Lack of Gender Effect on the Pharmacokinetics of Oxytetracycline in Fenneropenaeus chinensis After Intramuscular Administration
2015-04-05SUNMingLIJianCHANGZhiqiangGUOWentingZHAOFazhenandLIJitao
SUN Ming, LI Jian,, CHANG Zhiqiang, GUO Wenting, ZHAO Fazhen, and LI Jitao
1)Fishery College,Ocean University of China,Qingdao266003,P. R. China
2)Key Laboratory of Sustainable Development of Marine Fisheries of Ministry of Agriculture,Yellow Sea Fisheries Research Institute,Chinese Academy of Fishery Sciences,Qingdao266071P. R. China
3)College of Marine Sciences,Nanjing University of Information Science & Technology,Nanjing210044,P. R. China
Lack of Gender Effect on the Pharmacokinetics of Oxytetracycline in Fenneropenaeus chinensis After Intramuscular Administration
SUN Ming1),2), LI Jian2),*, CHANG Zhiqiang2), GUO Wenting3), ZHAO Fazhen2), and LI Jitao2)
1)Fishery College,Ocean University of China,Qingdao266003,P. R. China
2)Key Laboratory of Sustainable Development of Marine Fisheries of Ministry of Agriculture,Yellow Sea Fisheries Research Institute,Chinese Academy of Fishery Sciences,Qingdao266071P. R. China
3)College of Marine Sciences,Nanjing University of Information Science & Technology,Nanjing210044,P. R. China
Fenneropenaeus chinensis, an economically important shrimp species, currently suffers from epizootic diseases due to high density stocking and bacterial infections. Oxytetracycline (OTC) has been widely used to treat various systemic bacterial infections in shrimp farming. In the present study, the effect of gender on pharmacokinetics of OTC inF. chinensiswas investigated. The OTC concentrations in hemolymph of shrimp after single intramuscular administration (75 mg OTC per kg body weight) were analyzed by high performance liquid chromatography and best described with a two-compartment open model which is characterized by a short elimination half-life, low clearance, and a relatively large apparent volume of distribution. The pharmacokinetic equations wereCt= 58.54e-0.38t+ 11.67e-0.04tfor females; andCt= 27.94e-0.28t+ 14.87e-0.04tfor males. The distribution and elimination half-lives of OTC were 1.82 and 19.58 h, respectively, in females and 2.50 and 16.11 h, respectively, in males at 22℃. The areas under the drug concentration curve were 480 mg L-1h-1in females and 430 mg L-1h-1in males. The total body clearance of the drug was 157.11 mL kg-1h-1in females and 172.47 mL kg-1h-1in males. The apparent volume distribution was 4.44 in females and 4.01 L kg-1in males. There was no significant difference in pharmacokinetic parameters between female and male shrimps, indicating that there is no need to consider the gender effect in clinical use of OTC inF. chinensisfarming.
Fenneropenaeus chinensis; oxytetracycline; pharmacokinetics; gender
1 Introduction
Fenneropenaeus chinensisis an economically important shrimp species which is commonly cultured in China (Wanget al., 2011). It distributes widely along the coast stretching from Shandong Province, China to the Korean Peninsula (Liu, 1959).F. chinensisis of great importance to China’s mariculture industry and its total pond-production has reached 780000 t in 2003 (Wanget al., 2006).F. chinensisis an ideal mariculture species for rapid growth, resistance to low temperatures, superior nutritional properties, and ease of adaptation to artificial culture conditions (Liet al., 2006). However, the incidence of epizootic diseases in cultured shrimp has been increasing due to high density stocking and infections by bacteria for example those relating toVibrio anguillarumandAeromonas hydrophila(Zhanget al., 2004; Qinet al., 2006). Therefore, utilizing antimicrobials to prevent andcontrol relevant problems has become an important part of shrimp farming.
Chemotherapeutic drugs such as oxytetracycline (OTC), tetracyclines, sulfonamides, and quinolones are commonly used in shrimp farming. Of these, OTC is one of the antibiotics most widely used against vibriosis and furunculosis (Uno, 1996). The mode of OTC action is to interfere with bacterial protein synthesis by binding to bacterial 30S ribosomal subunit (Rigoset al., 2003). However, OTC residues have immunosuppressive effects and cause liver damage, and bacterial resistance has frequently been observed for OTC abuse under unknown condition of the drug pharmacokinetics in aquaculture (Zhanget al., 2007).
The pharmacokinetics and residues of OTC have been studied in various shrimp species, such asLitopenaeus vannamei,L. setiferusPenaeus japonicas,P. monodon, andP. chinensis(Gómez-Jimenez, 2008; Uno, 2004; Unoet al., 2006; Wanget al., 2004; Reedet al., 2006). Presently, the less understood is the effect of gender on OTC pharmacokinetics, which is known to play an importantrole in the pharmacokinetics in teleost (Bjǒrklundet al., 1990; Bjǒrklundet al., 1992; Luzzanaet al., 1994; Rigoset al., 2002). For large animals, the effect of gender on pharmacokinetics has been reported in goats (Witkampet al., 1992), cows (Witkampet al., 1992; Abdennebiet al., 1994), sheep (Bengtssonet al., 1997), and camels (Al Katheeriet al., 2000). Since 1993, the Food and Drug Administration of United States issued guidelines to include both genders in drug development (Food and Drug Administration, 1993). Considering the apparent differences between teleost and shrimp in terms of anatomy and physiology, the present study was performed to investigate the effect of gender on OTC pharmacokinetics inF. chinensis. Recommended dose regimen and withdrawal time of OTC forF. chinensiswere given to guarantee the safe use of this drug in shrimp culture.
2 Materials and Method
2.1 Chemicals
OTC standard (99% purity) was purchased from China Institute of Veterinary Drugs Control, and OTC hydrochloride was produced by Weifang Medicine Company, China. Organic solvents, including acetonitrile, methanol, and dimethyl formamide, were at high performance liquid chromatography (HPLC) grades (Merck, German). All other chemicals were at analytical or higher grades.
2.2 Animal Maintenance and Drug Exposure
HealthyF. chinensis(8-10 g) were obtained from a shrimp farm in Qingdao, China, rapidly transported to the laboratory in Yellow Sea Fisheries Research Institute, and cultured in tanks with aerated seawater. The shrimps were divided into two groups by gender, 120 individuals each. Water temperature was maintained at 22℃ and the salinity of seawater was 23. The shrimps were acclimatized for 7 d before experiment and fed continuously with commercial pellet feed at 3% body weight per day. No OTC was found in any tissue of shrimps before experiment. For OTC administration, OTC hydrochloride was dissolved in isotonic sterile phosphate buffered saline at a concentration of 75 mg mL-1. The OTC solution was injected into abdominal muscles of shrimp with a microliter syringe. The dose of OTC was 75 mg kg-1body weight (Wanget al., 2001) and the injection volume was 1.0 mL kg-1body weight.
2.3 Hemolymph Sampling
After OTC intramuscular administration, eight shrimps were sampled each group at 0.083, 0.25, 0.5, 1, 2, 6, 12, 24, 48, and 72 h. Hemolymph samples were taken immediately from the pericardinal cavity with 1-mL syringe and frozen at -20℃ until analysis.
2.4 Analytical Procedures
The OTC concentration in hemolymph was analyzed with a HPLC method (Liet al., 1997) with slight modifications. The Agilent 1100 HPLC system (USA) was comprised of a quaternary pump, a vacuum degasser, a manual injector, a variable wavelength detector set at 360 nm, and a reversed-phase analytical column (Hypersil ODS, 5 μm; 250 mm×4.6 mm I.D.) (UK). The mobile phase consisted of methanol, acetonitrile, dimethyl formamide, phosphate buffer solution at the volume fraction of 1:1.2:0.42:1.9. The flow rate was 1.0 mL min-1.
OTC residues in hemolymph were extracted with a 0.02 mol L-1Na2HPO4solution containing 4% trichloroacetic acid. Hemolymph samples (1 mL each) were mixed with 1 mL of chloroform and 3 mL of extraction solution. The mixtures were homogenized with a high-speed homogenizer at 16000 r min-1for 10 s and then centrifuged at 2655 g for 10 min. The supernatant was transferred to a clean test tube and the residue was extracted again with 3 mL of extraction solution. The extracts were combined and 2 mL of hexane was added to remove fat. The organic layer was discarded while the aqueous phase was retained and filtered through a 0.22-µm filter unit equipped with a cellulose acetate membrane. A 20-µL aliquot of each sample was injected into the HPLC system for OTC detection.
2.5 Data Analysis
All processes followed first-order kinetics. Pharmacokinetic analysis was performed in Drug and Statistics (DAS ver 2.0, Center for Clinical Drug Evaluation, Wannan Medical College, Wuhu, China). The program analyzes the concentration-time data for plasma with a compartmental open model based on non-linear regression analysis.
3 Results
A sensitive and specific HPLC method was used to quantify OTC residues inF. chinensishemolymph. The response of HPLC-UV detector was linearly correlated to the concentration of OTC standard within the range of 0.01-50 μg mL-1, with a correlation coefficient of a typical regression line of 0.9999. The detection limit of OTC inF. chinensishemolymph with the HPLC method was 0.01 µg mL-1. The intra- and inter-day precisions of the analytical method were 2.44%-3.11% and 3.16%-4.14%, respectively. Mean recoveries of OTC were higher than 84.99%.
Fig.1 shows the OTC concentrations in hemolymph of female and maleF. chinensisafter single intramuscular administration (75 mg kg-1body weight) at 22℃. The OTC concentration inF. chinensishemolymph peaked at 72.56 µg mL-1in females and 68.24 µg mL-1in males at 0.25 h. The OTC concentrations inF. chinensishemolymph can be best described by a two-compartment open model, and the equations for female and male shrimps are as follows:
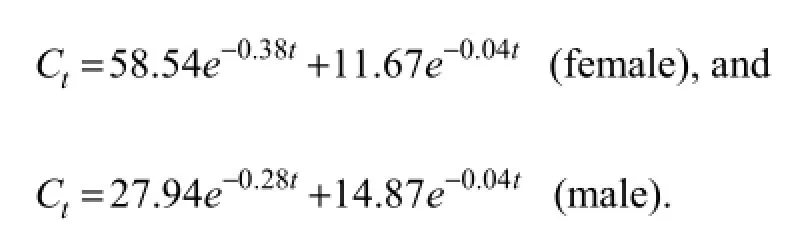
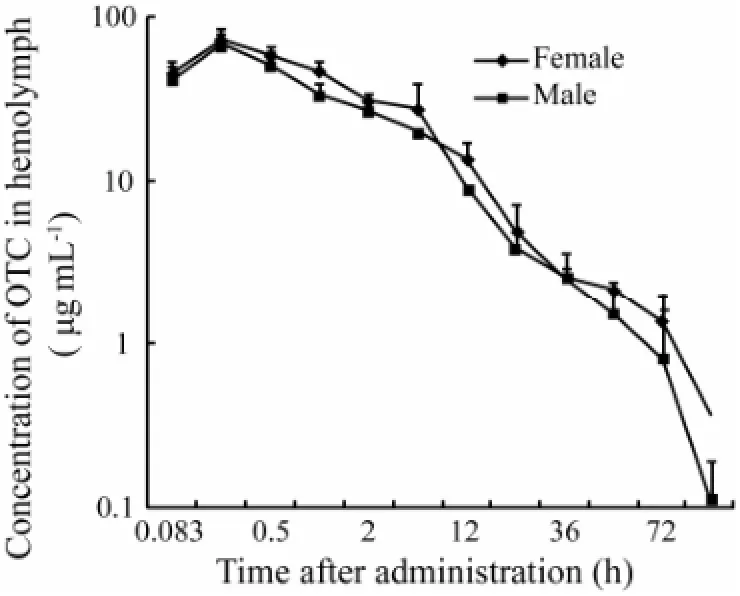
Fig.1 Hemolymph concentrations of oxytetracycline (OTC) in female and maleF. chinensisfollowing intramuscular administration (75 mg kg-1).

Table 1 Pharmacokinetic parameters of oxytetracycline in female and maleF. chinensisafter intramuscular administration (75 mg kg-1)
Pharmacokinetic parameters of OTC in female and maleF. chinensisafter single intramuscular administration are presented in Table 1. The distribution half-lives of the drug were 1.82 h in females and 2.50 h in males. The elimination half-lives of OTC were 19.58 h in females and 16.11 h in males. The apparent volumes of drug distribution were 4.44 L kg-1in females and 4.01 L kg-1in males. The AUC values were 480 mg L-1h-1in females and 430 mg L-1h-1in males. The total body clearance of the drug was 157.11 mL kg-1h-1in females and 172.47 mL kg-1h-1in males. We can conclude that there is no significant effect of gender on the elimination of OTC in shrimps.
4 Discussion
The pharmacokinetics of OTC inF. chinensisafter single intramuscular administration is best fitted by a two-compartment open model, which is characterized by a short elimination half-life, low clearance, and a relatively large apparent volume of distribution. The present study examined the pharmacokinetics of OTC in healthy shrimps under laboratory conditions, different from a field situation in which a sick shrimp population can be treated with medicated feed only. Still, the obtained data can guide the formulation of effective dose regimen and legitimate withdrawal time in field situation.
The distribution half-lives of OTC inF. chinensiswere 1.82 h in females and 2.50 h in males, both longer than those of antibacterial agents such as enroflxacin, florfenicol, sulfamethoxazole, marbofloxacin, and miloxacin (Lianget al., 2012; Liet al., 2006; Rubio-Langreet al., 2012; Liet al., 2008) but shorter than sulphamonomethoxine (Liet al., 2006) in other species.
Gender-related differences in the elimination half-life of chemotherapeutic drugs have been reported in crustacean. In American lobster (Homarus americanus), the elimination half-life of 2-naphthol was significantly longer in males (63.9 h) than in females (30.6 h) (Li and James, 1997). Likewise, in crab (Eriocheir Sinensis), the elimination half-life of ciprofloxacinum in males (40.34 h) is approximately 2-fold that in females (22.07 h) (Yanget al., 2003). In the present study, the elimination half-life of OTC inF. chinensiswas slightly longer in females (19.58 h) than in males (16.11 h) (Table 1), showing no significant effect of gender on the elimination of OTC in shrimps. These data suggest that the greater accumulation of drugs in male American lobster and crab could lead to a different and overall greater pharmacological profile than that in maleF. chinensis.
The apparent volumes of OTC distribution inF. chinensiswere 4.44 L kg-1in females and 4.01 L kg-1in males (Table 1), indicating that OTC is well-distributed throughout the shrimp body. The apparent volume of OTC distribution inF. chinensiswas much bigger than that in carp (2.1 L kg-1, Grondelet al., 1987), rainbow trout (2.1 L kg-1, Grondelet al., 1989), ayu (1.3 L kg-1, Uno, 1996), swine (1.7 L kg-1, Liuet al., 2011), and rabbit (1.99 L kg-1, Marínet al., 2013), but smaller than that in lactating goats (16.1 L kg-1, Ameret al., 2012). Thus, the organotropy of OTC inF. chinensisis higher than that in carp, rainbow trout, ayu, swine and rabbit, but lower than that in lactating goats. It demonstrates the use of OTC in shrimp shows even greater therapeutic efficacy with more durable responses.
Total body clearance is an important parameter for characterizing drug disposition and the only parameter for estimating the rate of clearance correctly (Denget al., 1992). In the present study, total body clearance of OTC inF. chinensiswas estimated to be 157-172 mL kg-1h-1(Table 1), approximately 10-fold higher than those reported in other species. Uno (1996), Grondelet al. (1987) and Grondelet al. (1989) estimated the total body clearance to be 17.4 mL kg-1h-1in ayu, 10.2 mL kg-1h-1in carp, 16.2 mL kg-1h-1in rainbow trout, and 11.4 mL kg-1h-1in African catfish. The results suggest thatF. chinensisclear drugs at a faster rate than ayu, carp, rainbow trout and African catfish.
There was no significant difference in any pharmacokinetic parameter of OTC inF. chinensisbetween female and male, indicating that gender effect on OTC pharmacokinetics lacked. This is not in accordance with the documented in other animals (Zouet al., 2007; Hernandezet al., 2006). The underlying mechanism of genderspecific pharmacokinetics has been related to molecular and physiological factors (Meibohmet al., 2002). The observed differences in the behavior of opposite genders under the influence of xenobiotics are most probably a consequence of the different activities of drug metabolic enzymes. As the main metabolic enzyme, CYP450 plays an important role in the elimination of xenobiotics. Gender-specific difference of CYP450 in rats is the most obvious, and gender-specific difference has been proven in CYP4503A in rats. The amount of CYP450 in female rats is 10%-30% lower than that in male rats (Zouet al., 2007). Hernandez (2006) examined the gender-specific effects of nonylphenol on hepatic CYP450 expression and found that in female mice nonylphenol induced CYP2d10 and CYP 2d13 and down-regulated female-specific p450s, CYP 3a41, and CYP 3a44, whereas male mice treated with nonylphenol showed increased expression of CYP 2a4, CYP 2b9, and CYP 2b10. It is inferred that the different result in our study may due to the lack of genderspecific effects of OTC on hepatic CYP450 expression. However, there has been no report on this subject. Further studies are required for elucidation of the detailed mechanisms.
Pharmacokinetic data can be applied to he development of clinical dosing regimens in which the plasma drug concentration can be maintained between the minimum inhibitory concentration and toxic concentration when repeated administration.In vitrominimum inhibitory concentrations for OTC against 12 strains of pathogenic bacteria isolated from diseased shrimps are reported to be 2.0 µg OTC mL-1(Mohney, 1992). The recommended dose of OTC is calculated using the following equation
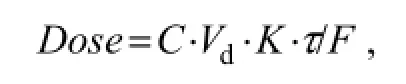
whereCis the desired steady-state hemolymph concentration;Vdis the apparent volume distribution;Kis the first-order rate constant for drug distribution;τis the dosing interval;Fis the bioavailability. Drugs can be fully utilized after intramuscular administration, so the bioavailability of OTC in shrimp after intramuscular administration is regarded as 1 in our study. When a desired steady-state hemolymph concentration of 2 µg OTC mL-1is achieved, the dose of intramuscular administration is calculated to be 23.67 mg OTC kg-1. The bioavailability of drug after oral administration is 30% of that after intramuscular administration (Yanget al., 2003). Therefore, the dose of oral administration is suggested to be 80 mg OTC kg-1for shrimp, similar to the level used in practice.
5 Conclusions
The plasma concentration-time data for OTC was best fit by a two-compartment open model in both female and male shrimps (F. chinensis). There was no significant effect of gender on OTC pharmacokinetics inF. chinensis, indicating that there is no need to consider the effect of gender in clinical use of OTC inF. chinensisfarming. The recommended clinical oral administration dosage is 80 mg OTC kg-1shrimp.
Acknowledgements
This study was supported by the Earmarked Fund for Modern Agro-industry Technology Research System, China (No. CARS-47) and the Special Fund for Agro-Scientific Research in the Public Interest of China (No. 201103034).
Abdennebi, E. H., Sawchuk, R. J., and Stowe, C. M., 1994. Thiamphenicol pharmacokinetics in beef and diary cattle.Journal of Veterinary Pharmacology and Therapeutics, 17:365-368.
Al Katheeri, N. A., Was fi, I. A., Lambert, M., Saeed, A., and Khan, I. A., 2000. Pharmacokinetics of ketoprofen enantiomers after intravenous administration of racemate in camels:E ff ect of gender.Journal of Veterinary Pharmacology and Therapeutics, 23: 137-143.
Amer, A. M. M., Constable, P. D., Goudah, A., and Badawy, S. A. El., 2012. Pharmacokinetics of tulathromycin in lactating goats.Small Ruminent Research, 108: 137-143.
Bengtsson, B., Jacobsson, S. O., Luthman, J., and Franklin, A., 1997. Pharmacokinetics of penicillin-G in ewes and cows in late pregnancy and in early lactation.Journal of Veterinary Pharmacology and Therapeutics, 20: 258-261.
Bjǒrklund. H. V., and Bylund. G., 1990. Temperature-Related absorption and excretion of oxytetracycline in rainbow trout (Salmo gairdneriR.).Aquaculture, 84: 363-372.
Bjǒrklund, H. V., Eriksson, A., and Bylund, G., 1992. Temperature related absorption and excretion of oxolinic acid and oxytetracycline in rainbow trout (Oncorhynchus mykiss).Aquaculture, 102: 17-27.
Deng, S. H., Zhang, X. Z., and Zou, L. J., 1992.Pharmacokinetics and Biopharmacy. Ocean University of Qingdao Publications, Qingdao, 37pp.
Food and Drug Administration, 1993. Guideline for the study and evaluation of gender difference in the clinical evaluation of drugs.Federal Register, 58: 39406-39416.
Grondel, J. L., Nouws, J. F. M., DeJong, M., Schutte, A. R., and Driessens, F., 1987. Pharmacokinetics and tissue distribution of oxytetracycline in carp,Cyprinus carpio L., following different routes of administration.Journal of Fish Disease, 10:153-163.
Gómez-Jimenez, S., Espinosa-Plascencia, A., Valenzuela-Villa, F., and Bermúdez-Almada, M. C., 2008. Oxytetracycline (OTC) accumulation and elimination in hemolymph, muscle and hepatopancreas of white shrimpLitopenaeus vannameifollowing an OTC-feed therapeutic treatment.Aquaculture, 274: 24-29.
Grondel, J. L., Nouws, J. F. M., Schutte, A. R., and Driessens, F., 1989. Comparative pharmacokinetics of oxytetracycline in rainbow trout (Salmo gairdneri) and African catfish (Clarias gariepinus).Journal of Veterinary Pharmacology & Therapeutics, 12: 157-162.
Hernandez, J. P., Chapman, L. M., Kretschmer, X. C., andBaldwin, W. S., 2006. Gender-specific induction of cytochrome P450s in nonylphenol-treated FVB/NJ mice.Toxicology and Applied Pharmacology, 216: 186-196.
Liang, J. P., Li, J., Zhao, F. Z., Liu, P., and Chang, Z. Q., 2012. Pharmacokinetics and tissue behavior of enrofloxacin and its metabolite ciprofloxacin in turbotScophthalmus maximusat two water temperatures.Chinese Journal of Oceanology and Limnology, 30: 644-653.
Liu, Y. M., Liu, Y. C., Ding, H. Z., Fang, B. H., Yang, F., Shan, Q., and Zeng, Z. L., 2011. Pharmacokinetics of mequindox and its metabolites in swine.Agricultural sciences in China, 10: 1968-1976.
Little, P., James, M., Pritchard, J., and Bend, J., 1985. Temperature dependent disposition of benzo(a)pyrene in the spiny lobster,Panulirus argus.Toxicology and Applied Pharmacology, 77: 325-333.
Li, M. T., Zhong, F., and Gou, L., 1997. Elimination law of oxytetracycline in eel tissues.Journal of Fisheries of China, 21: 39-43.
Liu, R. Y., 1959. Character of the economic macrurus crustacean fauna in the Yellow Sea and the East China Sea.Oceanologia et Limnologia Sinica, 2: 35-42.
Li, Y. Q., Li, J., and Wang Q. Y., 2006. The effects of dissolved oxygen concentration and stocking density on growth and non-specific immunity factors in Chinese shrimp,Fenneropenaeus chinensis.Aquaculture, 256: 608-616.
Luzzana, U., Serrini, G., Moretti, V. M., Maggi, C. L., Valfre, F., and Polidori, P., 1994. Effect of temperature and diet composition on residue depletion of oxytetracycline in cultured channel catfish.Analyst, 119: 2757-2759.
Li, J. Y., Li, J., Wang, Q., and Zhang, W. B., 2006. Single dose pharmacokinetics of florfenicol inFenneropenaeus chinensis.Marine Sciences, 30: 64-68.
Li, N., Li, J., and Wang, Q., 2008. Pharmacokinetics of miloxacin in the culture environment ofPenaeus chinensis.Journal of Anhui Agriculture Science, 36: 10480-10483.
Li, J. Y., Li, J., Wang, Q., and Zhan, W. B., 2006. Pharmacokinetics studies on sulphamonomethoxine inFenneropenaeus chinensis.Marine Fisheries Research, 27: 6-11.
Marín, P., Álamo, L. F., Escudero, E., Fernández-Varón, E., Hernandis, V., and Cárceles, C. M., 2013. Pharmacokinetics of marbo fl oxacin in rabbit after intravenous, intramuscular, and subcutaneous administration.Research in Veterinary Science, 94: 698-700.
Qin, L., Wang, Y. G., Zhang, Z., and Yang, S. L., 2006. The first report on fin rot disease of cultured turbotScophthalmus maximusin China.Journal of Aquatic Animal Health, 18: 83-89.
Rubio-Langre, S., Lucas, J. J. D., Litterio, N. J., Aguilar, S., Boggio, J., and Andrés, M. I. S., 2012. Pharmacokinetic behavior of marbo fl oxacin after intravenous, subcutaneous and intramuscular administrations in llamas (Lama glama).Small Ruminent Research, 106: 64-69.
Rigos, G., Nengas, I., Tyrpenou, A. E., Alexis, M., and Troisi, G. M., 2003. Pharmacokinetics and bioavailability of oxytetracycline in gilthead sea bream (Sparus aurata) after a single dose.Aquaculture, 221: 75-83.
Reed, L. A., Siewicki, T. C., and Shah, J. C., 2006. The biopharmaceutics and oral bioavailability of two forms of oxytetracycline to the white shrimp,Litopenaeus setiferus.Aquaculture, 258: 42-54.
Rigos, G., Alexis, M., Andriopoulou, A., and Nengas, I., 2002. Pharmacokinetics and tissue distribution of oxytetracycline in sea bass,Dicentrarchus labrax, at two water temperatures.Aquaculture, 210: 59-67.
Uno, K., 1996. Pharmacokinetic study of oxytetracycline in healthy and vibriosis-infected ayu (Plecoglossus altivelis).Aquaculture, 143: 33-42.
Uno, K., 2004. Pharmacokinetics of oxolinic acid and oxytetracycline in kuruma shrimp,Penaeus japonicus.Aquaculture, 230: 1-11.
Uno, K., Aoki, T., Kleechaya, W., Tanasomwang., V., and Ruangpan, L., 2006. Pharmacokinetics of oxytetracycline in black tiger shrimp,Penaeus monodon, and the effect of cooking on the residues.Aquaculture, 254: 24-31.
Wang, Q., Sun, X. T., Liu, D. Y., Liu, Q., and Li, J., 2001. Pharmacokinetic study of oxytetracycline in Black seabream (Sparus macrocephalus).Marine Fisheries Research, 22: 42-47.
Wang, Y., Li, J., Liu, P., Li, J. T., Zhang, Z., Chang, Z. Q., He, Y. Y., and Liu, D. Y., 2011. The responsive expression of a caspase gene in Chinese shrimpFenneropenaeus chinensisagainst pH stress.Aquaculture Research, 42: 1214-1230.
Wang, Q. Y., Zhuang, Z. M., Deng, J. Y., and Ye, Y. M., 2006. Stock enhancement and translocation of the shrimpPenaeus chinensisin China.Fisheries Research, 80: 67-79.
Wang, W. F., Lin, H., Xue, C. H., and Jamil, K., 2004. Elimination of chloramphenicol, sulphamethoxazole and oxytetracycline in shrimp,Penaeus chinensisfollowing medicatedfeed treatment.Environment International, 30: 367- 373.
Witkamp, R. F., Yun, H. I., van’t Klooster, G. A. E., van Mosel, J. F.,van Mosel, M., Ensink, J. M., Noordhoek, J., and van Miert, A. S. J. P. A. M., 1992. Comparative aspects and sex differentiation of plasma sulfamethazine elimination and metabolite formation in rats, rabbits, dwarf goats, and cattle.American Journal of Veterinary Research, 53: 1830-1835.
Yang, X. L., Liu, Z. Z., and Masahito, Y., 2003. Pharmacokinetics of ciprofloxacinum in Chinese mitten-handed crab,Eriocheir sinensis.Acta Hydrobiologica Sinica, 27: 18-22.
Zhang, Z., Wang, Y. G., Yang, G. P., and Li, Q. F., 2004. The present status of research on bacterial diseases of turbotScophthalmus maximus.Transactions of Oceanology and Limnology, 3: 83-89.
Zhang, Q. Z., and Li, X. M., 2007. Pharmacokinetics and residue elimination of oxytetracycline in grass carp,Ctenopharyngodon idellus.Aquaculture, 272: 140-145.
Zou, W., and Zhou, W., 2007. Research progress on species and gender differences of drug metabolism.Qilu Pharmaceutical Affairs, 26: 735-737.
Meibohm, B., Beierle, I., and Derendorf, H., 2002. How important are gender differences in pharmacokinetics?Clinical Pharmacokinetic, 41: 329-34.
(Edited by Qiu Yantao)
(Received May 23, 2013; revised July 9, 2013; accepted December 20, 2014)
© Ocean University of China, Science Press and Spring-Verlag Berlin Heidelberg 2015
* Corresponding author. E-mail: lijian@ysfri.ac.cn
杂志排行
Journal of Ocean University of China的其它文章
- Impacts of the Two Types of El Niño on Pacific Tropical Cyclone Activity
- Numerical Simulation of Typhoon Muifa (2011) Using a Coupled Ocean-Atmosphere-Wave-Sediment Transport (COAWST) Modeling System
- Estimating the Turbulence Characteristics in the Bottom Boundary Layer of Monterey Canyon
- Composition and Origin of Ferromanganese Crusts from Equatorial Western Pacific Seamounts
- Hydroelastic Analysis of a Very Large Floating Structure Edged with a Pair of Submerged Horizontal Plates
- A Storm Surge Intensity Classification Based on Extreme Water Level and Concomitant Wave Height
