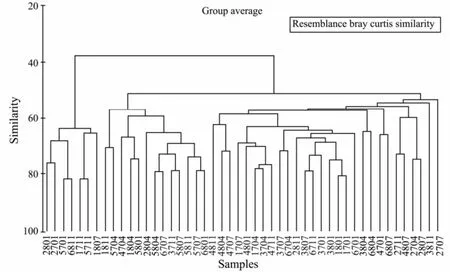
Fig.6 Dendrogram showing grouping of sections sampled during different seasons for macrobenthos. The four numbers designate four items: First number for section: 1, XX; 2, TG; 3, QJ; 4, HD; 5, WS; 6, XH. Second number for samplying year: 7, 2007; 8, 2008. The last two numbers for sampling month: 01, January; 04, April; 7, July; 4, November.
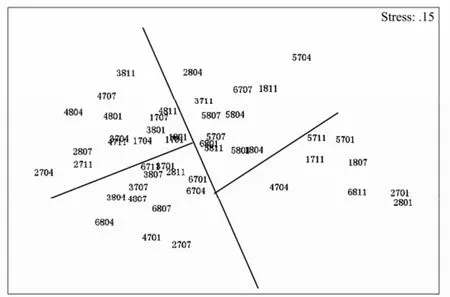
Fig.7 MDS plot for 48 stations sampled. The four numbers designate four items: First number for section: 1, XX; 2, TG; 3, QJ; 4, HD; 5, WS; 6, XH. Second number for samplying year: 7, 2007; 8, 2008. The last two numbers for sampling month: 01, January; 04, April; 7, July; 4, November.
3.4k-Dominance Curve
Multiplek-dominance plots facilitated the discrimination of macrobenthos according to species-relative contribution to standard stock (Fig.8). When all the sections belonging to all the seasons were plotted together, the curves for section 3701 (section QJ, winter 2007) and section 2704 (section TG, spring 2007), are lying low and is S-shaped, indicating the highest diversity, whereas the curve for station 1807 is lying high, showing the lowest diversity. Although the curve of section 1707 (section XX, summer 2007) is lying low, it is disturbed also, for the curve is not absolutely S-shaped; furthermore, the section TG was disturbed for Spring (see the curves 2701 and 2801). When thek-dominance plot is plotted according to seasons (Fig.9), it does not show any difference between the seasons. Thek-dominance plot was also plotted forthe sections (Fig.10), and the curves for HD, WS and XH with the same S-shape are lying low, indicating highest diversity, whereas the curves for the sections QJ and TG have irregular shapes, showing that the lower diversity, is lying high (Fig.10).
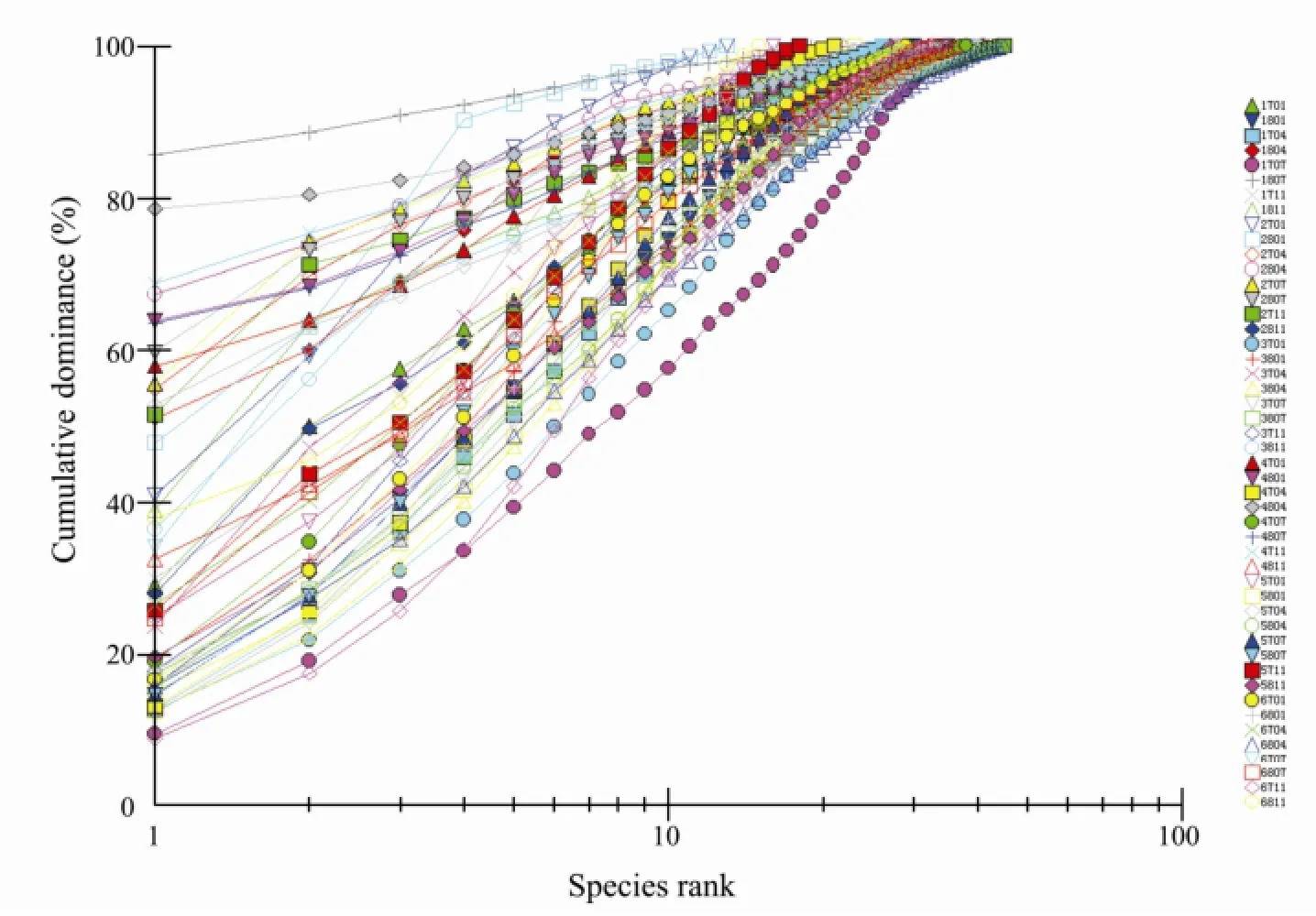
Fig.8k-Dominance curves for all stations. The four numbers designate four items: First number for section: 1, XX; 2, TG; 3, QJ; 4, HD; 5, WS; 6, XH. Second number for samplying year: 7, 2007; 8, 2008. The last two numbers for sampling month: 01, January; 04, April; 7, July; 4, November.
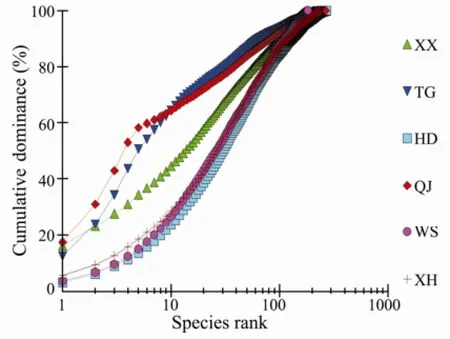
Fig.9k-Dominance cures for species abundance data in different sections.
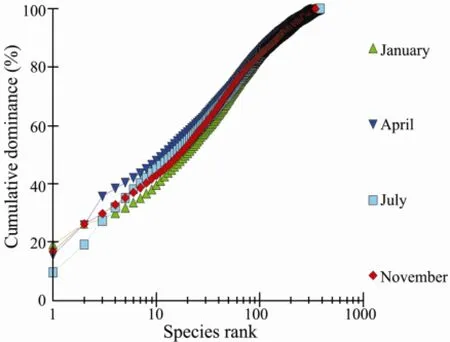
Fig.10k-Dominance curves for species abundance data in relation to season.
4 Discussion
Comparison of the present dataset with earlier reported literature for this area shows some changes in the faunal composition and abundance. Earlier average macrobenthos abundance and biomass were 288.18 ± 29.97 ind m-2and 84.23 ± 7.04 g m-2, respectively, which have both declined considerably (Shaoet al., 1996; Yanget al., 2008,). The declines of abundance and biomass are due to the changes of species composition surveyed in different periods.
Biotic structures can provide habitat for other species, which potentially enhances the bottom surface complexity and thus has implications on functioning (Pachecoet al., 2011); so the distribution of macrobenthos play key roles in biotic structures. In the present study, a great variability in the macrobenthos community have been discoveried, and species composition of macrobenthos shows the dominance in the order of molluscs (bivalves and gastropods), crustaceans and others (Figs.11 and 12), as observed earlier by Shaoet al., (1986). This is especially so in sections TG, HD, and XH, the three inner bays of Xiangshan Bay, where the sediments are mainly muddy and form suitable habitats for buried Bivalve. BuriedRuditapes philippinarumwith abundance of 294.58 ind m-2in section TG and 264.33 ind m-2in section HD was the dominant species, which contributed approximately 57.21% and 66.76% of total abundance, respectively. The reason of high abundance ofR.philippinarumwas that there were two seed reserves in these two inner bays. In section XH, though the Bivalve abundance was lowerthan that of Gastropod, their abundance reached 95.42 ind m-2and the biomass reached 34.29 g m-2. BuriedMuscuius senhouseiwas the dominant species in this inner bay. In contrast, compared to other species, the abundance and biomass of bivalve were the lowest in section XX, section QJ, and section WS. The sediment of section XX was hard bottom and the sediment of section QJ or section WS were both gravels, which confirms our conclusion about different macrobenthos composition in Xiangshan Bay and the theory of species compositions determined by the sediment types.
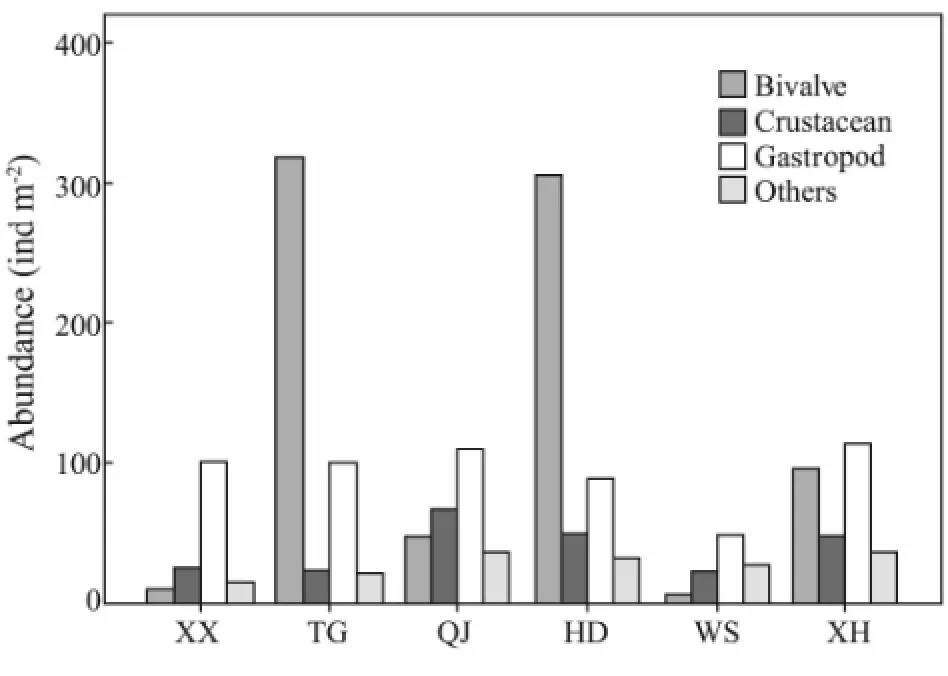
Fig.11 Composition of macrobenthos abundance in different sections.
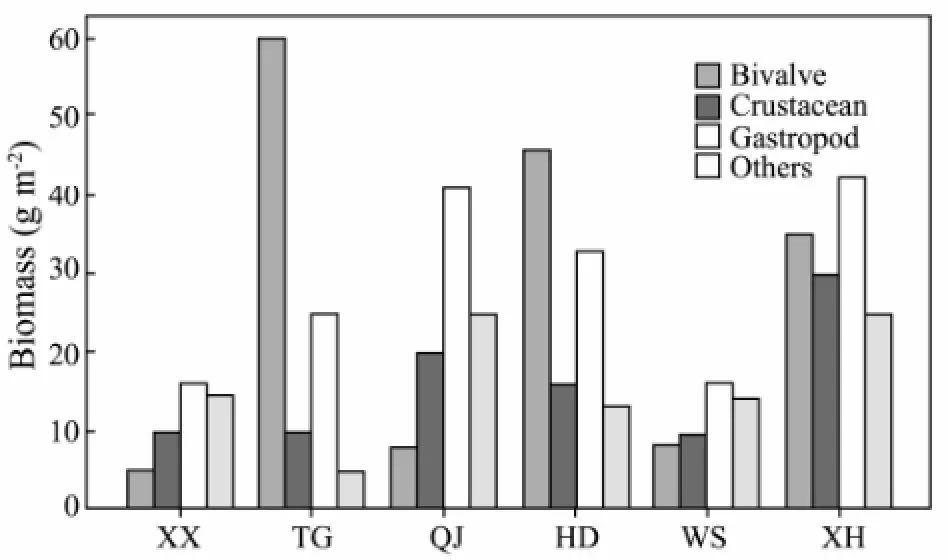
Fig.12 Composition of macrobenthos biomass in different sections.
It should be noted that the communities differentiated in the present study, as the macrobenthis compositions were affected by the environment factors (Sheng and Shi, 2002; Craeymeersch, 1991). Strong dominance by a few of these species was detected in sections, and the dominant species changed greatly in different sections. Only three species,Littorina brevicula,Ilyplax tansuiensisandCerithidea cingulatawere collected in all the sections.L. breviculawas a kind of Gastropod located at the high intertidal with hard bottom, especially rocky sediment.I. tansuiensis, andC. cingulatawere located in muddy bottom. Similar to the results of Shaoet al. (1990), only few Polychaeta were recorded in this survey. A total of 19 species, includingPatelloida pygmaea,Laternula marilina,Moerella iridescens,Macrophthalmus japonicus,Moerella culterandHaliplanella luciae, were recorded only in one section, which represents a different community among the six sections.Assiminea latericea,Ilyplax tansuiensisandLittorina breviculawere the three dominant species of Section XX located at the outer part of Xiangshan Bay.Ruditapes philippinarum,Ilyplax tansuiensis,Muscuius senhouseiandCerithidea cingulatawere the dominant species of the three inner bays (section TG, HD and XH). Compared to the early literature of Shaoet al. (1996), the community of macrobenthos did not changed greatly, except thatMacoma praerupla,Assiminea brevicula,Theora fragiliswere not recorded, andMuscuius senhousei,Nassariusbecame the dominant species in this study. The dominant species of Section QJ was the same as that of section WS but differed from other sections. For example,I.tansuiensisandC.cingulatawere the top two dominant species in sections QJ and WS.M.iridescenswas the definite dominant species of Xiangshan Bay in the 1990’s and recorded only in one section. This phenomenon is probably due to the decrease of artificial breeding ofMoerella iridescenson the intertidal flat in recent decades. Tilmanet al. (1997) showed that a strong linear relationship between functional and phylogenetic diversity pattern, and wider local taxonomic trees can support a wider range of species functions even in small spatial scales. However, a narrow range of species functions is maintained by certain species which are also present in the wider taxonomic trees. Furthermore, the results of this study con fi rms the hypothesis that some species are more important than others in ecosystem functioning.
Species diversity is a simple and useful measure of biological system. Sanders (1968) found a high level of agreement between species diversity and the nature of the environment and, hence, regarded the measure of species diversity as an ecologically powerful tool. Pearson and Rosenberg (1978) proposed that the use of diversity indices is advantageous for the description of faunas at different stages in the succession. Sanders (1968) postulated that the species diversity is mainly controlled by the fl uctuations in the environment that lead to narrow diversity. In previous literatures the humpshaped relation between functional diversity and species abundance was revealed, which denotes that functional diversity increases when low and intermediate levels of species abundance are involved; however, when species abundance reaches its maximum values, functional diversity decreases. Furthermore, it is universally accepted that areas with lower diversity and richness indicate polluted or stressed conditions (Magurran, 1988). According to Wilhm and Dorris (1966), species diversity index (H') value 〉3.00 bit ind-1indicates unpolluted conditions, 1.00 to 3.00 bit ind-1indicates moderately polluted, and 〈1.00 bit ind-1indicates heavily polluted condition of aquatic medium. The Shanonn-Wiener’s index ranges from 1.62 to 2.79 with an average value 2.26, which declined significantly to this value from 3.23 in comparison with the survey of the 1990’s (Shaoet al., 1996), showing that the study area has been polluted and macrobenthos community is under stress due to natural or anthropogenic factors. For comparison, the Shanonn-Wiener’s index is lowerthan that of Haitan Bay of Fujiang province (Lvet al., 2008), Jiaozhou bay of Shandong province (Liet al., 2006) and Gaoshaling of Tianjin City (Zhanget al., 2007), but higher than that of Hangzhou Bay of Zhejiang Province (Liet al., 2007) and Yangtze Estuary (Anet al., 2007).
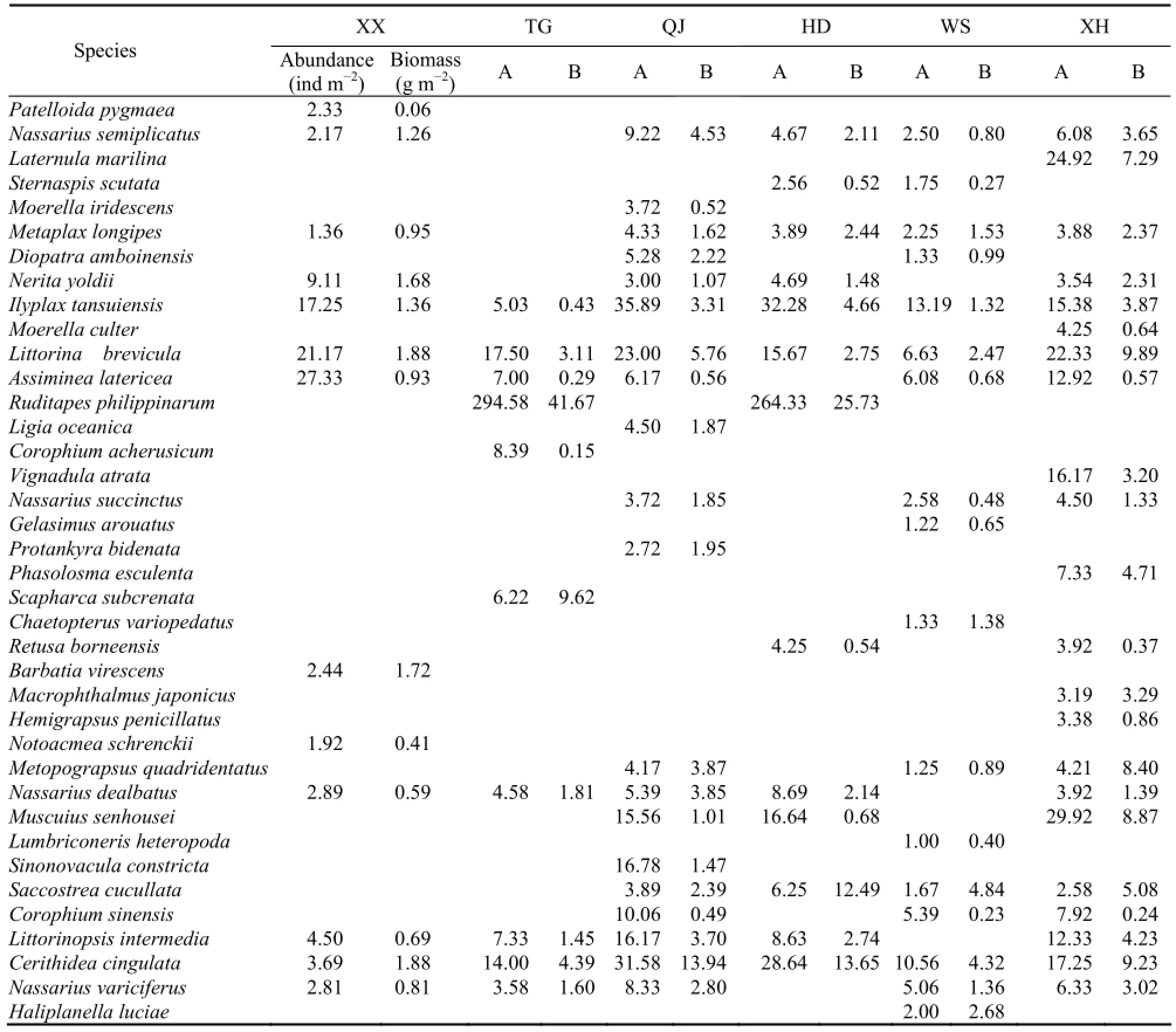
Table 2 The abundance and biomass of dominant species in different sections
Power and production plants are often built on sea coasts; more than one third of power plants in the United States are situated near the sea, and daily water intake by these plants has reached billions of liters since the 1970s (Young, 1971). Two thermal power plants are situated in Xiangshan Bay. The fouling of cooling systems at thermal power plants has been thoroughly investigated in China. The cooling systems of these plants take water from the benthic zone and mix it with coastal waters, usually in a 5 m layer of the pelagic zone. Cooling system biotopes are characterized by an absence of light and rapid water currents, thus excluding certain groups of foulers but favoring others (barnacles, hydroids, and mussels). In addition, the rapid water flow prevents the larval settlement of many motile animals that require a developed sessile fouling biotope as substrate. Water flow therefore defines the composition and quantitative characteristics of fouling communities on anthropogenic substrates (Sergy and Evans, 1975). Before the construction of thermal power plant, Yanget al. (2004) surveyed the abundance and biomass near Section QJ to be 839 ind m-2and 1753.92 g m-2, respectively, but they declined to 259.69 ind m-2and 81.87 g m-2in this study. On one hand, these changes might be due to the different sampling sections in the two studies. The sections surveyed in 2002 have been occupied by the power plant and we likely missed some species and their contribution to the functioning that could be obtained by enhancing the spatial replication. On the other hand, the changes may mainly be due to the deteriorating environmental conditions in the thermal power plant over the years, for a total of 80 m3s-1heat water have been discharged to the environment (You and Jiao, 2011). Meanwhile, coastal waters of Xiangshan Bay received industrial discharges up to 544 million ton and domestic wastes of around 1.29 billion ton per year (Huang, 2008). Furthermore, during the last decades, growing population and industries around Xiangshan Bay increased alarmingly the sewage dis-charge. The results of our study proves the conclusion of Liu’s (2008), which showed that the abundance and biomass obviously declined and the dominant species was markedly changed after the field run of power station. The pollution produced by thermal power plant, urbanization and industrial waste have obviously affected the marine biological environment. The recovery of disturbed macrobenthos community is slow and complicated. Oshurkovet al. (1994) found that the fouling organisms form the so-called ‘physically controlled’ communities, which are common in intertidal zones and estuaries and developed for 1-3 years and regain their original state relatively easily after stresses.
5 Conclusions
The present study provided information on the diversity and community structure of macrobenthos in Xiangshan Bay and showed the changes in abundance and community structure over the years. Species composition of the macrobenthos showed the dominance in the order of molluscs (bivalves and gastropods), crustaceans and others. Macrobenthos species density declined considerably in the last two decades, and the low benthic diversity index in all the observation sections indicated that the study area was under stresses. The multiplek-dominance plots showed that the curves for the sections HD, WS and XH with the same S-shape were lying low, indicating highest diversity, whereas the curves for the sections QJ and TG with irregular shape, show indicating the lower diversity, were lying high. The results of our study showed that the abundance and biomass obviously declined and the dominant species markedly changed after the field run of thermal power stations, for the pollution produced by power plants, urbanization and industrial wasted had affected the marine biological environment inevitably.
Acknowledgements
We gratefully acknowledge the financial support from the Ningbo Science and Technology Bureau and the Ningbo Oceanology and Fishery Bureau for Technology and Research of Marine Ecological Environmental Protection and Restoration of Xiangshan Bay (No. 2006C 10030).
An, C. G., Zhao, Y. L., Lin, L., Lu, G. T., and Chen, Y. Q., 2007. Primary investigation of seasonal characters of macrobenthic communities distribution in tidal flats of Jiuduansha wetland of Yangtze River Estuary.Journal of Fishery Sciences of China, 31: 52-58.
Bylyard, G. R., 1987. The value of benthic infauna in marine pollution monitoring studies.Marine Pollution Bulletin, 8:581-585.
Chen, K., Tian, S. Y., and Jiao, J. J., 2010. Macrobenthos community in Tolo Harbour Hong Kong and its relations with heavy metals.Estuaries and Coasts, 33: 600-608.
Clarke, E. M., Faeder, J. R., Langmead, C. J., Harris, L. A., Jha, S. K., and Legay, A., 2008. Statistical model checking in BioLab: Applications to the automated analysis of T-cell receptor signaling pathway.Lecture Notes in Computer Science, 5307: 231-250.
Clarke, K. R., and Gorley, R. N., 2006.Primer v6:User Manual/ Tutorial. PRIMER-E, Plymouth, 5-9.
Craeymeersch, J. A., 1991. Applicability of the abundance/ biomass comparison method to detect pollution effects on intertidal macrobenthic communities.Hydrobiology Bulletin, 24:133-140.
Dimitriadis, C., and Koutsoubas, D., 2011. Functional diversity and species turnover of benthic invertebrates along a local environmental gradient induced by an aquaculture unit: The contribution of species dispersal ability and rarity.Hydrobiologia, 670: 307-315.
Estacio, F. J., Garcia-Adiego, E. M., Fa, D. A., Garcia-Gomez, J. C., Daza, J. L., and Hortas, F., 1997. Ecological analysis in a polluted area of Algeciras Bay (southern Spain): External‘versus’ internal outfalls and environmental implications.Marine Pollution Bulletin, 34: 780-793.
Fan, M. S., Shao, X. Y., Cai, R. X., and Wang, H. M., 1996. Studies on intertidal zone ecology of the benthic fauna in Xiangshan Harbor and Sanmen Bay I. species composition and distribution.East Sea Marine Science, 14: 27-34.
Galil, B. S., 2000. A sea under siege alien species in the Mediterranean.Biological Invasions, 2: 177-186.
Gao, A. G., Chen, Q. Z., Hu, X. G., Yang, J. Y., Dong, Y. T., Zeng, J. N., Ning, X. R., and Zhang, J., 2005. Ecological characteristics on macrobenthos of net-cage cultural areas in the Xiangshan Bay.Acta Oceanologica Sinica, 27: 108-113.
Gao, A. G., Yang, J. Y., Chen, Q. Z., Wang, Z. P., Zhang, J., Dong, Y. T., and Ning, X. R., 2003. Comparative studies on macrobenthos between cultured and non-cultured areas in Xiangshan Bay.Journal of Fishery Sciences of China, 27: 25-31.
Gray, J. S., and Elliot, M., 2010.Ecology of Marine Sediments:From Science to Management. Oxford University Press, Oxford, 1-32.
Gu, X. Y., Tao, L., Shi, H. X., Lou, D., Jiao, H. F., and You, Z. J., 2010. Macrobenthic faunal diversity in Xiangshan Bay.Chinese Journal of Applied Ecology, 21: 1551-1557.
Gu, X. Y., Tao, L., You, Z. J., Jiao, H. F., Shi, H. X., and Lou, D., 2010. The macrobenthic community of the Xiangshan Bay.Oceanology and Limnology Sinica, 41: 208-213.
Huang, X. Q., Wang, J. H., and Jiang, X. S., 2008.Study of Marine Environmental Capacity and Total Pollutants Control of Xiangshan Bay. China Ocean Press, Beijing, 1-98.
Inagaki, Y., Takatsu, T., Ashida, Y., and Takahashi, T., 2012. Annual changes in macrobenthos abundance in Funka Bay, Japan.Fisheries Science, 78: 647-659.
Ingole, B., Sivadas, S., Nanajkar, M., Sautya, S., and Nag, A., 2009. A comparative study of macrobenthic community from harbors along the central west coast of India.Environmental Monitoring and Assessment, 154: 135-146.
Levins, R., and Lewontin, R., 1985.The Dialectical Biologist. Harvard University Press, Cambridge, 1-44.
Li, H. H., Bao, X. Y., Hu, Z. Y., and Ge, B. M., 2007. Seasonal dynamics of macrobenthic functional groups and trophic levels in the bridge construction zone at the South Bank of Hangzhou Bay, China.Acta Zoologica Sinica, 53: 1011-1023.
Li, X. Z., Li, B. Q., Wang, H. F., Wang, J. B., and Zhang, B. L., 2006. Macrobenthic community of the intertidal zone ofJiaozhou Bay.Acta Zoologica Sinica, 52: 612-618.
Liao, Y. B., Shou, L., Zeng, J. N., Gao, A. G., and Jiang, Z. B., 2011. A comparative study of macrobenthic community under different mariculture types in Xiangshan Bay China.Acta Ecologica Sinica, 31: 646-653.
Liu, L., Ren, M., Chen, D. Q., and Xiang, Y. T., 2008. Situation of benthos in sea area near Wusha Mountain Power Station in Xiangshan Bay.Marine Environmental Science, 27: 19-22.
Lu, X. M., Fang, S. H., Zhang, Y. P., and Wu, P. R., 2008. Community structure and secondary production of macrobenthos in the intertidal zone of Haitan Strait, Fujian Province.Acta Zoologica Sinica, 54: 428-435.
Magurran, A. E., 1988.Ecological Diversity and Its Measurement. Princeton University Press, Princeton, 1-45.
Mandal, S., and Harkantra, S. N., 2013. Changes in the soft bottom macrobenthos diversity and community structure from the ports of Mumbai, India.Environmental Monitoring and Assessment, 185: 653-672.
Naeem, S., 2002. Ecosystem consequences of biodiversity loss:The evolution of a paradigm.Ecology, 83: 1517-1552.
Neira, C., Mendoza, G., Levin, L. A., Zirino, A., Francisco, D. H., Porrachia, M., and Deheyn, D. D., 2011. Macrobenthic community response to copper in Shelter Island Yacht Basin San Diego Bay, California.Marine Pollution Bulletin, 62:701-717.
Ning, X. R., and Liu, Z. L., 1999. Standing crop and productivity of the benthic microflora living on tidal flats of the Xiangshan Bay.Acta Oceanologica Sinica, 21: 98-105.
Odling-Smee, F. J., Laland, K. N., and Feldman, M. W., 1996. Niche construction.American Naturalist, 147: 641-648
Pacheco, A. S., Gonzalez, M. T., Bremner, J., Oliva, M., Heilmayer, O., Laudien, J., Riascos, J. M., 2011. Functional diversity of marine macrobenthic communities from sublittoral softsediment habitats off northern Chile.Helgoland Marine Research, 65: 413-424.
Pearson, T. H., and Rosenberg, R., 1978 . Macrobenthic succession in relation to organic enrichment and pollution of the marine environment.Ocean Marine Biology, 16: 229-311.
Redding, J. M., and Cory, R. L., 1975. Macroscopic benthic fauna of three tidal creeks adjoining the Rhode River, Maryland.Water Resources Investment Representative USA, 39-75.
Rosenberg, R., 1973. Succession in benthic microfauna in a Swedish fjord subsequent to the closure of a sulphite pulp mill.Oikos, 24: 244-258.
Sanders, H. L., 1968. Marine benthic diversity: A comparative study.American Naturalist, 102: 243-282
Sergy, G. A., and Evans, J. W., 1975. The settlement and distribution of marine organisms fouling in a seawater pipe system.Veliger, 18: 87-92.
Shao, X. Y., Cai, R. X., and Wang, H. M., 1996. Studies on intertidal zone ecology of the benthic fauna in Xiangshan Harbor and Sanmen Bay Ⅲ . community structure.East Sea Ma-rine Science, 14: 42-47.
Sheng, G. Y., and Shi, B. Z., 2008.Marine Ecology. Science Press, Beijing, 1-33.
Snelgrove, P. V. R., 1998. The biodiversity of macrofaunal organisms in marine sediments.Biodiversity Conservation, 7:1123-1132.
Sumit, M., and Harkantra, S. N., 2013. Changes in the softbottom macrobenthic diversity and community structure from the ports of Mumbai, India.Environmental Monitoring and Assessment, 185: 653-672.
Tilman, D., Knopps, J., Wedin, D., Reich, P., Ritchie, M., and Siemann, E., 1997. The in fl uence of functional diversity and composition on ecosystem processes.Science, 277: 1300-1302.
Wang, J. H., Yang, C. W., Sun, Y. W., and Ang, Y. L., 2006. Secondary productivity and biodiversity of macrobenthos in Xiangshan Bay.Journal of Tianjin Agriculture University, 13:16-20.
Wilhm, J. L., and Dorris, T. C., 1966. Species diversity of benthic macroinvertebrates in a stream receiving domestic and oil refinery effluents.American Midland Naturalist, 76: 427-449.
Yang, J. Y., Gao, A. G., Chen, Q. Z., Hu, X. G., Yang, G. M., 2004. Ecology of benthic macrofauna in the intertidal zone near the Zhejiang Ninghai Power Station.East Sea Marine Science, 22: 48-55.
Yang, Y. F., Cai, Y. H., Wei, Y. G., Wang, X. B., Ye, X. S., Xiang, Y. T., and Zhou, Z. P., 2008. Research on marine macrofauna near Guohuaninghai power plant in Xiangshan Bay.Marine Environmental Science, 27: 79-82.
Young, C. S., 1971. Thermal discharges into the coastal waters of Southern California. Southern California Coastal Water Research Project, Technical Report 003, 30pp.
Zhang, Q. T., Hu, G. K., and Zhang, B., 2007. Analysis of macrobenthos in Gaoshaling intertidal zone.Journal of Chemical Industry, 36: 42-44.
(Edited by Ji Dechun)
(Received October 21, 2013; revised April 3, 2014; accepted May 24, 2014)
© Ocean University of China, Science Press and Springer-Verlag Berlin Heidelberg 2015
* Corresponding author. E-mail: xunianjun@nbu.edu.cn
杂志排行
Journal of Ocean University of China的其它文章
- Impacts of the Two Types of El Niño on Pacific Tropical Cyclone Activity
- Numerical Simulation of Typhoon Muifa (2011) Using a Coupled Ocean-Atmosphere-Wave-Sediment Transport (COAWST) Modeling System
- Estimating the Turbulence Characteristics in the Bottom Boundary Layer of Monterey Canyon
- Composition and Origin of Ferromanganese Crusts from Equatorial Western Pacific Seamounts
- Hydroelastic Analysis of a Very Large Floating Structure Edged with a Pair of Submerged Horizontal Plates
- A Storm Surge Intensity Classification Based on Extreme Water Level and Concomitant Wave Height
