Mechanical Stress Induces Neuroendocrine and Immune Responses of Sea Cucumber (Apostichopus japonicus)
2015-04-05TANJieLIFenghuiSUNHuilingGAOFeiYANJingpingGaiChunleiChenAihuaandWANGQingyin
TAN Jie, LI Fenghui, SUN Huiling GAO Fei YAN Jingping Gai Chunlei, Chen Aihua, and WANG Qingyin
1)College of Marine Life Sciences,Ocean University of China,Qingdao266003,P.R.China
2)Yellow Sea Fisheries Research Institute,Chinese Academy of Fishery Sciences,Qingdao266071,P.R.China
3)College of Fisheries and Life Science,Shanghai Ocean University,Shanghai201306,P.R.China
4)Key Laboratory of Mariculture Disease Treatment,Provincial Mariculture Institute of Shandong,Qingdao266002,P.R.China
5)Municipal Oceanic and Fishery Administration of Rushan,Rushan264500,P.R.China
Mechanical Stress Induces Neuroendocrine and Immune Responses of Sea Cucumber (Apostichopus japonicus)
TAN Jie1),2),#, LI Fenghui3),#, SUN Huiling2), GAO Fei2), YAN Jingping2), Gai Chunlei4), Chen Aihua5), and WANG Qingyin2),*
1)College of Marine Life Sciences,Ocean University of China,Qingdao266003,P.R.China
2)Yellow Sea Fisheries Research Institute,Chinese Academy of Fishery Sciences,Qingdao266071,P.R.China
3)College of Fisheries and Life Science,Shanghai Ocean University,Shanghai201306,P.R.China
4)Key Laboratory of Mariculture Disease Treatment,Provincial Mariculture Institute of Shandong,Qingdao266002,P.R.China
5)Municipal Oceanic and Fishery Administration of Rushan,Rushan264500,P.R.China
Grading procedure in routine sea cucumber hatchery production is thought to affect juvenile sea cucumber immunological response. The present study investigated the impact of a 3-min mechanical perturbation mimicking the grading procedure on neuroendocrine and immune parameters of the sea cucumberApostichopus japonicus. During the application of stress, concentrations of noradrenaline and dopamine in coelomic fluid increased significantly, indicating that the mechanical perturbation resulted in a transient state of stress in sea cucumbers. Coelomocytes concentration in coelomic fluid increased transiently after the beginning of stressing, and reached the maximum in 1 h. Whereas, coelomocytes phagocytosis at 3 min, superoxide anion production from 3 min to 0.5 h, acid phosphatase activity at 0.5 h, and phenoloxidase activity from 3 min to 0.5 h were all significantly down-regulated. All of the immune parameters recovered to baseline levels after the experiment was conducted for 8 h, and an immunostimulation occurred after the stress considering the phagocytosis and acid phosphatase activity. The results suggested that, as in other marine invertebrates, neuroendocrine/immune connections exist in sea cucumberA. japonicus. Mechanical stress can elicit a profound influence on sea cucumber neuroendocrine system. Neuroendocrine messengers act in turn to modulate the immunity functions. Therefore, these effects should be considered for developing better husbandry procedures.
Apostichopus japonicus; mechanical stress; neuroendocrine; immune response
1 Introduction
Sea cucumber,Apostichopus japonicus, is one of the most important species intensively cultured in China. Due to the continuously increasing demand for dried sea cucumber and overexploitation of natural resource, mass production of sea cucumber juveniles and their culture have evolved as a prosperous fraction of aquaculture sector in north China. The total number of juveniles produced in 2013 reached 73 billions (DOF, 2014).
Size grading is routinely practiced in commercial sea cucumber juvenile production in China, during which sea cucumbers are forced to pass through a sieve and thus they are separated by size and stocked separately. Such practice is believed to be able to enhance the growth of individuals in small size, avoid size variation (Donget al.,2010), and facilitate feeding and harvesting. However, grading may stress sea cucumbers seriously.
Early studies have demonstrated that environmental stressors, such as contaminants, rapid shift of temperature and salinity, hypoxia and mechanical perturbation, reduce the growth of marine invertebrates, suppress their immune system and increase their disease susceptibility (Chu and Hale, 1994; Chenget al., 2004; Liu and Chen, 2004; Changet al., 2009; Hooperet al., 2011). When marine invertebrates experience stresses, their sympathetic nervous system receive stimulation and release catecholamines (adrenaline, noradrenaline and dopamine) which then serve as neuroendocrine messengers, diverting bioenergetics resources from certain processes such as reproduction, growth and immunity to specific physiological functions that help them maintain homeostasis. Therefore, mass culture of marine animals requires a better understanding of stress physiology, so that husbandry practices can be optimized to reduce stress incurrence. Unfortunately, the neuroendocrine response and immune change of sea cucumber induced by grading process havenot been investigated.
In invertebrates, immune response is based on both cellular and humoral factors (Schmid-Hempel, 2003). Coelomocytes, locating mainly in the coelomic cavities, are the effector cells of echinoderm immune system (Coteuret al., 2004). Similar to their immune system homologues, haemocytes in molluscs, coelomocytes are capable of carrying out phagocytosis, encapsulation and intercellular respiratory burst, avoiding invasion of microbes (Guet al., 2010). For humoral defense of sea cucumber, the phenolxidase (PO) system is an important immune defense mechanism. PO activity has been detected in coelomic fluid and circulating coelomocytes ofIsostichopus badionotus(Klanian, 2013) andA. japonicus(Zhanget al., 2010; Zhaoet al., 2011; Liuet al., 2012).
The immune response of an animal can be assessed by measuring changes of immune variables. In this study, the response of fundamental immune components of sea cucumbers to acute mechanical stress was evaluated, aiming to optimize husbandry practice in the seedling raising of sea cucumber.
2 Materials and Methods
2.1 Animals
Juvenile sea cucumbersA. japonicus(5.09 g ± 0.14 g) were collected from a commercial farm in Qingdao, China, and acclimatized one month in three 500 L cylindrical tanks (200 animals each tank) with filtered, aerated water (14-15℃). During acclimation, sea cucumbers were fedad libitumwith a diet (7% protein and 3% lipid) consisting of sea mud andSargassum thunbergiipowder, and seawater was changed once a day.
2.2 Stress
In total, 140 sea cucumbers were taken from one acclimation tank and put into a wooden box (40 cm × 40 cm × 8 cm) with a plastic mesh with holes (0.8 cm in diameter) at bottom. The sieve was shaken by hand at a rate of approximately 2 times per second in seawater. Juveniles were just submerged in seawater and curled into spheres during shaking. After 3 min of simulated grading, sea cucumbers were allowed to recover in their original tank. Twenty individuals were selected randomly for testing at eight time points (0 min, 3 min, 0.5 h, 1 h, 2 h, 4 h, 8 h and 16 h). The shaking time of 3 min was selected because it is usually adopted for grading practice. Experiments were repeated three times. No sea cucumber died during experiments.
2.3 Sample Collection
Coelomic fluid was withdrawn from individuals each using a 2 mL sterile syringe through body wall. The procedure was finished within 20 s to minimize the effect of sampling on immune response. Coelomic fluid from 5 sea cucumbers was pooled, yielding 2 mL of coelomic fluid. Coelomocytes were immediately counted 4 times using a haemocytometer and calculated as cells per mL coelomic fluid. For the measurement of phagocytosis and intracellular superoxide anion production, 200 μL of coelomic fluid was removed and the coelomocyte concentration was rapidly adjusted to 106cells mL-1with anticoagulant solution (0.02 mol L-1EGTA, 0.48 mol L-1NaCl, 0.019 mol L-1KCl, 0.068 mol L-1Tri-HCl, pH 7.6) (Guet al., 2010). The remaining coelomic fluid was stored at -80℃for enzyme activity and catecholamines quantity analyses.
2.4 Phagocytosis Assay
Phagocytosis assay was performed according to Ballarinet al. (2003) with some modifications. In brief, 100 microliters coelomic fluid containing 106cells mL-1in anticoagulant solution was placed on a glass slide. The coelomocytes were left to adhere for 30 min in a moist incubation chamber before rinsing with anticoagulant solution and adding 100 μL yeast (Saccharomyces cerevisiae) suspension in FSW (yeast/coelomocyte ratio= 100:1). After a further 60 min incubation, the glass slides were repeatedly dipped in FSW to remove uningested yeast cells and coelomocytes were fixed for 30 min at 4℃in a solution of 1% saccharose and 1% glutaraldehyde in FSW, washed in phosphate-buffered saline (0.03 mol L-1KCl, 1.37 mol L-1NaCl, 0.065 mol L-1Na2HPO4, 0.015 mol L-1KH2PO4) and stained using 5% Giemsa solution in distilled water. The Giemsa solution was removed after 10 min and coverslips were placed over the slide. Three counts of 200 cells were made under a Nikon 80i light microscope from each slide and the percentage of coelomocytes with ingested yeast cells was then calculated.
2.5 Measurement of Intracellular Superoxide Anion Production
Intracellular superoxide anion production was measured according to the method described early (Bussellet al., 2008). One hundred microliters of coelomocytes suspension and equal volume of nitroblue tetrazolium (NBT, Sigma) solution (2 mg mL-1in Tris/HCl buffer containing 2% NaCl, pH 7.6) were added to triplicate wells of a 96 well plate for each sample point. The remaining wells were filled with 100 μL of NBT and 100 μL of Tris/HCl as negative controls. The plate was incubated in dark for 1 h, centrifuged at 120 r min-1for 10 min with the supernatant discarded. The cells were washed twice with Tris-HCl (pH 7.6) and fixed with 100 μL of methanol for 10 min. After fixation, the plate was centrifuged at 300 r min-1with supernatant removed and cells air-dried. The cells were then carefully rinsed five times with 100 μL of 50% methanol before adding 120 μL of 2 mol L-1KOH and 140 μL of DMSO. The optical density (OD) value at 620 nm was measured on a microplate reader spectrophotometer and the results were expressed as OD value/106cells mL-1.
2.6 Acid Phosphatase Activity
Frozen coelomic fluid was thawed at 4℃, disrupted with an ultrasonic cell disruptor, centrifuged at 4000 r min-1and 4℃ for 10 min. Supernatant was collected foracid phosphatase (ACP) activity assay using a commercial kit (Nanjing Jiancheng, China) (Zhaoet al., 2011). One unit of ACP activity was defined as the degradation of 1 mg phenol per 100 mL coelomic fluid at 37℃ within 30 min.
2.7 Phenoloxidase Activity
Samples were prepared as described in Section 2.6. Phenoloxidase (PO) activity was measured spectrophotometrically using L-3, 4-dihydroxyphenylalanine (LDOPA; Sigma, USA) as substrate and trypsin (Sigma, USA) as elicitor according to Klanian (2013). Briefly, 50 μL of coelomic fluid and 50 μL of 0.1% trypsin in cacodylate buffer (CAC, 10 mmol L-1sodium cacodylate, 10 mmol L-1CaCl2, pH 7.0) were added to triplicate wells of a 96 well plate for each sample point, incubated at 25℃for 20 min, and then 50 μL of L-DOPA (0.3% in CAC buffer) was added. Optical density was measured at 490 nm. One unit of enzyme activity was expressed as an increase in absorbance of 0.001 min-1.
2.8 Measurement of Catecholamines
Catecholamines, including noradrenaline (NA) and dopamine (DOP), in coelomic fluid were quantified using HPLC with electrochemical detection as Quet al. (2009) described. Coelomic fluid was thawed at 4℃ in refrigerator, centrifuged at 600 r min-1and 4℃ for 10 min to remove the cellular debris. 500 μL of coelomic fluid was placed into a clean Eppendorf tube, to which 500 μL of TRIS buffer (1.5 mol L-1, pH 8.6, containing 0.07 mol L-1EDTA), 50 μL of 5 nmol L-1sodium metabisulfite, 100 μL of 10 ng mL-1internal standard-dihydroxybenzylamine (DHBA) and 10 mg acid-washed alumina were added. The mixture was agitated for 15 min, centrifuged at 1000 r min-1for 2 min and the supernatant was discarded. The alumina was washed with distilled water and then centrifuged for 2 min at 1000 r min-1before removal of the supernatant. Then, 100 μL 0.2 mol L-1acetic acid was added to the alumina, mixed for 10 min and centrifuged for 2 min at 1000 r min-1. The supernatant was carefully collected, stored at -20℃ and analysed within 2 weeks. Catecholamines were separated on an Agilent XDB C18 column (150 mm × 4.6 mm, 5 μm) with a mobile phase (50 mmol L-1citric acid, 0.05 mmol L-1EDTA, 50 mmol L-1sodium dihydrogen-phosphate, 3 mmol L-1sodium chloride, 0.4 mmol L-1octanesulfonic acid, and 5% methanol, pH 3.0) at a flow rate of 1 mL min-1. An Agilent ESA electrochemical detector was used at 0.7 V with sensitivity of 5 nAFS.
2.9 Statistical Analysis
Data are expressed as mean ± SD (n=3). One-way analysis of variance (ANOVA) was performed to compare data among sample points. The homogeneity of data were tested before the analysis and the difference was significant ifP≤ 0.05. When the ANOVA detected a significant difference, multiple comparison test of Tukey was carried out. The analyses were performed using SPSS 17.0 software.
3 Results
3.1 Catecholamines
A 3 min mechanical stress significantly changed NA and DOP concentrations in sea cucumber coelomic fluid. NA (Fig.1a) and DOP (Fig.1b) concentrations changed in the same way over time. During exposure to the mechanical stressor, noradrenaline and dopamine concentration increased (P〈 0.05) to seven- and three-folds of the basal value, respectively. After stress, NA and DOP concentrations decreased rapidly, returning to the basal values 1 h after beginning of experiment.

Fig.1 Effect of a 3-min mechanical stress on noradrenaline (a) and dopamine (b) concentrations in coelomic fluid of sea cucumberA.japonicus. Black bars represent values recorded during stress and hatched bars represent values recorded after stress. The concentration is expressed as mean ± SE (n= 12). Asterisks denote significant differences from basal values (sample time 0;P〈 0.05).
3.2 Coelomocytes Concentration in Coelomic Fluid
Coelomocytes concentration in coelomic fluid showed a non-significant increase during application of the stress, from 8.97 × 106cells mL-1at the beginning to 10.23 × 106cells mL-1at the end (Fig.2a). After stress, it increased significantly (P〈 0.05) and reached a maximum of 16.16 × 106cells mL-1at 1 h after the onset of stress and thenbegan to decrease and return to the initial level in 3 h.
3.3 Phagocytosis Activity
As indicated in Fig.2b, a significant (P〈 0.05) decrease, from 14.86% to 5.48%, in the percentage of phagocytic cells was observed during stress. Following stress the percentage of phagocytic cells tended to increase significantly (P〈 0.05) in 1 h and then decreased to roughly the same value (12.79%) as beginning of experiment in one hour.
3.4 Measurement of Intracellular Superoxide Anion Production
Intracellular superoxide anion production significantly decreased (P〈 0.01) from 0.206 OD values to 0.108 OD values per 106cells mL-1in 3 min stress (Fig.2c). Intracellular superoxide anion production began to increase after stress and returned to the initial value in 1 h.
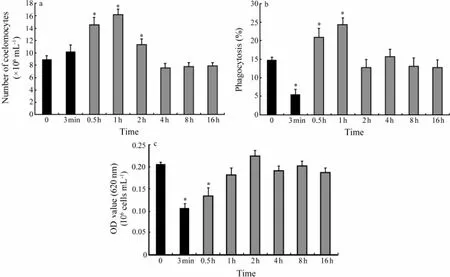
Fig.2 Effect of a 3-min mechanical stress on (a) coelomocytes concentration, (b) phagocytosis activity and (c) the production of intracellular superoxide anion in coelomic fluid ofA. japonicus. Black bars represent values recorded during stress and hatched bars represent values recorded after the stress. Each bar represents mean ± SE (n= 12). Asterisks denote significant (P〈 0.05) differences from basal values (sample time 0).
3.5 ACP Activity
At the start point of the experiment, ACP activity was around 3.59 U per 100 mL coelomic fluid (Fig.3a). During stress, ACP activity decreased slightly. When stress ended, ACP activity decreased significantly (P〈 0.05) and reached a minimum of 2.05 U per 100 mL coelomic fluid in 0.5 h. After that, it began to increase to 4.41 U per 100 mL coelomic fluid at 4 h, which was significantly higher than the initial value, and then decreased to initial value in 4 h.
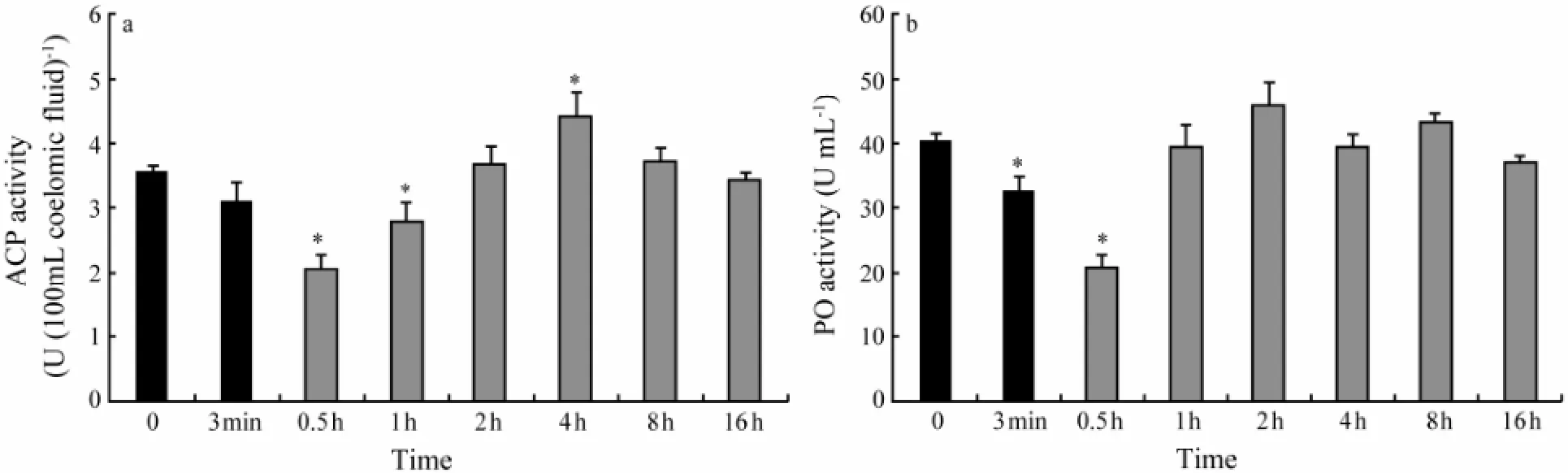
Fig.3 Effect of a 3-min mechanical stress on (a) acid phosphatase (ACP) and (b) phenoloxidase (PO) activity in coelomic fluid ofA. japonicus. Black bars represent values recorded during the stress period and hatched bars represent values recorded after the disturbance. Each bar represents mean ± SE (n= 12). Asterisks denote significant (P〈 0.05) differences from basal values (sample time 0).
3.6 PO Activity
PO activity decreased significantly in 0.5 h after the beginning of stress, returned to initial value in 1 h and remained unchanged until the end of experiment.
4 Discussion
The release of neurohormones such as glucocorticoids and catecholamines is a primary response to stress in vertebrates (Chrousos and Gold, 1992; Ottaviani and Franceschi, 1996). There are increasing evidences that this neuroendocrine response system also exists in aquatic invertebrates (Lacosteet al., 2001a, 2001b; Pequeuxet al., 2002; Quet al., 2009; Simonet al., 2010). Previous studies have demonstrated thatA. japonicuschanges activities of catecholamines in response to temperature and salinity variations during aestivation (Wanget al., 2008). The present study also proved that NA and DOP concentrations increased significantly in coelomic fluid when sea cucumber encountered a mechanical stress. Presumably, neuroendocrine stress-response axis has been preserved during the course of evolution.
In molluscs, a decline of the number of circulating haemocytes was identified after the start of a mechanical stress (Lacosteet al., 2002; Malhamet al., 2002, 2003). Noradrenaline and dopamine injections were also demonstrated to decrease the haemocyte count in hemolymph in white shrimpLitopenaeus vannamei(Chenget al., 2006; Panet al., 2011). In contrast, there was no reduction in coelomocytes concentration during application of the stress in our experiment was found. According to a study in starfish, the coelomic epithelium was identified as one of the sources of new coelomocytes, which releases coelomocytes upon LPS injection (Holmet al., 2008). It is possible that mechanical stress also induced proliferation of coelomocytes in coelomic epithelium, and then coelomocytes entered coelomic fluid. The following decrease in the number of coelomocytes in coelomic fluid is proposed to be the result of coelomocytes fixation in tissues (Changet al., 2011). An alternative suggestion is that during stress, immunoactive coelomocytes migrated from connective tissues into coelomic fluid. However, the reasons for this phenomenon remained to be unknown.
In invertebrates, phagocytosis represents an important mechanism of host defense (Bayne, 1990). During phagocytosis of coelomocytes, several reactive oxygen species (ROS) are produced, such as superoxide anion (O2-), hydrogen peroxide (H2O2), hydroxyl radical (OH), and singlet oxygen. All these short-living compounds can be directly toxic to pathogens (Roch, 1999). ROS production by phagocytes may be an important mechanism of antimicrobial protection of holothurians (Dolmatovaet al., 2003). In the present study, phagocytosis activity and intracellular superoxide anion production decreased within 5 min after the beginning of stress. A similar phenomenon is observed in abaloneHaliotis tuberculata(Malhamet al., 2003), oysterCrassostrea gigas(Lacosteet al., 2002) and oysterPinctada imbricata(Kuchelet al., 2010) subjected to a mechanical stressor. Conversely, Malhamet al. (2002) observed an increase in phagocytosis activity and intracellular superoxide production in the octopusEledone cirrhosaduring an air exposure. These facts suggest that the effects of stress hormones on invertebrate immune function are diverse, and usually depend on the type of the stressor, the animal species considered and the physiological condition of the animal (Husmannet al., 2011; Adamo, 2012). The reduction in phagocytosis activity and superoxide anion production observed in disturbed sea cucumber may be due to the β-adrenergic receptors expressed by coelomocytes, which permit noradrenaline to exert a dose-dependent inhibitory effect on these coelomocyte functions (Lacosteet al., 2001c, 2001d, 2001e).
Stresses were reported to depress the activity of enzyme involved in immune defense in many marine invertebrates. PO activity was inhibited in oysters exposed to handling practices (Kuchelet al., 2012). A significant decrease in acid phosphatase activity was observed in Venus clamsChamelea gallinaexposed to anoxic stress (Pampaninet al., 2002). In addition, noradrenaline injections inhibited acid phosphatase activity inS. glomerata(Aladailehet al., 2008). Dopamine injection resulted in a significant decrease in PO activity (Panet al., 2011). The decline in the activity of ACP and PO maybe the result of several events,e.g., change in lysosomal integrity, reduction in the frequency of defensive coelomocytes and depression of proPO-activating enzyme and PO activity by NA (Pampaninet al., 2002; Changet al., 2011; Kuchelet al., 2012).
Importantly, the fact that immunological functions are down-regulated during and after the mechanical disturbance could be highly detrimental to the sea cucumbers. Previous study has shown that oysters infected with the oyster pathogenVibrio splendiduscarried higher bacterial loads and experienced higher mortalities following a 15-min mechanical disturbance than infected unstressed animals (Lacosteet al., 2001a). The farmers anecdotally reported mechanical stress linked with disease outbreaks and mortality and reduced growth in sea cucumbers. Thus, it seems that mechanical stress depresses the immunity of sea cucumber and augments the susceptibility of the animal to certain pathogens. Furthermore, interactive effects of various stresses might also depress the immunity and disease-resistance ability of sea cucumbers. Wanget al. (2012) reported that there were interactive effects of hypoxic and hyposaline stresses on immune responses in musselPerna viridis. During farming, various stresses were bound to challenge the immune system of sea cucumbers. It is possible that mechanical stress increases the susceptibility of sea cucumbers to other forms of stress such as acute temperature or salinity stress. Further work should aim at testing this hypothesis.
In conclusion, the present study demonstrated that mechanical stress induced quantifiable alterations in neuroendocrine and immune response inA. japonicus. Concentrations of noradrenaline and dopamine in coelomic fluidelevated significantly during stress, and returned to the baseline levels at 2 h. All immune parameters investigated were significantly down-regulated by stress, except coelomocyte concentration, which increased transiently after the beginning of the stress. All of the immune parameters had recovered to baseline levels by 8h after the start of experiment. To further understand the neuroimmune processes inA. japonicusexposed to environmental stressors, more studies should be conducted on adrenergic receptors involved in stress-induced immune changes in these animals.
Acknowledgements
This work was financially supported by the 863 High Technology Project of the Ministry of Science and Technology (No. 2012AA10A412-4), the Special Funds for the Basic B & D Program in the Central Non-profit Research Institutes (No. 2010-cb-03), Science and Technology Development Planning Project of Shandong Province (No. 2012GGA06021) and Science and Technology Development Fund of Shinan district of Qingdao (No. 2011-5-023-QT).
Adamo, S. A., 2012. The effects of the stress response on immune function in invertebrates: An evolutionary perspective on an ancient connection.Hormones and Behavior, 62: 324-330.
Aladaileh, S., Mohammad, M. G., Ferrari, B., Nair, S. V., and Raftos, D. A., 2008.In vitroeffects of noradrenaline on the Sydney rock oyster (Saccostrea glomerata).Comparative Biochemistry and Physiology Part A, 151: 691-697.
Ballarin, L., Pampanin, D. M., and Marin, M. G., 2003. Mechanical disturbance affects haemocyte functionality in the venus clamChamelea gallina.Comparative Biochemistry and Physiology Part A, 136: 631-640.
Bayne, B. L., 1990. Phagocytosis and non-self recognition in invertebrates. Phagocytosis appears to be an ancient line of defense.Bioscience, 40: 723-731.
Bussell, J. A., Gidman, E. A., Causton, D. R., Gwynn-Jones, D., Malham, S. K., Jones, M. L., Reynolds, B., and Seed, R., 2008. Changes in the immune response and metabolic fingerprint of the mussel,Mytilus edulis(Linnaeus) in response to lowered salinity and physical stress.Journal of Experimental Marine Biology and Ecology, 358: 78-85.
Chang, C. C., Hung, M. D., and Cheng, W., 2011. Norepinephrine depresses the immunity and disease-resistance abilityviaα1- and β1-adrenergic reciptors ofMacrobrachium rosenbergii.Developmental and Comparative Immunology, 35: 685-691.
Chang, C. C., Yeh, M. S., and Cheng, W., 2009. Cold shockinduced norepinephrine triggers apoptosis of haemocytesviacaspase-3 in the white shrimpLitopenaeus vannamei.Fish & Shellfish Immunology, 27: 695-700.
Cheng, W., Chieu, H. T., Ho, M. C., and Chen, J. C., 2006. Noradrenaline modulates the immunity of white shrimpLitopenaeus vannamei.Fish & Shellfish Immunology, 21: 11-19.
Cheng, W., Hsiao, I. S., Hsu, C. H., and Chen, J. C., 2004. Change in water temperature on the immune response of Taiwan abaloneHaliotis diversicolor supertextaand its susceptibility toVibrio parahaemolyticus.Fish & Shellfish Immunology, 17: 235-243.
Chrousos, G. P., and Gold, P. W., 1992. The concepts of stress and stress system disorders. Overview of physical and behavioural homeostasis.Journal of the American Medical Association, 267: 1244-1252.
Chu, F. L. E., and Hale, R. C., 1994. Relationship between pollution and susceptibility to infectious disease in the eastern oyster,Crassostrea virginica.Marine Environmental Research, 38: 243-256.
Coteur, G., Corriere, N., and Dubois, P. H., 2004. Environmental factors influencing the immune response of the common European starfish (Asterias rubens).Fish & Shellfish Immunology, 16: 51-63.
Department of Fisheries (DOF), 2014.China Fisheries Statistic Yearbook 2014. China Agriculture Press, Beijing, 64pp (in Chinese).
Dolmatova, L. S., Eliseykina, M. G., Timchenko, N. F., Kovaleva, A. L., and Shitkova, O. A., 2003. Generation of reactive oxygen species in different fractions of the coelomocytes of holothurianEupentacta fraudatrixin response to the thermostable toxin ofYersinia pseudotuberculosisin vitro.Chinese Journal of Ocean and Limnology, 21: 293-304.
Dong, S., Liang, M., Gao, Q., Wang, F., Dong, Y., and Tian, X., 2010. Intra-specific effects of sea cucumber (Apostichopus japonicus) with reference to stocking density and body size.Aquaculture Research, 41: 1170-1178.
Gu, M., Ma, H. M., Mai, K. S., Zhang, W. B., Ai, Q. H., Wang, X. J., and Bai, N., 2010. Immune response of sea cucumberApostichopus japonicuscoelomocytes to several immunostimulantsin vitro.Aquaculture, 306: 49-56.
Holm, K., Dupont, S., Skold, H., Stenius, A., Thorndyke, M., and Hernroth, B., 2008. Induced cell proliferation in putative haematopoietic tissues of the sea star,Asterias rubens(L.).Journal of Experimental Biology, 211: 2551-2558.
Hooper, C., Day, R., Slocombe, R., Benkendorff, K., and Handlinger, J., 2011. Effect of movement stress on immune function in farmed Australian abalone (hybridHaliotis laevigataandHaliotis rubra).Aquaculture, 315: 348-354.
Husmann, G., Philipp, E. E. R., Rosenstiel, P., Vazquez, S., and Abele, D., 2011. Immune response of the Antarctic bivalveLaternula ellipticato physical stress and microbial exposure.Journal of Experimental Marine Biology and Ecology, 398:83-90.
Klanian, M. G., 2013. Physiological and immunological conditions of the sea cucumberIsostichopus badionotus(Selenka, 1867) during dormancy.Journal of Experimental Marine Biology and Ecology, 444: 31-37.
Kuchel, R. P., McCarthy, A., and Raftos, D. A., 2012. Phenoloxidase activity as an indicator of stress in the silver-lip pearl oyster,Pinctada maxima.Aquaculture, 364-365: 224-229.
Kuchel, R. P., Raftos, D. A., and Nair, S., 2010. Immunosuppressive effects of environmental stressors on immunological function inPinctada imbricata.Fish & Shellfish Immunology, 29: 930-936.
Lacoste, A., Jalabert, F., Malham, S. K., Cueff, A., and Poulet, S. A., 2001a. Stress and stress-induced neuroendocrine changes increase the susceptibility of juvenile oysters (Crassostrea gigas) toVibrio splendidus.Applied and Environmental Microbiology, 67: 2304-2309.
Lacoste, A., Malham, S. K., Cueff, A., Jalabert, F., Gelebart, F., and Poulet, S. A., 2001b. Evidence for a form of adrenergic response to stress in the molluskCrassostrea gigas.The Journal of Experimental Biology, 204: 1247-1255.
Lacoste, A., Malham, S. K., Cueff, A., and Poulet, S. A., 2001c. Noradrenaline modulates hemocyte reactive oxygen species productionviaβ-adrenergic receptors in the oysterCrassostrea gigas.Developmental and Comparative Immunology, 25: 285-289.
Lacoste, A., Malham, S. K., Cueff, A., and Poulet, S. A., 2001d. Noradrenaline inhibits phagocytosis by hemocytes of the oysterCrassostrea gigasviaa β-adrenoceptor/cAMP signaling pathway.General and Comparative Endocrinology, 122:252-259.
Lacoste, A., Malham, S. K., Cueff, A., and Poulet, S. A., 2001e. Noradrenaline reduces the stimulatory effect of interleukin-1 alpha on reactive oxygen species production by oyster immunocytes.Invertebrate Biology, 120: 358-364.
Lacoste, A., Malham, S. K., Gelebart, F., Cueff, A., and Poulet, S. A., 2002. Stress-induced immune changes in the oysterCrassostrea gigas.Developmental and Comparative Immunology, 26: 1-9.
Liu, C. H., and Chen, J. C., 2004. Effect of ammonia on the immune response of white shrimpLitopenaeus vannameiand its susceptibility toVibrio alginolyticus.Fish & Shellfish Immunology, 16: 321-334.
Liu, Z. M., Ma, Y. X., Yang, Z. P., Li, M., Liu, J., and Bao, P. Y., 2012. Immune responses and disease resistance of the juvenile sea cucumberApostichopus japonicusinduced byMetschnikowiasp. C14.Aquaculture, 368-369: 10-18.
Malham, S. K., Lacoste, A., Gelebart, F., Cueff, A., and Poulet, S. A., 2002. A first insight into stress-induced neuroendocrine and immune changes in the octopusEledone cirrhosa.Aquatic Living Resources, 15: 187-192.
Malham, S. K., Lacoste, A., Gelebart, F., Cueff, A., and Poulet, S. A., 2003. Evidence for a direct link between stress and immunity in the molluscHaliotis tuberculata.Journal of Experimental Zoology Part A, 295: 136-144.
Ottaviani, E., and Franceschi, C., 1996. The neuroendocrinology of stress from invertebrates to man.Progress in Neurobiology, 48: 421-440.
Pampanin, D. M., Ballarin, L., Carotenuto, L., and Marin, M. G., 2002. Air exposure and functionality ofChamelea gallinahaemocytes: Effects on haematocrit, adhesion, phagocytosis and enzyme contents.Comparative Biochemistry and Physi-ology Part A. 131: 605-614.
Pan, L., Hu, F., and Zheng, D., 2011. Effect of dopamine injection on the hemocyte count and prophenoloxidase system of the white shrimpLitopenaeus vannamei.Journal of Ocean University of China, 10: 280-286.
Pequeux, A., Le Bras, P., Cann-Moisan, C., Caroff, J., and Seberet, P., 2002. Polyamines, indolamines, and catecholamines in gills and haemolymph of the euryhaline crab,Eriocheir sinensis, effects of high pressure and salinity.Crustaceana, 75: 567-578.
Qu, Y., Li, X., Yu, Y., Vandepeer, M., Babidge, P., Clarke, S., Bott, K., and Li, H., 2009. The effect of different grading equipment on stress levels assessd by catecholamine measurements in Pacific oysters,Crassostrea gigas(Thunberg).Aquacultural Engineering, 40: 11-16.
Roch, P., 1999. Defense mechanisms and disease prevention in farmed marine invertebrates.Aquaculture, 172: 125-145.
Schmid-Hempel, P., 2003. Variation in immune defence as a question of evolutionary ecology.Proceedings of the Royal Society of London Series B, 270: 375-466.
Simon, B. A., Pinon, M., Racotta, R., and Racotta, I. S., 2010. Neuroendocrine and metabolic responses of Pacific whiteleg shrimpLitopenaeus vannameiexposed to acute handling stress.Aquaculture, 298: 308-314.
Wang, F. Y., Yang, H. S., Gabr, H. R., and Gao, F., 2008. Immune condition ofApostichopus japonicusduring aestivation.Aquaculture, 285: 238-243.
Wang, Y. J., Hu, M. H., Cheung, S. G., Shin, P. K. S., Lu, W. Q., and Li, J. L., 2012. Immune parameter changes of hemocytes in green-lipped musselPerna viridisexposure to hypoxia and hyposalinity.Aquaculture, 356-357: 22-29.
Zhang, Q., Ma, H. M., Mai, K. S., Zhang, W. B., Liufu, Z. G., and Xu, W., 2010. Interaction of dietaryBacillus subtilisand fructooligosaccharide on the growth performance, non-specific immunity of sea cucumber,Apostichopus japonicus.Fish & Shellfish Immunology, 29: 204-211.
Zhao, Y. C., Ma, H. M., Zhang, W. B., Ai, Q. H., Mai, K. S., Xu, W., Wang, X. J., and Liufu, Z. G., 2011. Effects of dietary β-glucan on the growth, immune responses and resistance of sea cucumber,Apostichopus japonicusagainstVibrio splendidusinfection.Aquaculture, 315: 269-274.
(Edited by Qiu Yantao)
(Received July 5, 2013; revised November 1, 2013; accepted January 15, 2015)
© Ocean University of China, Science Press and Spring-Verlag Berlin Heidelberg 2015
* Corresponding author. Tel: 0086-532-85822959 E-mail: qywang@public.qd.sd.cn
# These authors contributed equally to this work.
杂志排行
Journal of Ocean University of China的其它文章
- Impacts of the Two Types of El Niño on Pacific Tropical Cyclone Activity
- Numerical Simulation of Typhoon Muifa (2011) Using a Coupled Ocean-Atmosphere-Wave-Sediment Transport (COAWST) Modeling System
- Estimating the Turbulence Characteristics in the Bottom Boundary Layer of Monterey Canyon
- Composition and Origin of Ferromanganese Crusts from Equatorial Western Pacific Seamounts
- Hydroelastic Analysis of a Very Large Floating Structure Edged with a Pair of Submerged Horizontal Plates
- A Storm Surge Intensity Classification Based on Extreme Water Level and Concomitant Wave Height
