Evaluation of Cytotoxicity and Genotoxicity of Insecticide Carbaryl to Flounder Gill Cells and Its Teratogenicity to Zebrafish Embryos
2015-04-05PANDEYManishRajandGUOHuarong
PANDEY Manish Raj, and GUO Huarong
1)Laboratory of Evolution & Development,Institute of Evolution & Marine Biodiversity,Ocean University of China,Qingdao266003,P. R. China
2)Key Laboratory of Marine Genetics and Breeding ofMinistry of Education,College of Marine Life Sciences,Ocean University of China,Qingdao266003,P. R. China
Evaluation of Cytotoxicity and Genotoxicity of Insecticide Carbaryl to Flounder Gill Cells and Its Teratogenicity to Zebrafish Embryos
PANDEY Manish Raj1), and GUO Huarong2),*
1)Laboratory of Evolution & Development,Institute of Evolution & Marine Biodiversity,Ocean University of China,Qingdao266003,P. R. China
2)Key Laboratory of Marine Genetics and Breeding ofMinistry of Education,College of Marine Life Sciences,Ocean University of China,Qingdao266003,P. R. China
In this study, we determined the cytotoxicity and genotoxicity of carbamate insecticide carbaryl to flounder gill (FG) cells and its teratogenicity to zebrafish embryos. The cytotoxicity of carbaryl to FG cells was determined with methods including MTT and neutral red uptaking (NRU), lactate dehydrogenase (LDH) releasing and Hoechst 33342 and propidium idodide (PI) double staining. Moderate cytotoxicity in a concentration-dependent manner was observed. The 24 h-IC50value of 53.48 ± 1.21, 59.13 ± 1.19 and 46.21 ± 1.24 mg L-1carbaryl was obtained through MTT, NRU and LDH assays, respectively. Double fluorescence staining demonstrated that carbaryl induced the death of FG cells mainly through necrosis. There was no significant genotoxicity found in the FG cells exposed to the highest testing concentration of carbaryl (20 mg L-1,P〉 0.05) as was demonstrated by Comet assay. Zebrafish embryos exposed to carbaryl at concentrations ≥10 mg L-1displayed moderate toxic effects on the survival, spontaneous movement, hatching, heart rates of the embryos and their development, which were evidenced by yolk and pericardial sac edemas, body length reduction and tail flexure in time- and concentration-dependent manners at specific stages. The 24 h-, 48 h- and 96 h-LC50values of carbaryl to zebrafish embryos were 41.80 ± 1.10, 17.80 ± 1.04 and 14.46 ± 1.05 mg L-1, respectively. These results suggested that carbaryl is moderately toxic to FG cells culturedin vitroand zebrafish embryos, and the FG cells were similar to zebrafish embryos in their sensitivity to carbaryl as 24 h-IC50and LC50indicated.
carbaryl; cytotoxicity; genotoxicity; teratogenicity; flounder gill cell; zebrafish
1 Introduction
Carbaryl (1-naphthyl-N-methylcarbamate) is the most frequently used carbamate insecticide because of its relatively low mammalian oral and dermal toxicity and broad control spectrum (Agrawal and Sharma, 2010; Kuhr and Dorough, 1976). It has been widely used to the control of a variety of pests on fruits, vegetables, cereals, forage, cotton, forests, lawns, ornamentals and many other crops as well as poultry, livestock and pets in China. It is also used as a molluscicide and an acaricide as well as insecticide against the ectoparasites of humans and animals (Tomlin, 2000; USEPA, 2012). Carbaryl, along with other carbamates for animals, functions to inhibit the activity of acetylcholinesterase (AChE) at synaptic junctions in nervous system, resulting in the accumulation of acetylcholine in nerve synapses, thus uncontrolled movement, paralysis, convulsions and tetany, and possible death (Cox,1993; Gruber and Munn, 1998; Gunasekaraet al., 2008; Tomlin, 2000). As was classified by the World Health Organization (WHO), carbaryl is moderately hazardous (WHO, 2002). Carbaryl is not persistent in environment but is more stable in seawater than in freshwater (Pelletieret al., 2006; Xu, 2000). Its wide use also increased the risk of being leached into groundwater thus imposing adverse effects on human health (Schocket al., 2012; Todd and van Leeuwen, 2002).
The toxicity of carbaryl to fish is species-specific, high to slight to freshwater fish species, and moderate to ocean and estuary fish species on an acute basis (Beyerset al., 1994; McKimet al., 1987; Sinhaet al., 1991). Salmon, trout, and perch are reported as the most sensitive species, which will die when the concentration of carbaryl varies between 250 and 970 ng mL-1(USEPA, 2002). The 96 h-LC50value indicated that the acute toxicity of carbaryl was 9.26 mg L-1to zebrafish (Danio rerio), 2.52 mg L-1to guppy (Poecilia reticulata), 6.4 mg L-1to Indian carp (Catla catla), 5.5 mg L-1to climbing perch (Anabas testudineus), 4.6 mg L-1to Gangetic mystus (Mystus cavasius) and 2.4 mg L-1to striped catfish (Mystus vittatus) (Galloet al., 1995; Tilaket al., 1981). Carbaryl is also found to impose physiological and behavioral toxic effects on fish, such as a decrease in amino acid contents in muscle, damage to gills and liver cells, kidney lesions, and slowing fin regeneration (Cox, 1993; Gillet al., 1988; Peteret al., 2013; Pfeifferet al., 1997), neuromuscular abnormality and defect in intra-embryonic circulation (Crawford and Guarino, 1985; Weis and Weis, 1974), heart malformations, irregular heartbeat and oscillating blood in heart (Solomon and Weis, 1979).
Fish embryos and larvae are generally the most sensitive to chemicals in life cycle of teleosts (Laale and Lerner, 1981; Lele and Krone, 1996), and they are ideal for evaluating the toxicological endpoints of environmental pollutants. Fish embryo toxicity (FET) test was developed to evaluate the developmental toxicity of toxicants on fish, and most of these tests were carried out in zebrafish embryo because of its transparency and easy maintenance. The transparent nature of eggs and embryos allows the easy visualization of morphological and structural abnormalities in the whole body following exposure to chemicals (Brannenet al., 2010; Fraysseet al., 2006; OECD, 2006). Several studies have been carried out on the developmental toxicity of carbaryl to zebrafish embryo. Carbaryl exposure retarded embryonic development, delayed hatching, defect in heart formation, red blood cell accumulation, bradycardia and decreased embryo size in zebrafish at non-lethal concentrations (Linet al., 2007; Schocket al., 2012; Todd and van Leeuwen, 2002). But further global investigations of the developmental and teratogenic toxicity of carbaryl to izebrafish embryos are still needed for the safety assessment of carbaryl for aquatic organisms.
In vitrocell cultures, as an alternative of whole animals, provide us a useful tool of examining the cytotoxic and genotoxic effects of chemicals and environmental pollutants in a rapid and cost-effective way (Bolset al., 2005). Toxicity of carbaryl and intracellular localization of this insecticide in cell cultures derived from goldfish has been assessed (Shea and Berry, 1983). Langeet al. (1995) examined the carbaryl’s cytotoxic effect on rainbow trout gonad-derived RTG-2 cells and obtained a 72 h-IC50value of 100 mg L-1which indicated a much lower sensitivity than that of embryo toxicity test (96 h-LC50= 6 mg L-1). Carbaryl-induced genotoxic effects have been reported byin vitrostudies as mitotic aberrations (Renglinet al., 1999) and sister-chromatid exchanges (Önfelt and Klasterska, 1984) in V79 Chinese hamster cells. However, information regarding the genotoxicity of carbaryl to fish cell line is lacking. Gill cell is a goodin vitromodel for toxicity testing because these cells are functionally important for fish and are in direct contact with the surrounding environment (toxicants). The continuous marine flatfish cell line FG derived from flounder gill was maintained in our laboratory since its establishment by Tonget al. (1997). It has already been successfully used to studying the toxic effects and mechanisms of environmental pollutants on fish species (Guo and Zhang, 2002; Li and Zhang, 2001; Naet al., 2009; Xiaoet al., 2011; Xiaoet al., 2007; Yanget al., 2010; Yinet al., 2007). However, the toxic effects of carbaryl on FG cells have not yet been evaluated.
The main objective of present study was to examine the toxic effects of carbaryl toin vitrocultured FG cells and zebrafish embryos with a view to record its cytotoxicity, genotoxicity and teratogenicity, and then compare the suitability and sensitivity of FG cells for toxicity testing of toxicants with zebrafish embryos.
2 Material and Methods
2.1 Chemicals
Carbaryl (1-naphthyl-N-methylcarbamate, 99.8% pure), MTT (3-(4,5-dimethylthazol-2-yl)-2,5-diphenyl tetrazolium bromide), neutral red (NR), dimethyl sulphoxide (DMSO), low melting point agarose (LMPA), 4’,6-diamidino-2-phenylindole dihydrochloride (DAPI), ethylene diamine tetraacetic acid (EDTA), trypsin and tris base were purchased from Sigma-Aldrich (St. Louis, MO, USA). All other chemicals used were at analytical grade. Plastic cell culture flasks (25 cm2) and 24- and 96-well culture plates were from Corning Incorporated (NY, USA).
A 100 µg µL-1stock solution of carbaryl was prepared in DMSO and stored at 4℃ before use. Working solutions were prepared by diluting the stock into culture media immediately before use. The final concentration of DMSO in working solutions was always ≤ 0.5%.
2.2 Cell line and Culture Conditions
The continuous flounder gill (FG) cell line was derived from the gill tissue of flounder,Paralichthys olivaceus, and has been maintained in our laboratory since 1993 (Tonget al., 1997), which was used for cytotoxicity and genotoxicity assays. Briefly, the cells were cultured at 20℃ in minimal essential medium (MEM; Gibco BRL, New York) supplemented with 10% bovine calf serum (BCS; Hyclone, USA), 100 IU mL-1penicillin, and 100 mg L-1streptomycin, buffered to pH 7.0 in plastic cell culture flasks at 20℃.
2.3 Zebrafish and Eggs
Adult zebrafish were purchased from a fish dealer, and were acclimatized in five glass aquaria each filled with 10 L matured water. They were maintained in a light:dark photoperiod cycle of 14 h:10 h and at 26℃ ± 1℃. Fish were fed with live nematodes twice a day and a part of the water was exchanged every day. In the evening, male and female fish (2:1) were placed in a spawning box. Spawning was triggered once the light was turned on the next morning and the fertilized eggs were collected and examined under a stereomicroscope.
2.4 MTT and Neutral Red (NR) Uptake Assays
The cytotoxicity of carbaryl was determined by MTT assay according to Borenfreundet al. (1988) and was performed on FG cells according to the method described previously (Yanget al., 2010). Briefly, 200 µL per well of FG cells at 1.0 × 105cells mL-1were seeded into 96-wellmicroplate and incubated at 20℃ for 24 h. Then the old medium was removed and the cells were exposed to new medium containing 0 (control), 0.1, 0.5, 1, 10, 25, 50, 75, 100 and 150 mg L-1carbaryl. After 24 h of exposure, the medium was replaced and MTT assay was performed. The absorbance was measured by spectrophotometer (Tecan, GENios, Austria) at 490 nm. The 24 h-IC50(50% inhibitory concentration after a 24 h exposure period of the test agent) value was determined by logistic nonlinear regression analysis using the GraphPad Prism 5 software. Further validation of the cytotoxicity of carbaryl was done by a time-dependent MTT assay using sublethal concentration of 30 mg L-1carbaryl, which is about 60% of 24 h-IC50value of concentration-dependent MTT assay. Briefly, after the removal of media from 24 h incubation of 1.0 × 105FG cells mL-1per well, the cells were exposed to 30 mg L-1carbaryl in MEM. The exposed cells were sampled at 3, 6, 9, 12, and 24 h and subjected to MTT assay.
NR uptake assay was carried out based on the procedure described by Naet al.(2009). This assay measures the inhibition of cell growth, which is based on the absorbance of the vital dye NR by living, but not by dead cells. All concentrations were tested in triplicate and the mean absorbance at each concentration was calculated and expressed as the percentage of absorbance of treated cells against that of control.
2.5 LDH Release Assay
LDH release assay measures the integrity of cell membrane in the exposed cells by the activity of LDH released from the cytosol of damaged or lysed cells in the supernatant of culture wells (Korzeniewski and Callewaert, 1983). The LDH activity is evaluated through the loss of NADH and formation of NAD+during the oxidation of pyruvate to lactate. Untreated cells should retain LDH in their cells and have minimal LDH leakage. The LDH release percentage for each sample was then calculated according to the following equation:
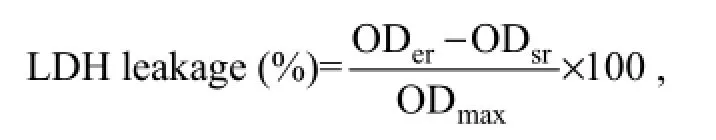
where ODeris experimental release OD, ODsris spontaneous release OD, and ODmaxis maximal release OD. Briefly, following exposure of FG cells to varied concentrations (0, 1, 10, 25, 50, 75, 100 mg L-1) of carbaryl for 24 h, LDH release assay was performed using a commercial LDH detection kit (Nanjing Jiancheng Bioengineering Institute, Nanjing, China) according to the manufacturer’s instructions. Further validation of the results was done by a time-dependent LDH release assay using sublethal concentration of 30 mg L-1carbaryl. Briefly, following exposure of FG cells to 30 mg L-1carbaryl in MEM, the exposed cells were sampled at 3, 6, 9, 12, and 24 h and subjected to LDH release assay.
2.6 Hoechst 33342 and Propidium Iodide (PI) Double Staining Assay
The mode of cell death was determined by Hoechst 33342 and PI double staining assay which distinguishes the percentage of dead cells after exposure to varied concentrations of carbaryl for 24 h with or without a fragmented nucleus, thus quantifying the percentage of necrotic and apoptotic cells. Briefly, cells were seeded into 24-well plates and exposed to different concentrations (0, 0.1, 0.5, 1, 10, 50, 100 mg L-1) of carbaryl for 24 h followed by staining the exposed cells using a Hoechst 33342/PI double staining kit (Shanghai Majorbio Bio-Pharm Technology Co. Ltd., Shanghai, China) according to the manufacturer’s instructions. The blue and red fluorescence of these cells were separately examined under fluorescence microscope (Carl Zeiss). The number of live, apoptotic and necrotic cells were recorded and the percentages of damaged cells were calculated in contrast to total cells.
2.7 DNA Fragmentation Assay
In order to delineate whether the cell damage was associated with apoptosis or necrosis at genome level by carbaryl, DNA fragmentation assay was performed. Briefly, following exposure of FG cells to varied concentrations (0, 10, 50, and 100 mg L-1) of carbaryl for 24 h, DNA was isolated from the exposed cells using a DNA extraction kit (CoWin Biotech Co., Ltd., Beijing, China) according to the manufacturer’s instructions. The isolated DNA was electrophoresed in 2% agarose gel containing ethidium bromide and visualized under UV-illuminated camera (Peiqing, China).
2.8 Comet Assay
Comet assay allows detecting DNA breakages induced by genotoxic agents and has been modified variously to suit different types of cell assays (Collins, 2004). In this study, this assay was conducted under alkaline conditions as described by Singhet al. (1988). In order to avoid false positive response of the toxicant due to cytotoxicity, cell viabilities exceeding 75% were further processed for the evaluation (Hendersonet al., 1998). The Comet assay was carried out according to the method described previously (Yanget al., 2010). The cells were exposed to 0, 0.5, 1, 5, 10, and 20 mg L-1carbaryl for 24 h and subjected to Comet assay. As a positive control, the FG cells were exposed to 5 mg L-1bleomycin prior to Comet assay and an unamended medium was used as a negative control. For the time-dependent genotoxicity test, 10 mg L-1of carbaryl was chosen based on the results of cytotoxicity assay above where about 80% survival was observed at this concentration. The exposed cells were sampled at 3, 6, 12, 24, 48, 72, and 96 h, respectively and subjected to Comet assay. The cells were scored randomly and analyzed visually according to the relative intensity of the fluorescence in the tail that classified the comets from grades 0-3.
2.9 Teratogenicity Assay
The assay was based on the OECD guidelines on Fish Embryo Toxicity (FET) test (OECD, 2006) and was car-ried out as described by Fraysseet al. (2006). A total of 45 eggs per treatment were selected and grouped into three experiments. For each concentration and control, experiments were performed in triplicate. The test started with newly fertilized eggs exposed to different concentrations of 0, 1, 10, 20, 40, 80, 100 and 120 mg L-1of carbaryl. Embryos and larvae were observed daily under a stereomicroscope connected to a camera device (Olympus SZX12, Japan) at specific time points (4, 8, 12, 24, 48-60, 72-84, and 96 h). During the periods of 48-60 h and 72-84 h, records were made every 2 h and 4 h, respectively, for hatching rate calculation. In the embryo phase, the parameters evaluated included egg coagulation, malformations, eye development, body pigmentation, otolith formation, spontaneous movement, heartbeat and total body length as well as hatching rate, heart rate and mortality.
2.10 Data Analysis and Statistics
Significant (P〈 0.05) data were obtained by usingpost hocrange tests expressed as mean ± SEM and pairwise multiple comparisons were made using the Tukey HSD test, depending on the nature of the test, parametric or non-parametric, respectively to verify differences between the tested concentrations and control. The statistical tools used for the comparison between groups depend on the type of the data. The data were tested for homogeneity and normality. If these assumptions were met, one-way analysis of variance (ANOVA) was performed to detect the significant differences between the groups for normally distributed data set. Otherwise, the non-parametric test viz. the Kolmogorov-Smirnov (KS) normality test and χ2tests were performed in order to evaluate the non-normal distribution of group data to assess the homogeneity between the tested concentrations. All the statistical analyses were carried out using GraphPad Prism 5 (GraphPad Software, Inc., San Diego, USA) and SPSS 16.0 (SPSS Inc., Chicago, IL). All the experiments were conducted at least in triplicate.
3 Results
3.1In VitroCytotoxicity of Carbaryl to FG Cells
3.1.1 Toxic effects of carbaryl on the growth of FG cells
Carbaryl inhibited the proliferation of FG cells in a concentration-dependent fashion (Fig.1A); the viability of FG cells decreased with the increase of carbaryl concentration. Significant effect was observed at concentrations of ≥ 10 mg L-1. The 24 h-IC50value of carbaryl to FG cells determined by MTT and NRU assays was 53.48 ± 1.21 mg L-1and 59.13 ± 1.19 mg L-1, respectively. A time-dependent cytotoxic effect was also observed when FG cells were exposed to sublethal concentration of carbaryl (30 mg L-1) for 3-24 h as was determined by MTT assay (Fig.1B). Significant (P〈 0.05) toxic effect was obtained only after 24 h exposure in time-course toxic test.
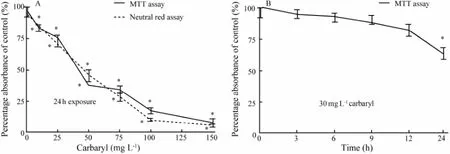
Fig.1In vitrocytotoxicity of carbaryl to FG cells. A, concentration-dependentin vitrocytotoxicity after 24 h exposure; B, time-course cytotoxicity of carbaryl at the sublethal concentration (30 mg L-1) to FG cells during a 24 h exposure period. The percentage absorbance was calculated in comparison with the untreated cells (set as 100%). The values are expressed as mean ± SEM (n=3; *,P〈 0.05).
3.1.2 Toxic effect of carbaryl to the membrane integrity of FG cells
The toxic effect of carbaryl to the membrane integrity of FG cells were examined by LDH release assay. As shown in Fig.2A, the cells displayed increasingly significant LDH release when exposed to 25 mg L-1or higher carbaryl. The obtained 24 h-IC50value was 46.21 ± 1.24 mg L-1carbaryl, which is consistent with the value obtained by MTT assay. Similar to MTT assay, a time-dependent cytotoxic effect on the membrane integrity of the FG cells after exposed to the sublethal concentration of 30 mg L-1carbaryl for 3-24 h was also observed (Fig.2B). And statistically significant (P〈 0.05) toxic effect in the timecourse toxic test was obtained only after 24 h exposure.
The above findings were further confirmed by morphological changes. Prominent morphological changes were observed in the FG cells after being exposed to 25 mg L-1or higher carbaryl for 24 h (Fig.3), inferring that the LDH leakage was coincident with the occurrence of obvious morphological changes. At lower concentrations of carbaryl (〈 25 mg L-1), the cells showed the same morphology as that of control cells. With further increase of the concentration, however, the treated cells started toshrink and distort into irregular shape, and eventually detached from the substrate surface and lysed.
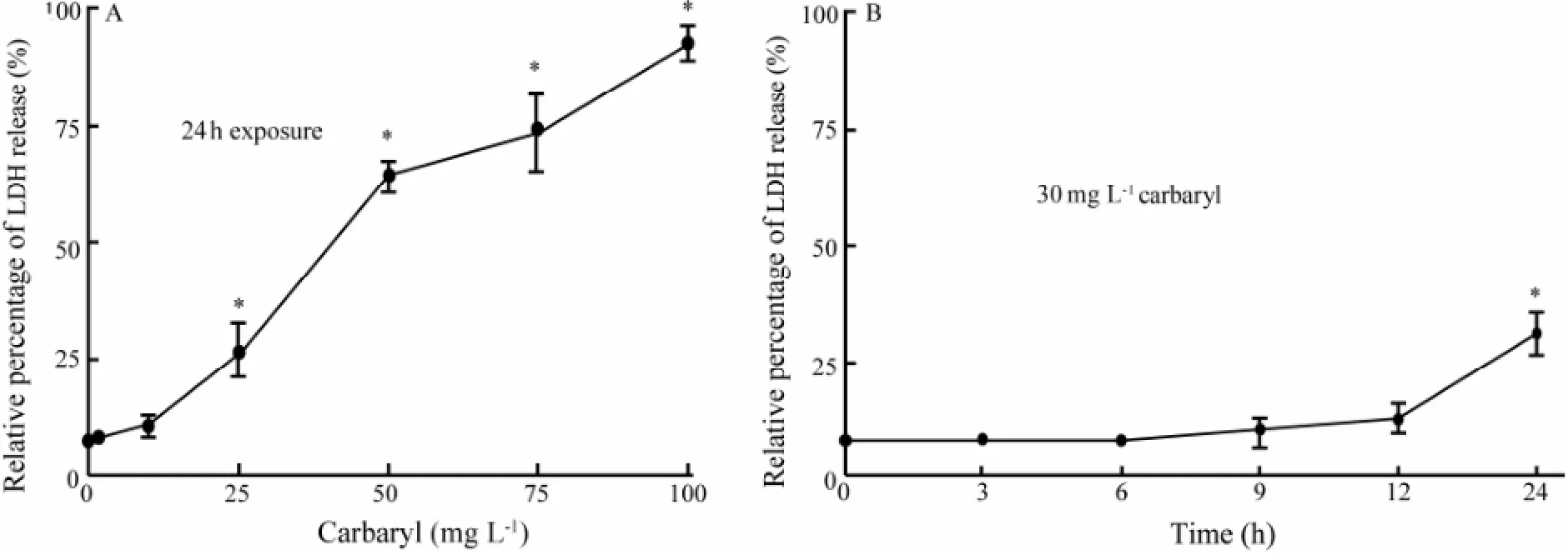
Fig.2 Effects of carbaryl on LDH release in FG cells. (A) Concentration-dependentin vitrocytotoxicity of carbaryl to FG cells. (B) Time-course cytotoxicity of carbaryl at a sublethal concentration (30 mg L-1) to FG cells within 24 h exposure period. The values are expressed as mean ± SEM (n=3; *,P〈 0.05).
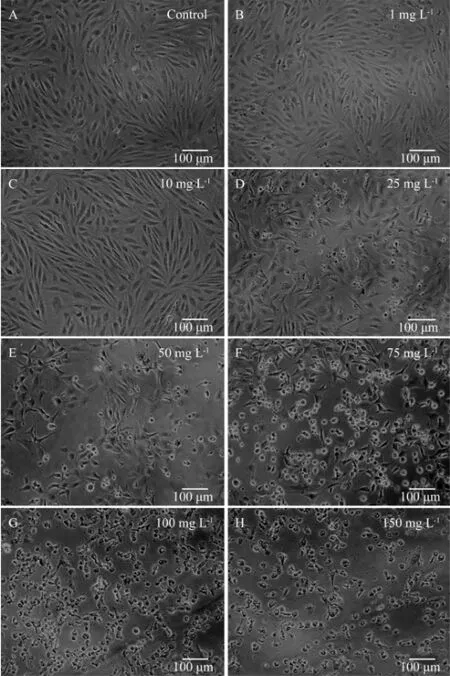
Fig.3 Morphological changes of FG cells after being exposed to carbaryl at various concentrations for 24 h. A through H correspond to 0 (control), 1, 10, 25, 50, 75, 100 and 150 mg L-1carbaryl, respectively.
3.1.3 Carbaryl induced necrosis rather than apoptosis of FG cells
In order to investigate the toxicological mechanism of carbaryl, living, necrotic and apoptotic FG cells were discriminated by Hoechst 33342 and PI double fluorescence staining after the cells were exposed to various concentrations of carbaryl for 24 h. The morphology and the intensity of blue and red fluorescence of the nuclei (Figs.4 and 5, respectively) indicated that carbaryl causedthe necrosis of the cells in a concentration-dependent way rather than the apoptosis of the cells as apoptotic cells were noticed sparsely. Significant cell damage was observed when the concentration of carbaryl was ≥ 10 mg L-1.
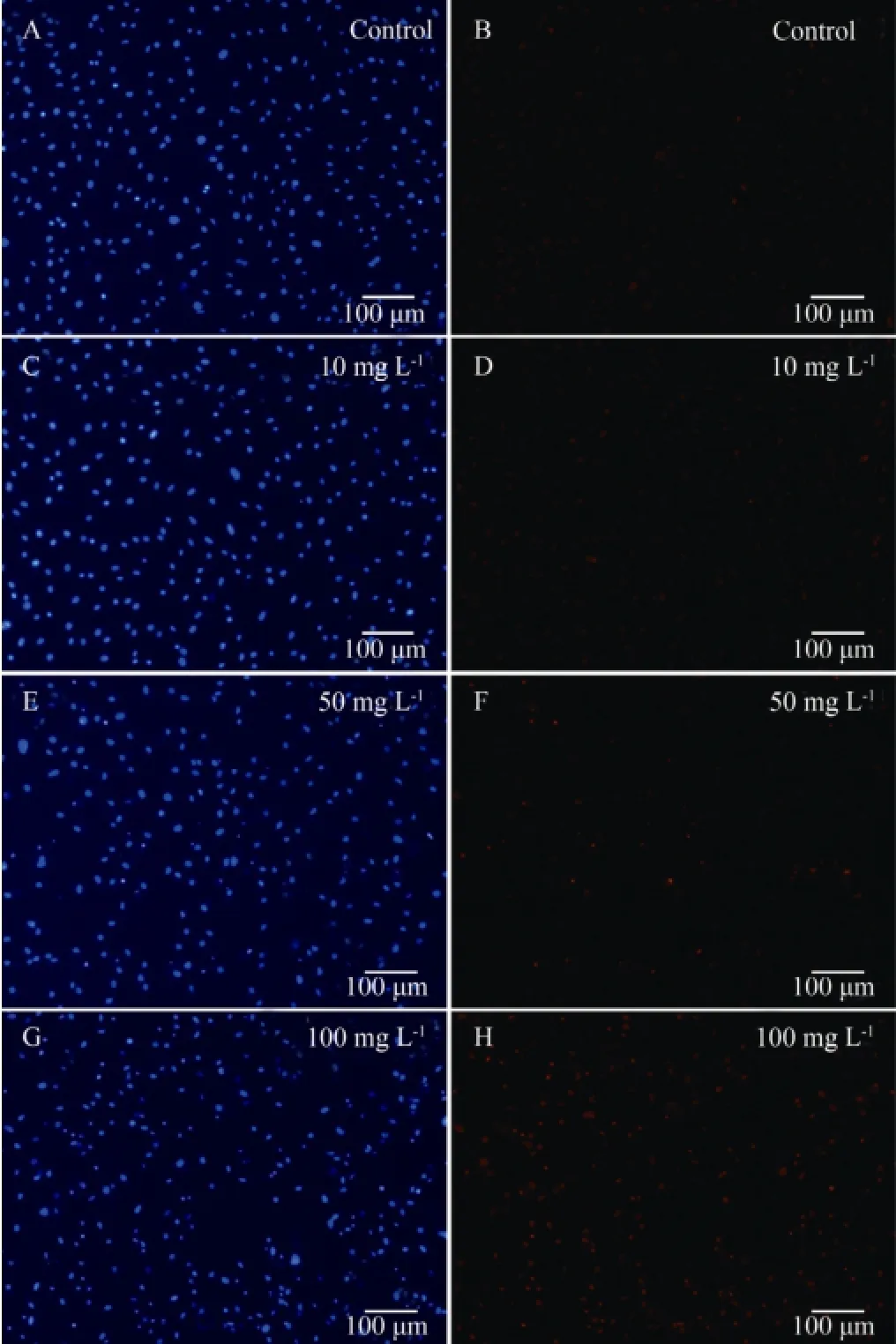
Fig.4 Representative fluorescent micrographs showing the morphology and intensity of blue and red fluorescence of live, necrotic and apoptotic cells after they were exposed to various concentrations of carbaryl for 24 h with Hoechst 33342 and PI double staining. A and B, controls; C and D, E and F, and G and H, cells treated with carbaryl at concentrations of 10, 50 and 100 mg L-1, respectively; A, C, E and G, Hoechst 33342 stained; B, D, F and H, PI stained. Live cell nuclei are seen in light blue and light red, dead cell nuclei are in light blue and bright red, and apoptotic cell nuclei are in bright blue and light red.

Fig.5 Percentage of FG cells showing necrosis and apoptosis after being exposed to various concentrations of carbaryl for 24 h as was determined by Hoechst 33342 and PI double staining. Data are expressed as mean ± SEM (n=3; *,P〈 0.05).
3.2 Carbaryl Did Not Elicit Any Significant DNA Damage in the FG Cells
In FG cells, carbaryl did not significantly induce DNA damage. Comet assay has not found significant DNA damage to FG cells by 0.5-20 mg L-1of carbaryl although comet assay scores (au) ranged from 18 ± 4.58 to 31 ± 8.02.
3.3 Embryotoxicity of Carbaryl to Zebrafish
3.3.1 Malformation of zebrafish embryos induced by carbaryl
As shown in Fig.6, malformation appeared in 8 h post fertilization (hpf) after carbaryl treatment, pronounced in yolk and pericardial sac edema from 24 to 96 hpf, and delayed epiboly progression from 8 to 12 hpf. Malformation in body length and tail flexure was also observed at 96 hpf. The number of malformed embryos increased with carbaryl concentration increase in a time-dependent manner (Fig.7A).
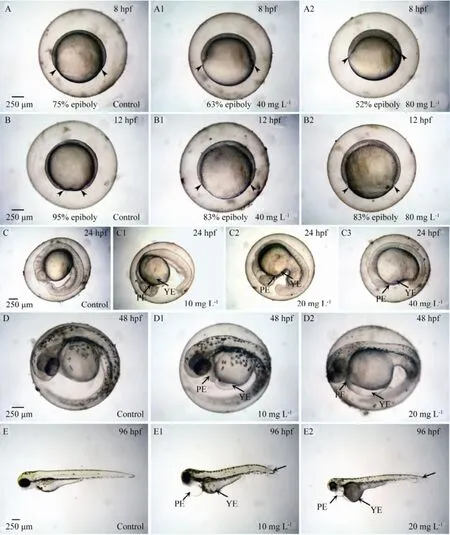
Fig.6 Representative micrographs showing the teratogenicity of carbaryl to zebrafish embryos. A and B, controls; A1 and A2, epiboly slowdown at 8 hpf; B1 and B2, epiboly slowdown at 12 hpf; A1 and B1, 40 mg L-1of carbaryl; A2 and B2, 80 mg L-1of carbaryl; Arrowheads, the degree of epiboly; C, D and E, controls; C1, D1 and E1, 10 mg L-1of carbaryl; C2, D2 and E2, 20 mg L-1of carbaryl; C3, 40 mg L-1of carbaryl; YE, PE and TF, yolk sac edema, pericardial sac edema and tail flexure, respectively.
As shown in Fig.6, slowdown of epiboly was first evident in the exposed zebrafish embryos at 8 hpf at 40 and 80 mg L-1of carbaryl concentrations, at which 84.44% ± 24.65% and 0% exposed embryos reached at the stage of≥ 75% epiboly, respectively, whereas all the remaining exposed embryos reached at the stage of about 45%-65% epiboly. Similar type of effects were also observed significantly at 12 hpf at which only 55.56% ± 16.74% and 0% exposed embryos reached at the stage of ≥ 90% epiboly for 40 and 80 mg L-1carbaryl, respectively, whereas all the remaining exposed embryos were observed at the stage of about 40%-85% epiboly instead. In contrast, 97.78% ± 28.28% of the control groups reached at the stage of ≥ 90% epiboly.
Fig.6 shows also the effects of various concentrations of carbaryl on the yolk and pericardial sacs of zebrafish embryos at 24, 48 and 96 hpf. At 24 hpf, the significant percentage effects on the yolk sac for 10, 20 and 40 mg L-1carbaryl were observed in ≥ 40% of the exposed embryos, whereas that on the pericardial sac were found in ≥27% of the survived embryos (P〈 0.05). At 48 hpf, the percentage effects on yolk and pericardial sacs for 10 and 20 mg L-1carbaryl exposure were ≥ 37%, and ≥ 30%, respectively, however, ≥ 44% of the survived embryos were affected by 10 mg L-1carbaryl exposure. But all survived exposed embryos were observed affected significantly (P〈 0.05) at the concentration of 20 mg L-1carbaryl.
At 96 hpf total body length was markedly affected by carbaryl at exposure levels of 10 and 20 mg L-1, causing significant reduction in body length to ≥ 81% of the em-bryos that survived (P〈 0.05) (Fig.6). Tail flexure was also observed at 96 hpf and significantly affected at 10 and 20 mg L-1carbaryl to ≥ 73% of the exposed embryos.
3.3.2 Zebrafish egg/embryo mortality following exposure to carbaryl
As shown in Figs.7A and B, zebrafish embryos exposed to increasing concentrations of carbaryl for 8, 12, 24, 48 and 96 hpf demonstrated elevated mortality rates in both concentration- and time-dependent manners. The early mortality of embryos were first observed at 8 and 12 hpf with LC50(50% lethal concentration after the exposure of test agent at certain time period) values of 80.01 ± 2.5 mg L-1and 62.08 ± 1.05 mg L-1, respectively. The lowest observed effect concentration (LOEC) of carbaryl was 10 mg L-1(P〈 0.05) with 24 h-, 48 h- and 96 h-LC50values of 41.80 ± 1.10 mg L-1, 17.80 ± 1.04 mg L-1and 14.46 ± 1.05 mg L-1, respectively.
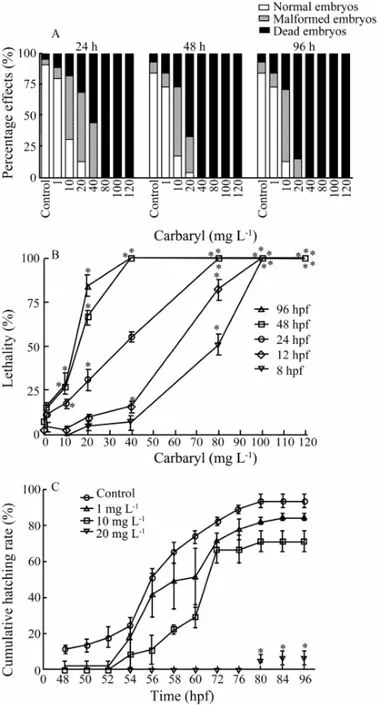
Fig.7 Embryotoxic effects of carbaryl on zebrafish embryos exposed for 8, 12, 24, 48 and 96 hpf. A, the total percentages of normal, malformed and dead embryos after 24, 48 and 96 hpf exposure (n= 45 for each concentration); B, the concentration- and time-dependent lethal toxicity of carbaryl; C, the cumulative hatching rates of the exposed embryos observed from 48 to 96 hpf. The values are expressed as mean ± SEM. (n= 45 for each concentration; *,P〈 0.05).
As shown in Figs.7A and B, on the average, embryos exposed to lower concentration (i.e., 1 mg L-1) of carbaryl showed low rates of malformation (≤ 11.11% ± 0.02% up to 96 hpf) and mortality (≤ 15.56% ± 0.02% up to 96 hpf). However, the percentage of malformation and mortality increased as the concentration of carbaryl administered increased, with malformation of ≤ 57.78% ± 0.02% and mortality of ≤ 28.89% ± 0.06% of the total embryos at 10 mg L-1up to 96 hpf. With further increase of mortality at the concentration of 20 mg L-1carbaryl, the malformation observed were ≤ 55% of the total exposed embryos at 24, 48, and 96 hpfs.
3.3.3 Hatching success following exposure to carbaryl
Cumulative hatching rates from 48 to 96 hpf are shown in Fig.7C. The statistically significant effects on hatching were observed at 10 and 20 mg L-1carbaryl when compared to the control at 96 hpf (P〈 0.05). However, the toxic effects increased with the increasing concentration of carbaryl and in a concentration-dependent manner.
The value of median hatching time (HT50) calculated from the cumulative hatching rates in Fig.7C is shown in Table 3. At carbaryl concentration of 20 mg L-1, the HT50was not calculated because of the lower hatching rates and/or no hatching. Significant difference between the control and exposed groups was observed only at 10 mg L-1(P〈 0.05).

Table 3 Median hatching time (HT50) of zebrafish embryoson exposure to carbaryl
3.3.4 Carbaryl affected spontaneous movement
Effects of different concentrations of carbaryl on spontaneous movement of embryos were recorded from the movements of each embryo observed for 20 s (Fig.8A). Resulting effects were discrete and the distribution was not symmetric over the mean. The data revealed that the movement frequencies and the corresponding percentage of the embryos were almost similar in control and 1 mg L-1group with 2 times of spontaneous movements per 20 s recorded in ≤ 28.89% ± 2.22%, and 3 times of movements recorded in ≤ 26.67% ± 10.18% of the embryos. However, the maximum movement frequency was observed up to 8 times in control and 7 times in 1 mg L-1groups in ≤ 4.44% ± 2.22%. For 10 and 20 mg L-1, the movement frequencies recorded and the corresponding percentage were found to be 0, 1 and 2 times in ≤ 31.11% ± 5.88% of the embryos. But at 40 mg L-1carbaryl exposure, all exposed embryos showed no spontaneous movement. The results showed that the frequency ofmovement decreased with the increasing concentrations of carbaryl. As the obtained data were not suitable for parametric law, the comparison between the movement counts was done with Kolmogorov-Smirnov (KS) and χ2tests for the non-normal distribution of group data. KS test showed that there was no uniform distribution between the spontaneous movements among all tested concentration groups (P〈 0.05). χ2test also indicated the statistical difference between the number of spontaneous movements (P〈 0.05).
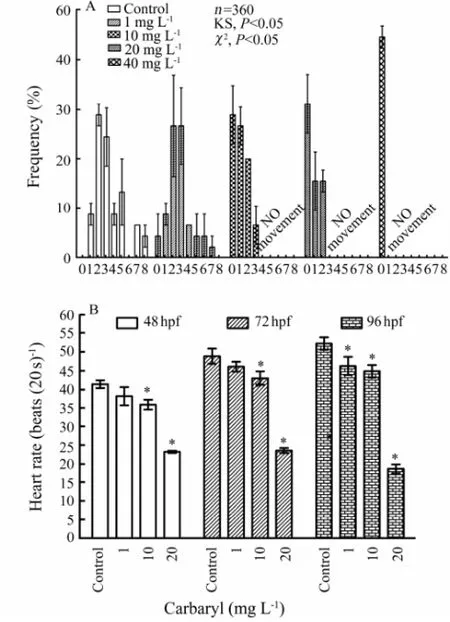
Fig.8 Embryotoxic effects of various concentrations of carbaryl on the frequency of spontaneous movement of un-hatched embryos per 20 s monitored at 24 hpf (A) and heart beat rate of exposed embryos or larvae monitored at 48, 72 and 96 hpf, respectively (B). Data from the spontaneous movement counts are compared using non-parametric tests Kolmogorov-Smirnov (KS) and χ2tests for the non-normal distribution of group data.n, number of values;P, probability. The values are expressed as mean ± SEM (n=360; *,P〈 0.05).
3.3.5 Reduction of heart rates in zebrafish embryos following exposure to carbaryl
Mean of heart rates of the embryos at 48, 72 and 96 hpf following exposure to carbaryl at different concentrations (n= 45 for each concentration) are given in Fig.8B. The mean heart beats ranged from 41.42 ± 1.06 to 23.24 ± 0.33 beats per 20 s at 48 hpf; 48.83 ± 2.03 to 23.58 ± 0.65 beats per 20 s at 72 hpf; and 52.22 ± 1.66 to 18.67 ± 1.20 beats per 20 s at 96 hpf for control and 20 mg L-1, respectively.
Significant decline in heart rates were observed at carbaryl concentrations of 10 and 20 mg L-1(P〈 0.05) in comparison to control at 48 and 72 hpf. But heart rates were observed declined significantly at all the tested concentrations from 1 mg L-1and above at 96 hpf. Moreover, it was noted that the heart rates of exposed embryos showing yolk sac edema and pericardial edema were feeble and irregular, and reduced to as low as 19 beats per 20 s at 48 hpf, 22 beats per 20 s at 72 hpf, and 17 beats per 20 s at 96 hpf for the higher carbaryl concentration of 20 mg L-1.
No abnormality was observed in other developmental endpoints, such as tail detachment, otolith formation, somite formation, eye development and body pigmentation in the treated embryos (Table 4).
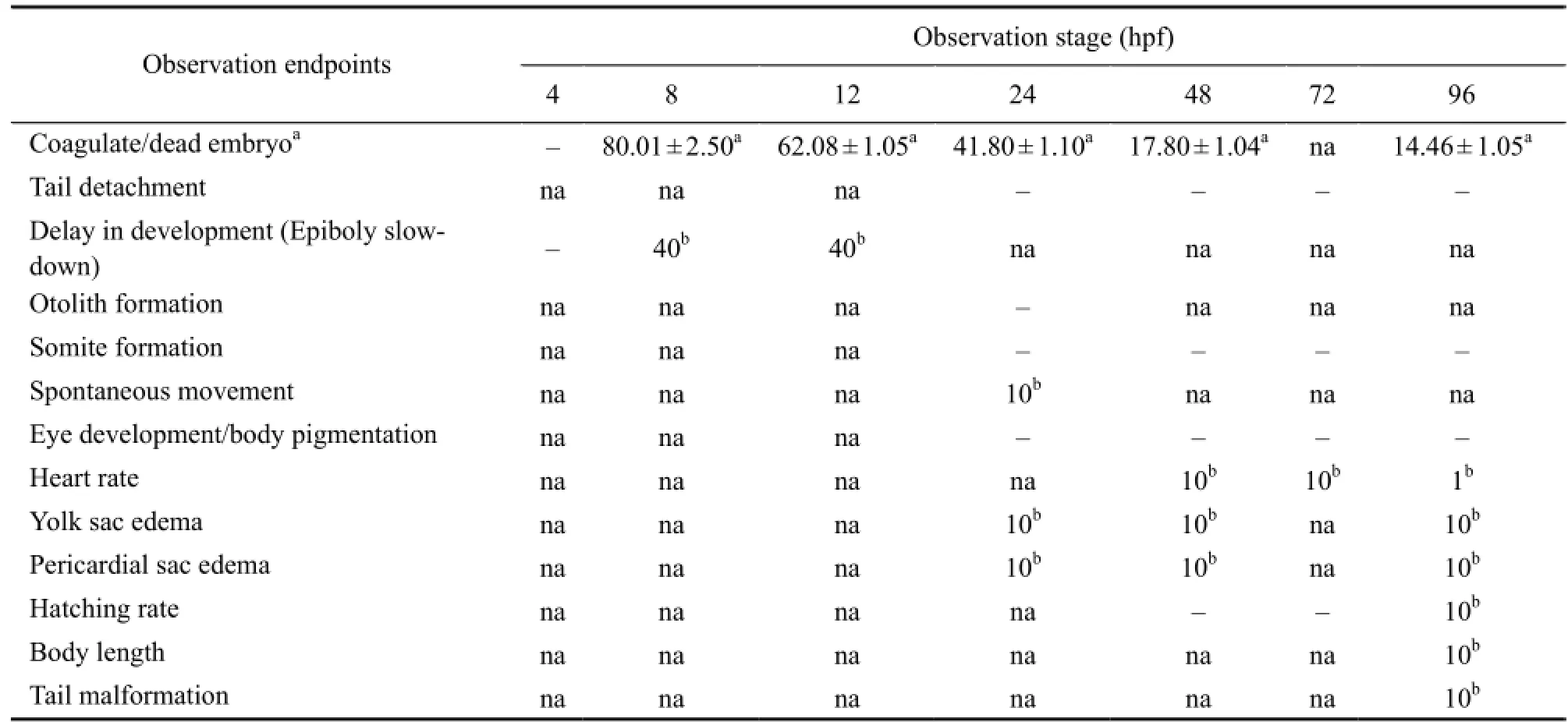
Table 4 Summary of toxic effects of carbaryl on different stages of developing zebrafish embryos (hpf)
4 Discussion
The cytotoxic and genotoxic effects of carbaryl toin vitrocultured fish cell line of FG and its teratogenic effect on zebrafish embryos were assessed. The findings may aid to clarifying the possible adverse effects of this pesticide and its toxic potential.
The cytotoxicity of carbaryl inin vitrocultured fish cells has been examined previously. Carbaryl showed similar levels of toxicity to goldfish fin-derived CAR cells, and goldfish air bladder-derived ABIII cells at 200 mg L-1at day seven, while 25 mg L-1demonstrated marginal cytotoxicity (Shea and Berry, 1983). Langeet al. (1995) exposed RTG-2 cells to different compounds including carbaryl and determined the same 72 h-IC50values of 100 mg L-1for both MTT and NRU assays. In the present study, FG cells showed higher sensitivity to carbaryl exposure. Close correlations of cytotoxicity value range were obtained with the 24 h-IC50values of 53.48 ± 1.21 mg L-1, 59.13 ± 1.19 mg L-1and 46.21 ± 1.24 mg L-1carbaryl in FG cells for MTT, NRU and LDH leakage assays, respectively. It was also demonstrated that LDH leakage and MTT assays are more sensitive than NRU assay in the cytotoxicity detection of carbaryl. Both the different mechanisms of toxicity detection for these assays and the toxic mechanism of carbaryl in FG cells may account for the above-mentioned different sensitivity. According to their 24 h-IC50values, the order of sensitivity for these three assays, was LDH 〉 MTT 〉 NRU. Taken together, carbaryl imposed moderate cytotoxicity on FG cells. In addition, the cytotoxicity of carbaryl on FG cells was closely correlated in all these assays, and was independent of the different cytotoxic endpoints. The results support the views of Ekwall (1995), Li and Zhang (2001) and Xiaoet al. (2011) that most of the cell lines have a similar response to toxicants when toxicity is measured with various endpoints relating to basal functions and structures. The results also suggest that the endpoints employed in the present study are useful to predict acute cytotoxicity. Further, gills are very important fish organs that are involved in the exchange of oxygen and CO2(respiration), ion and water. Dysfunction of gills is often lethal for fish, for example, suffocation. Gills contact directly with surrounding environment (toxicants) and thus are very sensitive to toxicant stress. The functional importance and direct toxicant-exposure property of gills may account for the higher sensitivity of FG cells than other fish cells.
Morphological alterations are the primary indications of cytotoxicity and the underlying mechanisms. Boranet al. (2010) studied the diverse effects of carbaryl on the gills of rainbow trout (Oncorhynchus mykiss), and observed the histopathological changes of the gill lamellae, including edema, separation of epithelium from lamellae, lamellar fusion, swelling of the epithelial cells, and epithelial cell necrosis. By exposing goldfish to 10 mg L-1carbaryl for 96 h, Pfeifferet al. (1997) also observed a variety of cellular changes such as secondary lamellar fusion, distortion, thinning, mucus release, enlargement of subepithelial lymphatic spaces and mitochondrial disruption and distortion of the lamellar covering epithelium. Pronounced changes in branchial epithelia by carbaryl were also observed with stretching of secondary gill lamellae and migration of mitochondria-rich cells throughout the lamellar surface in climbing perch (Peteret al., 2013). Here, FG cells also demonstrated prominent morphological alterations when exposed to 25 mg L-1or higher carbaryl for 24 h. The significant LDH leakage was observed in the FG cells after 24 h exposure to 25 mg L-1and higher carbaryl, indicating that the LDH leakage was coincident with the occurrence of obvious morphological changes. Carbaryl at the concentrations up to 50 mg L-1did not induce the apoptosis of FG cells significantly. Furthermore, no apparent DNA laddering was detected by agarose gel electrophoresis analysis. The Hoechst 33342 and PI double staining assay showed the necrotic appearance rather than apoptosis. Thus the main pathway involved in the cytotoxicity was not apoptosis, but the necrosis of the cells.
Little is known about the genotoxic effects of carbaryl on fish cells, and there are limited and inconsistent data in the literature about the genotoxic effects of carbaryl with the Comet assay. Carbaryl was not genotoxic in mostin vitroassays except a cytogenic assay in cultured Chinese hamster ovary cells with metabolic activation, and unscheduled DNA synthesis in human fibroblast cells (PMRA, 2009). In the present study, despite the non-significant DNA damage, Comet assay was sensitive for analyzing the genotoxicity of carbaryl to exposed FG cells for both concentration- and time-dependent tests up to 96 h.
Previous studies demonstrated the toxic potency of carbaryl on different fish species and the embryotoxicity to fish embryos (Beyerset al., 1994; Galloet al., 1995; McKimet al., 1987; Sinhaet al., 1991). In this study, we also observed some toxic effects of carbaryl on developing zebrafish. Among the selected endpoints, carbaryl affected the epiboly, spontaneous movement, yolk and pericardial sac area, heart rate and hatching success, body length and tail malformation in developing zebrafish embryo. The insecticide effects were manifested at different stages of development of zebrafish in a concentrationdependent manner (Table 4). The earliest developing processes were disrupted at 8 hpf with 40 and 80 mg L-1of carbaryl with a slowdown of epiboly. The 24 h-LC50for zebrafish embryo in our study was 41.80 ± 1.10 mg L-1. It was similar with the value obtained by Linet al. (2007), which was 44.66 mg L-1along with EC50of 7.52 mg L-1. Galloet al. (1995) also reported a 96 h-LC50of 9.26 mg L-1in adult zebrafish, however, 96 h-LC50value was 14.46 ± 1.05 mg L-1in the present study.
Furthermore, the delayed or unfinished epiboly at 8 and 12 hpf later resulted in either malformation or mortality. The induction of spontaneous movements of the tail at 24 hpf are considered as the result of the development of the motoneurons without any control by the central nervous system. The joint development of muscular and motoneural systems are responsible for arising the spontaneousmovements (Kimmelet al., 1995; Myerset al., 1997). Spontaneous movements in the developing embryos indicate the uncontrolled action potential of the motorneurons (Fraysseet al., 2006). The result of the present study revealed the decrease in the frequency of spontaneous movement and embryo activity per 20 s at 24 hpf in a concentration-dependent manner. The AChE inhibitory ability of carbaryl may account for the toxic effects on the motoneural system in the developing zebrafish embryos (Behraet al., 2002; Cox, 1993; Linet al., 2007). Yolk and pericardial sac edemas, at the tested higher concentrations, were the observed malformations along with some reduced body length and tail malformation. Fish embryos have shown slowed development as well as the reduction of the size of the embryo (Cox, 1993). It has been reported that carbaryl shortened body length of medaka embryos significantly with growth stage-dependent influences at 10 mg L-1(Kashiwadaet al., 2008), and delayed hatching time and decreased embryo size in zebrafish at 5.3-21.33 mg L-1(Linet al., 2007; Todd and van Leeuwen, 2002). Similar effects were also observed in our experiments with LOECs of 10 mg L-1at 96 hpf. But at 20 mg L-1, all survived embryos showed reduced body length which may further suffer the consequences due to the malformation as suggested by Todd and van Leeuwen (2002). We also found that pericardial edema was a common feature in carbaryl-exposed zebrafish embryos as reported in medaka (Kashiwadaet al., 2008; Solomon and Weis, 1979), common carp,Cyprinus carpio(Kaur and Dhawan, 1993) and zebrafish (Linet al., 2007). As reported earlier, the significant decreased heart beat was observed from 48 hpf onwards; however, Linet al. (2007) examined significantly decreased heartbeat in 1 to 4 days post fertilization (dpf) embryos after a short period of exposure to carbaryl. There are various biochemical and molecular mechanisms which occur among cells, receptors, tissues and organs during embryogenesis, and these mechanisms could be specifically influenced by the multitude of pollutants (Fraysseet al., 2006). Previous studies have pointed out the toxicity of carbaryl to zebrafish and compared with other fish species, in which the lack of butyrylcholinesterase (BChE) gene might be the main reason, although the presence or absence of BChE in most fish is unknown (Bertrandet al., 2001; Linet al., 2007).
The lowering of heart rates were also observed from 10 mg L-1at 48 hpf to 1 mg L-1at 96 hpf, which might be caused by general metabolic retardation due to toxic effect of the toxicant at the higher concentrations tested. The AChE inhibitory ability of the toxicant may induce the lowering of heart action. Such effects were also exhibited when exposed to zebrafish, medaka and killifish (Kashiwadaet al., 2008; Linet al., 2007; Schocket al., 2012; Solomon and Weis, 1979; Weis and Weis, 1974), but the effects can be toxicant- and concentration-specific. Linet al. (2007) also suggested that besides inhibition of the AChE, carbaryl may work via inhibition of calcium ion channels to induce bradycardia along with other alternate mechanisms. The observed delayed and retarded hatching of zebrafish embryos at the higher concentrations of cabaryl might be due to the disturbance of the hatching enzyme and hypoxia induced by carbaryl. The proteolytic hatching enzyme, secreted from hatching gland cells of the embryo, has the role of digesting the chorion during the normal hatching process of teleost embryos. Moreover, the interference of hatching-enzyme activity by osmotic disturbances, increased consumption of oxygen, and weakened spontaneous muscular movement can all affect the hatching (David and Pancharatna, 2009; Haendelet al., 2004; Strmacet al., 2002). Similar type of delayed hatching time was also observed earlier in carbaryl-exposed zebrafish embryos (Linet al., 2007; Todd and van Leeuwen, 2002).
In summary, the current study demonstrates that the exposure of FG cells to carbaryl for 24 h could induce moderate cytotoxic and no significant genotoxic effects in a concentration-dependent manner. FG cell lines also reacted differently to different cytotoxicity assays. These results confirm the suitability of this cell lines and the methods used for the screening of cytotoxic and genotoxic effects of this type of pesticide. Using zebrafish embryo as a model, our research also provided more information on the effects of exposure to carbaryl on its early embryo development in a concentration-dependent manner. The embryos exhibited a series of effects, including mortality, slowdown of epiboly, decreased spontaneous movement, yolk and pericardial sac edemas, decreased heart rates, delayed hatching rates, reduced body length and tail flexure. The concentration responsive endpoints analyzed in all the tests indicate that the significant effects were usually observed at 10 mg L-1. However, in LDH assay for cytotoxicity test, the significant effect was observed from 25 mg L-1, and no significant effects were observed in Comet assay for genotoxicity test. The effect on cumulative hatching rate of developing zebrafish and tail malformation at 96 hpf were significant only at the concentration of 10 mg L-1. Likewise, the effect on heart rate was significant at 1 mg L-1at 96 hpf. Moreover, epiboly slowdown was significant at the concentration of 40 mg L-1at both 8 and 12 hpf.
Acknowledgements
We thank Professor Shicui Zhang of Ocean University of China for his kind support and advice. This work was supported by the grants from National High-tech R&D Program of China (863 Program, Grant No. 2012AA 10A402), National Natural Science Foundation of China (Grant Nos. 31172391 and 31472274), Key Laboratory Open Foundation of Marine Bioactive Substances and Modern Analytical Technology, State Oceanic Administration (No. MBSMAT-2011-01), and Fundamental Research Funds for the Central Universities of China (Grant No. 201122005).
Agrawal, A., and Sharma, B., 2010. Pesticides induced oxida-tive stress in mammalian systems: A review.Journal of International Medical Research, 1: 90-104.
Behra, M., Cousin, X., Bertrand, C., Vonesch, J. L., Biellmann, D., Chatonnet, A., and Strähle, U., 2002. Acetylcholinesterase is required for neuronal and muscular development in the zebrafish embryo.Nature Neuroscience, 5: 111-118.
Bertrand, C., Chatonnet, A., Takke, C., Yan, Y. L., Postlethwait, J., Toutant, J. P., and Cousin, X., 2001. Zebrafish acetylcholinesterase is encoded by a single gene localized on linkage group 7. Gene structure and polymorphism; molecular forms and expression pattern during development.Journal of Biological Chemistry, 276 (1): 464-474.
Beyers, D. W., Carlson, C. A., and Keefe, T. J., 1994. Toxicity of carbaryl and malathion to two federally endangered fishes, as estimated by regression and ANOVA.Environmental Toxicology and Chemistry, 13: 101-107.
Bols, N., Dayeh, V., Lee, L., and Schirmer, K., 2005. Use of fish cell lines in the toxicology and ecotoxicology of fish. Piscine cell lines in environmental toxicology. In:Biochemistry and Molecular Biology of Fishes. Moon, T. W., and Mommsen, T. P., eds., Elsevier Science, Amsterdam, 43-84.
Boran, H., Altinok, I., and Capkin, E., 2010. Histopathological changes induced by maneb and carbaryl on some tissues of rainbow trout,Oncorhynchus mykiss.Tissue Cell, 42: 158-164.
Borenfreund, E., Babich, H., and Martin-Alguacil, N., 1988. Comparisons of twoin vitrocytotoxicity assays-The neutral red (NR) and tetrazolium MTT tests.Toxicology in Vitro, 2: 1-6.
Brannen, K. C., Panzica-Kelly, J. M., Danberry, T. L., and Augustine-Rauch, K. A., 2010. Development of a zebrafish embryo teratogenicity assay and quantitative prediction model.Birth Defects Research Part B: Developmental and Reproductive Toxicology, 89: 66-77.
Collins, A. R., 2004. The comet assay for DNA damage and repair: Principles, applications, and limitations.Molecular Biotechnology, 26: 249-261.
Cox, C., 1993. The problems with Sevin (carbaryl).Journal of Pesticide Reform, 13: 31-36.
Crawford, R. B., and Guarino, A. M., 1985. Effects of environmental toxicants on development of a teleost embryo.Journal of Environmental Pathology Toxicology and Oncology, 6: 185-194.
David, A., and Pancharatna, K., 2009. Developmental anomalies induced by a non-selective COX inhibitor (ibuprofen) in zebrafish (Danio rerio).Environmental Toxicology and Pharmacology, 27: 390-395.
Ekwall, B., 1995. The basal cytotoxicity concept. In:Alternative Methods in Toxicology. Goldberg, A., and Zutphen, L., eds., Mary Ann Liebert, Inc., New York, 721-726.
Fraysse, B., Mons, R., and Garric, J., 2006. Development of a zebrafish 4-day embryo-larval bioassay to assess toxicity of chemicals.Ecotoxicology and Environmental Safety, 63: 253-267.
Gallo, D., Merendino, A., Keizer, J., and Vittozzi, L., 1995. Acute toxicity of two carbamates to the guppy (Poecilia reticulata) and the zebrafish (Brachydanio rerio).Science of the Total Environnent, 171: 131-136.
Gill, T. S., Pant, J. C., and Pant, J., 1988. Gill, liver, and kidney lesions associated with experimental exposures to carbaryl and dimethoate in the fish (Puntius conchoniusHam.).Bulletin of Environmental Contamination and Toxicology, 41: 71-78.
Gruber, S. J., and Munn, M. D., 1998. Organophosphate and carbamate insecticides in agricultural waters and cholinesterase (ChE) inhibition in common carp (Cyprinus carpio).Archives of Environmental Contamination and Toxicology, 35:391-396.
Gunasekara, A. S., Rubin, A. L., Goh, K. S., Spurlock, F. C., and Tjeerdema, R. S., 2008. Environmental fate and toxicology of carbaryl.Reviews of Environmental Contamination and Toxicology, 196: 95-121.
Guo, H. R., and Zhang, S. C., 2002. Cytotoxicity and genotoxicity of polyethylenimine and nickel chloride in red sea bream (Pagrosomus major) fin cell line RSBF.Chinese Journal of Oceanology and Limnology, 20: 323-331.
Haendel, M. A., Tilton, F., Bailey, G. S., and Tanguay, R. L., 2004. Developmental toxicity of the dithiocarbamate pesticide sodium metam in zebrafish.Toxicological Sciences, 81:390-400.
Henderson, L., Wolfreys, A., Fedyk, J., Bourner, C., and Windebank, S., 1998. The ability of the Comet assay to discriminate between genotoxins and cytotoxins.Mutagenesis, 13: 89-94.
Kashiwada, S., Tatsuta, H., Kameshiro, M., Sugaya, Y., Sabo-Attwood, T., Chandler, G. T., Ferguson, P. L., and Goka, K., 2008. Stage-dependent differences in effects of carbaryl on population growth rate in Japanese medaka (Oryzias latipes).Environmental Toxicology and Chemistry, 27: 2397-2402.
Kaur, K., and Dhawan, A., 1993. Variable sensitivity ofCyprinus carpioeggs, larvae, and fry to pesticides.Bulletin of Environmental Contamination and Toxicology, 50: 593-599.
Kimmel, C. B., Ballard, W. W., Kimmel, S. R., Ullmann, B., and Schilling, T. F., 1995. Stages of embryonic development of the zebrafish.Developmental Dynamics, 203: 253-310.
Korzeniewski, C., and Callewaert, D. M., 1983. An enzymerelease assay for natural cytotoxicity.Journal of Immunological Methods, 64: 313-320.
Kuhr, R. J., and Dorough, H. W., 1976.Carbamate Insecticides:Chemistry,Biochemistry,and Toxicology. CRC Press, Inc., Cleveland, Ohio, 301pp.
Laale, H. W., and Lerner, W., 1981. Teratology and early fish development.American Zoologist, 21: 517-533.
Lange, M., Gebauer, W., Markl, J., and Nagel, R., 1995. Comparison of testing acute toxicity on embryo of zebrafish,Brachydanio rerioand RTG-2 cytotoxicity as possible alternatives to the acute fish test.Chemosphere, 30: 2087-2102.
Lele, Z., and Krone, P. H., 1996. The zebrafish as a model system in developmental, toxicological and transgenic research.Biotechnology Advances, 14: 57-72.
Li, H., and Zhang, S., 2001.In vitrocytotoxicity of the organophosphorus pesticide parathion to FG-9307 cells.Toxicology in Vitro, 15: 643-647.
Lin, C. C., Hui, M. N., and Cheng, S. H., 2007. Toxicity and cardiac effects of carbaryl in early developing zebrafish (Danio rerio) embryos.Toxicology and Applied Pharmacology, 222: 159-168.
McKim, J. M., Schmieder, P. K., Niemi, G. J., Carlson, R. W., and Henry, T. R., 1987. Use of respiratory-cardiovascular responses of rainbow trout (Salmo gairdneri) in identifying acute toxicity syndromes in fish: Part 2. Malathion, carbaryl, acrolein and benzaldehyde.Environmental Toxicology and Chemistry, 6: 313-328.
Myers, P. Z., Sipple, B. A., Hasaka, T., and Qutub, H., 1997. Automated analysis of spontaneous motor activity in the embryonic zebrafish,Danio rerio.Journal of Computer-Assisted Microscopy, 9: 169-181.
Na, N., Guo, H. R., Zhang, S. C., Li, Z., and Yin, L., 2009.In vitroandin vivoacute toxicity of fenpyroximate to flounderParalichthys olivaceusand its gill cell line FG.Aquatic Toxicology, 92: 76-85.
OECD (Organization for Economic Cooperation and Development), 2006.Fish Embryo Toxicity (FET) Test. OECD draft proposal for a new guideline (1st version). Guideline for the testing of chemicals, Paris. http://www.oecd.org/env/ehs/ testing/36817070.pdf.
Önfelt, A., and Klasterska, I., 1984. Sister-chromatid exchanges and thioguanine resistance in V79 Chinese hamster cells after treatment with the aneuploidy-inducing agent carbaryl ± S9 mix.Mutation Research, 125: 269-274.
Pelletier, E., Sargian, P., Payet, J., and Demers, S., 2006. Ecotoxicological effects of combined UVB and organic contaminants in coastal waters: A review.Photochemistry and Photobiology, 82: 981-993.
Peter, V. S., Babitha, G. S., Bonga, S. E., and Peter, M. C., 2013. Carbaryl exposure and recovery modify the interrenal and thyroidal activities and the mitochondria-rich cell function in the climbing perchAnabas testudineusBloch.Aquatic Toxicology, 126: 306-313.
Pfeiffer, C. J., Qiu, B., and Cho, C. H., 1997. Electron microscopic perspectives of gill pathology induced by 1-naphthyl-N-methylcarbamate in the goldfish (Carassius auratusLinnaeus).Histology and Histopathology, 12: 645-653.
PMRA (Pest Management Regulatory Agency), 2009. Proposed re-evaluation decision carbaryl. PRVD2009-14. PMRA, Health Canada, Ottawa, Ontario.
Renglin, A., Härmälä-Brasken, A. S., Eriksson, J. E., and Önfelt, A., 1999. Mitotic aberrations induced by carbaryl reflect tyrosine kinase inhibition with coincident up-regulation of serine/threonine protein phosphatase activity: Implications for coordination of karyokinesis and cytokinesis.Mutagenesis, 14: 327-333.
Schock, E. N., Ford, W. C., Midgley, K. J., Fader, J. G., Giavasis, M. N., and McWhorter, M. L., 2012. The effects of carbaryl on the development of zebrafish (Danio rerio) embryos.Zebrafish, 9: 169-178.
Shea, T. B., and Berry, E. S., 1983. Toxicity and intracellular localization of carbaryl and 1-naphthol in cell cultures derived from goldfish.Bulletin of Environmental Contamination and Toxicology, 30: 99-104.
Singh, N. P., McCoy, M. T., Tice, R. R., and Schneider, E. L., 1988. A simple technique for quantitation of low levels of DNA damage in individual cells.Experimental Cell Research, 175: 184-191.
Sinha, N., Lal, B., and Singh, T. P., 1991. Carbaryl-induced thyroid dysfunction in the freshwater catfishClarias batrachus.Ecotoxicology and Environmental Safety, 21: 240-247.
Solomon, H. M., and Weis, J. S., 1979. Abnormal circulatory development in medaka caused by the insecticides carbaryl, malathion and parathion.Teratology, 19: 51-62.
Strmac, M., Oberemm, A., and Braunbeck, T., 2002. Effects of sediment eluates and extracts from differently polluted small rivers on zebrafish embryos and larvae.Journal of FishBiology, 61: 24-38.
Tilak, K. S., Rao, D. M., Devi, A. P., and Murty, A. S., 1981. Toxicity of carbaryl and 1-naphthol to four species of freshwater fish.Journal of Biosciences, 3: 457-461.
Todd, N. E., and van Leeuwen, M., 2002. Effects of Sevin (carbaryl insecticide) on early life stages of zebrafish (Danio rerio).Ecotoxicology and Environmental Safety, 53: 267-272.
Tomlin, C. D. S., 2000.The Pesticide Manual: A World Compendium. 12th edition. British Crop Protection Council, Farnham, Surrey, 1457pp.
Tong, S. L., Li, H., and Miao, H. Z., 1997. The establishment and partial characterization of a continuous fish cell line FG-9307 from the gill of flounderParalichthys olivaceus.Aquaculture, 156: 327-333.
USEPA (U.S. Environmental Protection Agency), 2002. Environmental fate and ecological risk assessment for the reregistration of carbaryl. Office of Pesticide Programs, Environmental Fate and Effects Division, USEPA, Washington DC, USA.
USEPA (U.S. Environmental Protection Agency), 2012. Aquatic life ambient water quality criteria for carbaryl-2012. EPA-820-R-12-007. Office of Water, Health and Ecological Criteria Division, USEPA, Washington DC, USA. http://water. epa.gov/scitech/swguidance/standards/criteria/aqlife/upload/ carbaryl2012.pdf.
Weis, P., and Weis, J. S., 1974. Cardiac malformations and other effects due to insecticides in embryos of the killifish,Fundulus heteroclitus.Teratology, 10: 263-267.
WHO (World Health Organization), 2002. The WHO classification of pesticides by hazard and guidelines to classification, 2000-2002, Geneva, Switzerland. http://www.who.int/ipcs/ publications/en/pesticides_hazard.pdf.
Xiao, Q., Li, D., and Liu, H., 2011. A flounder (Paralichthys olivaceus) gill cell line asin vitroacute assay system of nonylphenol cytotoxicity.Environmental Monitoring and Assessment, 175: 315-319.
Xiao, Q., Zhang, S., Guo, H., Su, F., and Xu, Y., 2007. Nonylphenol causes decrease in antioxidant enzyme activities, increase in O2-content, and alteration in ultrastructures of FG cells, a flounder (Paralichthys olivaceus) gill cell line.Toxicology Mechanisms and Methods, 17: 127-134.
Xu, S., 2000. Environmental fate of carbaryl. California Environmental Protection Agency, Department of Pesticide Regulation, Sacramento, CA. http://www.cdpr.ca.gov/docs/emon/ pubs/fatememo/carbaryl.pdf.
Yang, F., Zhang, Q., Guo, H., and Zhang, S., 2010. Evaluation of cytotoxicity, genotoxicity and teratogenicity of marine sediments from Qingdao coastal areas usingin vitrofish cell assay, comet assay and zebrafish embryo test.Toxicology in Vitro, 24: 2003-2011.
Yin, L. C., Guo, H. R., Zhang, S. C., and Wang, J., 2007. Study on the acute toxicity and genotoxicity of herbicide butachlor in flounder,Paralichihys olivaceus, and flounder gill (FG) cells.Journal of Ocean University of China, 37: 167-171.
(Edited by Qiu Yantao)
(Received July 4, 2013; revised December 15, 2013; accepted January 19, 2015)
© Ocean University of China, Science Press and Spring-Verlag Berlin Heidelberg 2015
∗Corresponding author. Tel: 0086-532-82031932 E-mail: huarongguo@ouc.edu.cn
杂志排行
Journal of Ocean University of China的其它文章
- Impacts of the Two Types of El Niño on Pacific Tropical Cyclone Activity
- Numerical Simulation of Typhoon Muifa (2011) Using a Coupled Ocean-Atmosphere-Wave-Sediment Transport (COAWST) Modeling System
- Estimating the Turbulence Characteristics in the Bottom Boundary Layer of Monterey Canyon
- Composition and Origin of Ferromanganese Crusts from Equatorial Western Pacific Seamounts
- Hydroelastic Analysis of a Very Large Floating Structure Edged with a Pair of Submerged Horizontal Plates
- A Storm Surge Intensity Classification Based on Extreme Water Level and Concomitant Wave Height
