Primary Discussion of a Carbon Sink in the Oceans
2015-04-05MACaihuaYOUKuiJIDechunMAWeiweiandLIFengqi
MA Caihua, YOU Kui,, JI Dechun MA Weiwei and LI Fengqi
1)Ocean University of China,Qingdao266100,P.R.China
2)Dalian University of Technology,Panjin124221,P.R.China
3)Woods Hole Oceanographic Institution,Woods Hole,MA02543
Primary Discussion of a Carbon Sink in the Oceans
MA Caihua1),3), YOU Kui2),3),*, JI Dechun1), MA Weiwei1), and LI Fengqi1)
1)Ocean University of China,Qingdao266100,P.R.China
2)Dalian University of Technology,Panjin124221,P.R.China
3)Woods Hole Oceanographic Institution,Woods Hole,MA02543
As a consequence of global warming and rising sea levels, the oceans are becoming a matter of concern for more and more people because these changes will impact the growth of living organisms as well as people’s living standards. In particular, it is extremely important that the oceans absorb massive amounts of carbon dioxide. This paper takes a pragmatic approach to analyzing the oceans with respect to the causes of discontinuities in oceanic variables of carbon dioxide sinks. We report on an application of chemical, physical and biological methods to analyze the changes of carbon dioxide in oceans. Based on the relationships among the oceans, land, atmosphere and sediment with respect to carbon dioxide, the foundation of carbon dioxide in shell-building and ocean acidification, the changes in carbon dioxide in the oceans and their impact on climate change, and so on, a vital conclusion can be drawn from this study. Specifically, under the condition that the oceans are not disturbed by external forces, the oceans are a large carbon dioxide sink. The result can also be inferred by the formula:C=A-BandG=E+Fwhen the marine ecosystem can keep a natural balance and the amount of carbon dioxide emission is limited within the carrying capacity of the oceans.
biological ecosystems; Carbon Dioxide sinks; marine carrying capacity
1 Introduction
The oceans constitute the dominant feature on earth and a resource bank that could provide a variety of biological, energetic, mineral and ‘space’ resources. Global carbon dioxide emissions soared from 22.7 billion tons in 1990 to 33.9 billion tons in 2011, despite 20 years of attempted mitigation. Approximately one-third of the carbon dioxide released into the atmosphere as a result of human activity has been absorbed by the oceans (Sabineet al., 2004), where it is partitioned into the constituent ions of carbonic acid. This process leads to ocean acidification, one of the major threats to marine ecosystems (Fabryet al., 2008), and particularly to calcifying organisms such as corals (Gattusoet al., 1998; Kleypaset al., 1999), foraminifera (Barker and Elderfield, 2002; Moyet al., 2009; de Moelet al., 2009) and coccoli- thophores(Riebesellet al., 2000). The oceans absorb approximately one quarter of the carbon dioxide emitted into the atmosphere each year. This process makes the water more acidic and can affect sea creatures, even weakening the calcium carbonate shells or skeletons of organisms such as corals, oysters and some marine plankton. The acidity of seawater has increased by 30 percent over the past 150 years, and some regions have already become corrosive enough to inhibit the growth of corals and other species for part of the year.With increasing levels of carbon dioxide in the air, the global temperature will increase by a minimum of two degrees before the end of this century, and carbon dioxide emissions will affect the climate for tens of thousands of years. It is generally believed that atmospheric carbon dioxide and the earth’s climate are closely coupled over the earth’s history (Royeret al., 2006).
The oceans cover approximately 70 percent of the earth’s surface and has played a dominant role in buffering the global carbon dioxide generated by fossil fuel combustion to date, governing Earth’s climate system and helping to regulate global cycles of heat, water, and so on. Approximately half of the oxygen we breathe and approximately 80 percent of the water vapor in our atmosphere come from ocean processes; the oceans also take up more than 80 percent of the heat generated by rising levels of greenhouse gases in the atmosphere. Excess carbon dioxide mixed into the upper ocean reduces the pH of seawater, making it more acidic and increasing the potential for large-scale change at the base of the marine food chain and in the coral reef ecosystems. These ecosystems are considered the breadbasket of the tropical oceans and an important source of biodiversity and income for many regions. The oceans are also sources of food, fresh water, various other materials, and beauty on this earth. This paper will mainly show that the oceans are a large carbon sink without human activity.
The oceans are responsible for half our oxygen and a large portion of our food. They also regulate the climateand can be used to water crops and provide inexpensive transportation for goods, and they are the source between new materials and pharmaceuticals that improve quality of life around the world. For processes that emit carbon dioxide and use oxygen, the difference of the former and the latter will play a decisive role in oceanic changes, climate changes and to ecosystem changes in the future. According to a recent study, the carbon discharged to the oceans is only a fraction of that which enters rivers from terrestrial ecosystems via soil respiration, leaching, chemical weathering, and physical erosion (Aufdenkampeet al., 2011; Aaltoet al., 2003). The climate in general and the oceans in particular are complicated systems. The latter currently plays a number of roles in climate change because this phenomenon also affects marine function and further influences people’s lives and other organisms’survival. In this paper, we discuss two of the most important factors. First, the oceans moderate the climate by taking in heat when the overlying atmosphere is warmer and storing or releasing heat when the atmosphere is colder. The oceans redistribute the heat through the large-scale ocean circulation to keep the balance between the oceans and the atmosphere. In addition, the ocean generally has a lower albedo than the land, and if all oceans were replaced by land, the planet as a whole would be cooler. The mean global oceanic uptake of anthropogenic carbon dioxide between 1990 and 2007 is bounded by the Circulation and Climate of the Ocean Model ECCO-based estimate of 2.3 Pg C yr-1(1 Pg = 1.015×103g) and the Community Climate System Models CCSM-based estimate of 1.7 Pg C yr-1(Gravenet al., 2012). Human activities substantially affect all of the processes involved.
Another important function of oceans is that they transport heat from the tropics to higher latitudes. The warming of the sea surface releases carbon dioxide, and that increased amount of carbon dioxide contributes to further warming. Ocean circulation changes driven by climate changes affect the amount of carbon dioxide sequestered in the deep ocean, with, in turn, influences surface temperature (Brook, 2013). The exchange of carbon dioxide between atmosphere and ocean is a critical process of the global carbon cycle that determines the future of the earth system. However, how the marine biosphere will react to the uptake of additional carbon dioxide cannot be predicted. A survey of the available literature shows that the ocean has removed approximately 118 ± 19 Pg C from the atmosphere from 1800 to 1994. The figure means that the oceans annually removed approximately 0.70 ± 0.51 Pg C during this period. Moreover, with the rapid development of industries and the fast growth of the human population, the function of the oceans has become weaker and has ultimately resulted in global warming.
Many other factors (including solar radiation, wind, and ocean currents) also influence the climate change. Moreover, the interaction among the various factors is very complex and numerous open questions remain. Incentives for using biomass to mitigate climate change currently focus on replacing fossil fuels in combustion. A key attraction of bio-char is that it can enhance the fertility and resilience of crop land. However, how long bio-char remains stable in the soil is still not completely resolved (Sohi, 2012). Therefore, scientists are making extensive measurements to determine how much human-made carbon dioxide is being absorbed and released by the oceans as a carbon sink or carbon source.
2 The Relationships Among Oceans, Land, Atmosphere and Sediment in Connection with CO2
Carbon is the element of life. Plants on land and algae in ocean assimilate it in the form of carbon dioxide from the atmosphere or water and transform it through photosynthesis into energy-rich molecules, such as sugars and starches. Carbon constantly changes its state through the metabolism of organisms and natural chemical processes, and it is also stored in particulate, dissolved inorganic and organic forms. Carbon can be transported into the oceans via water and atmosphere.
The four most important repositories (i.e. the atmosphere, terrestrial biosphere, ocean and sediment) within the context of anthropogenic climate change are constantly exchanging carbon with one another. Although the process can occur over time spans of up to centuries, given that carbon dioxide has remained present in the earth’s crust for millions of years, the exchange between the four repositories mentioned above is relatively rapid. The amount of carbon stored in the individual reservoirs can be estimated fairly accurately. The ocean, with approximately 3.8×1014tons of carbon, contains sixteen times as much carbon as the terrestrial biosphere and approximately sixty times as much as the pre-industrial atmosphere (Fig.1). Researchers estimate that ocean acidity has increased by approximately 30 percent since the Industrial Revolution in the nineteenth century, although this result requires further confirmation. Even so, we still firmly believe that the oceans are the greatest of the carbon reservoirs, which essentially determines atmospheric carbon dioxide levels.
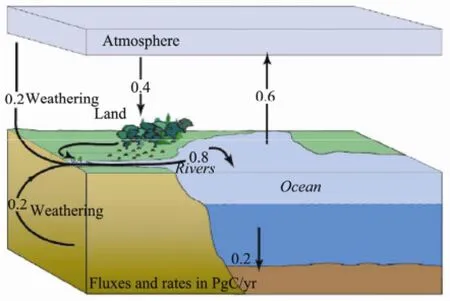
Fig.1 Pre-industrial fluxes of carbon dioxide.
Calcification is one of the primary targets for many studies of the impact of carbon dioxide-driven climatechange in the oceans because the calcium carbonate shells or skeletons of many organisms make them potentially susceptible to dissolution in acidic waters (Orret al., 2005). The pH shift consequent to carbon dioxide dissolution in seawater changes the equilibrium between bicarbonate and carbonate and depletes the available carbonate pool (Gattuso and Lavigne, 2009). This process increases the rate of dissolution of deposited calcium carbonate, which depends on the crystalline form of calcium carbonate. For example, the aragonite found in corals and mollusks is twice as soluble as the calcite found in crustaceans (Mucci, 1983).
Overall, changes in the atmospheric carbon content induced by the oceans also occur over a time frame of centuries. This process is quite fast in reference to geological time, yet it is too slow to extensively buffer climate change from a human perspective because this process includes carbon exchange, river input, organic carbon burial in bottom sediments, atmospheric deposition, point sources, fisheries, and net carbon dioxide exchange between sea water and the atmosphere (Fig.2). The results reported in the literature are ambiguous (Thomas and Schneider, 1999; Algestenet al., 2006; Kusset al., 2006; Wesslanderet al., 2010) because the net carbon dioxide exchange is temporally and spatially highly variable. Therefore, it is better to calculate the net amount of carbon dioxide by using the mass balance model.
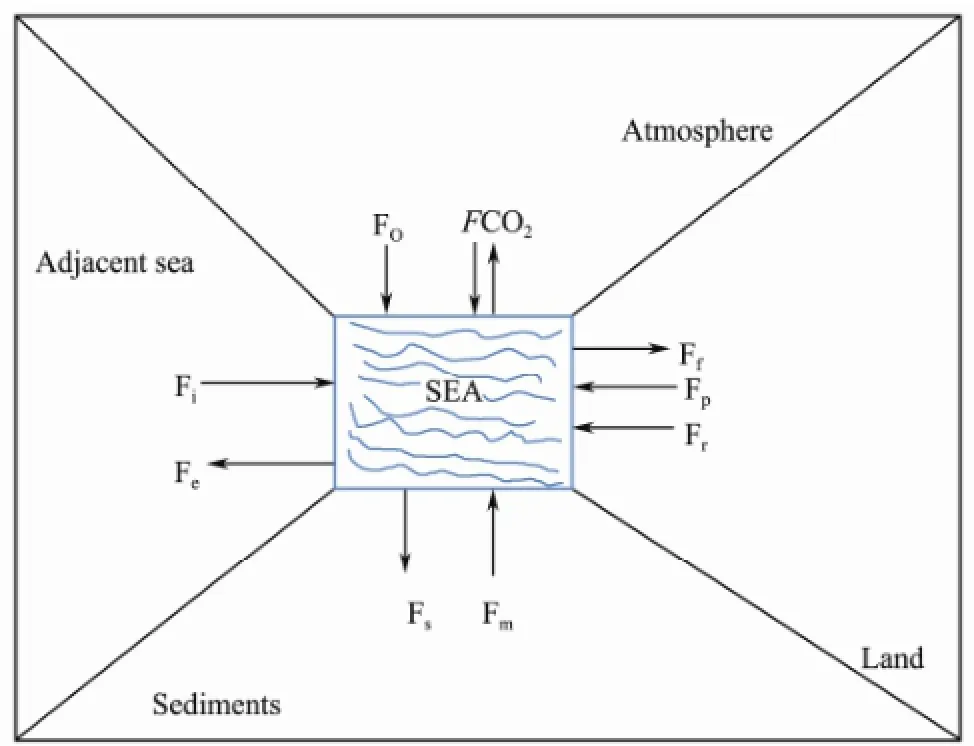
Fig.2 Carbon sources and sinks in the oceans. Symbols: Fe, export to the Oceans; Fi, import from the Oceans; Ff, fisheries; Fp, point sources; Fr, river input; Fs, accumulation in sediments; FCO2, net CO2exchange between seawater and the atmosphere; Fm, return flux from sediments to the water column; Fo, atmospheric deposition.
In this model as given above, A is the carbon dioxide exported from the oceans, B is the carbon dioxide imported to the oceans and C is net carbon dioxide exchange between export from and import to the oceans:

In cases with C greater than 0, the ocean is a carbon sink; on the contrary, when C is less than 0, the ocean is carbon source.
In the above chart (Fig.3), there are two types of balances between the surface and interior of oceans. One balance is the reaction of production and export of Corgand calcium carbonate, and the other is respiration and dissolution of Corgand calcium carbonate. Balance between calcium carbonate burial and river-inputting is ultimately achieved and is called the ocean calcium carbonate budget. The amount of this budget is determined by calculating the balance of carbon dioxide between the amount of sea surface and interior through the same method. The result shows that the oceans are a carbon sink if the amount carbon dioxide in the interior is greater than that going out from the surface. In this situation, the oceans absorb carbon dioxide, and vice versa.
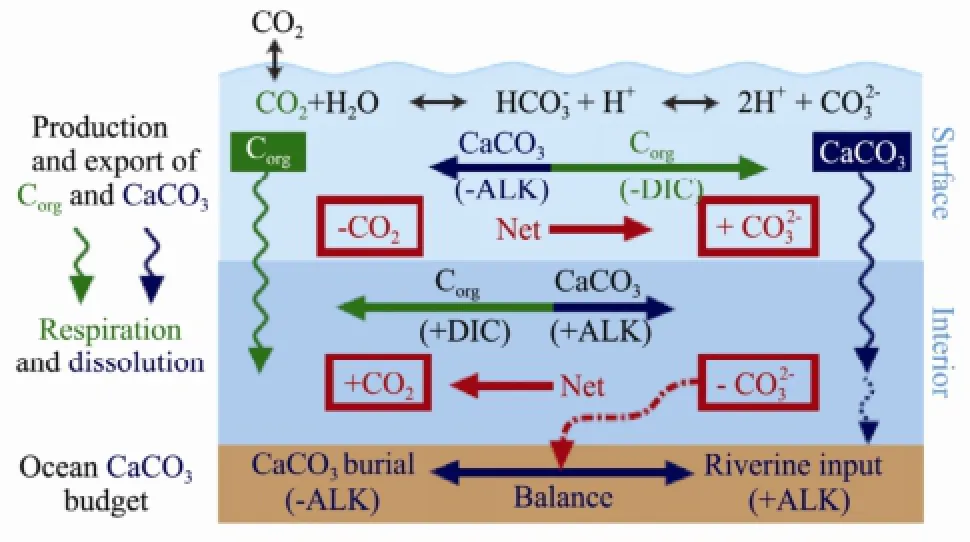
Fig.3 The process in ocean calcium carbonate budget.
3 The Function of Carbon Dioxide in Shell-Building and Ocean Acidification
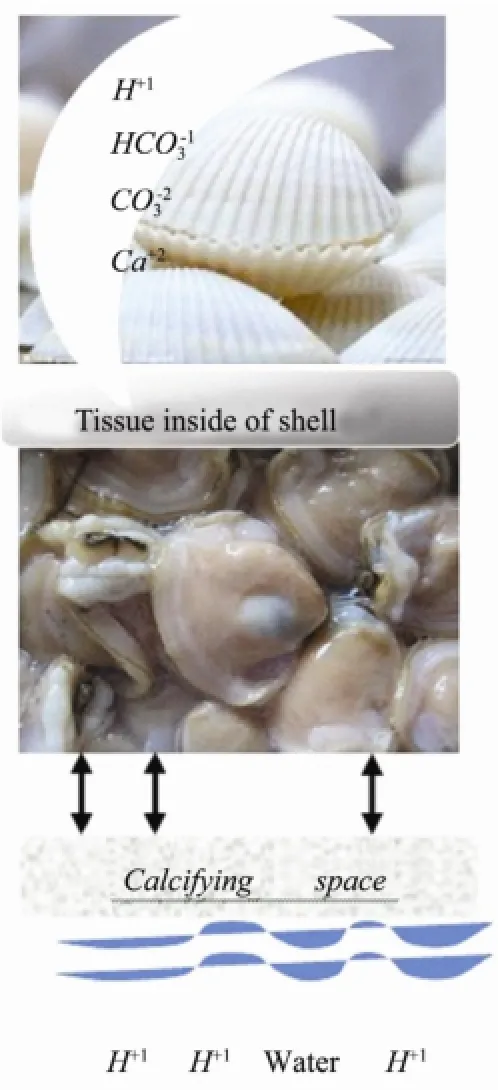
Fig.4 The exchange process of Ocean CO2..
Marine organisms that build shells and skeletons extract calcium ions and carbonate ions from seawater and combine them into solid carbonate crystal that makesup their shells. Seawater is a soup of dissolved substances, chemicals, and ions. All living organisms strive to maintain pH fluctuations within a tolerable range. To maintain a proper pH, the organisms must either enhance their expulsion of excessive protons or take up additional buffering substances, such as bicarbonate ions, which bind protons to compensate for an increase in acidity due to carbon dioxide. Most marine animals employ special epithelial cells that line body cavities, blood vessels, or the gills and intestine for the necessary ion regulation processes (Fig.4).
The specific process of carbon dioxide exchange between the oceans and organisms is demonstrated in Fig.4. Overall, carbon dioxide dissolves in the oceans and reacts with water to form carbonic acid, which, in turn, generates bicarbonate, carbonate, and hydrogen ions as the following equation indicates:
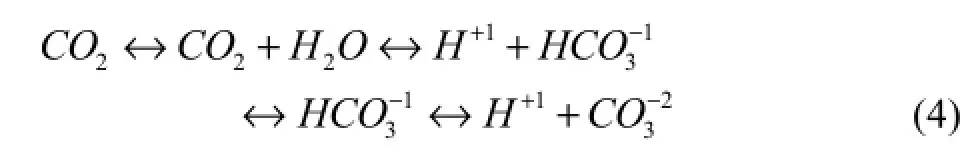
All of these ions in seawater surround shell-building marine life. According to one hypothesis, seawater containing ions at the molecular level passes through the organisms’ tissues into the ‘calcifying spaces’ next to their shells. Hydrogen ions in seawater interfere with shellbuilding because they tend to bond with carbonate ions and reduce the carbonate available for shell-building. As a result, organisms must expend energy to pump hydrogen out and increase the carbonate concentration (Fig.5).

Fig.5 The process of new shell-building.
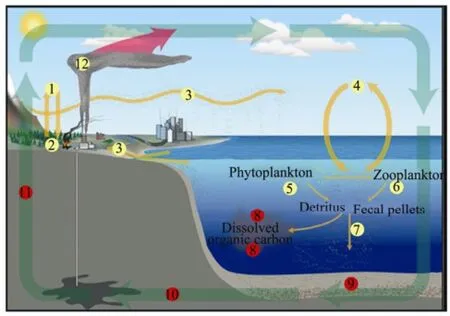
Fig.6 The carbon cycle of oceans.
Fig.6 sheds light on the need for organisms to absorb a great quantity of carbon dioxide from the seawater for approximately two or three years to make shells. To date, it has been demonstrated that oceans are a carbon sink. However, shells will also emit carbon dioxide when the organisms die or are caught. It takes longer for shell to emit carbon dioxide than the shell-forming organisms take to absorb it, and the degree to which the former process occurs is much higher than that in the latter because the processes are totally different as a result of a non-equilibrium state of absorbing and emitting carbon dioxide. The same assertion was also confirmed in Science Magazine in 2012 with results consistent with ours.
4 The Revelation of Carbon Cycle
On the basis of the above analysis, we believe that carbon dioxide plays a crucial role in the oceans and everywhere on the earth. The relevant three stages and specific functions are illustrated in Fig.6.
During the first stage of the process, carbon dioxide improves growth and is utilized. This stage includes the events: ①plants absorb carbon dioxide to grow, ⑤phytoplankton take up carbon dioxide to grow, and ⑥zooplankton eat phytoplankton and expire carbon dioxide.
During the second stage of the process, carbon dioxide is stored. This stage includes the following: ②plants decompose into soil, ③soil is carried by wind and rivers to the ocean, ⑦decaying biota and fecal pellets sink, and⑧organic carbon dissolves in seawater. Some of this carbon is used by organisms at shallower depths, but dissolved organic carbon in the deep sea remains in the water for long periods of time, ⑨organic carbon particles are buried in sediments, and ⑩carbon is incorporated into rocks over millions of years.
During the final stage of the process, the carbon diox-ide is changed or released to the atmosphere. There are two major changes: one is ④ when the air and sea exchange carbon dioxide, another is (11) when rocks are eventually uplifted onto continents and gradually weathered to release carbon into the soil or atmosphere.
If E, F and G stand for the first, second and final stages, respectively, then equation (5) is:

From equation (5), we can determine that the amount of carbon dioxide inGis far less than that ofE. Equation (6) is:

Therefore, we can also hypothesize that the oceans are a carbon sink, which should be experimentally validated by chemists and physicists in the future. This point of view is well-known, and the behavior of the oceans constitute a crucial aspect of global warming.
5 The Relationship Between Carbon Dioxide and Climate Change in the Oceans
Humankind released approximately 4 × 1012tons of carbon in the form of carbon dioxide from the early 19thcentury to the end of the 20thcentury. In addition to terrestrial plants, the oceans also absorb a lot of carbon dioxide. This process has created a serious imbalance in today’s carbon cycle.
The carbon dioxide migrating from the atmosphere into the oceans can react chemically with water molecules to form carbonic acid because carbon dioxide is immediately processed in the oceans. The amount of carbon dioxide entering the oceans in this form is ten times larger than that of freshwater; this type of assimilation of carbon dioxide is referred to as a sink. The ability of oceans to absorb carbon dioxide that humans release is primarily attributed to carbonation, which represents a significant proportion (ten percent) of the dissolved inorganic carbon in the oceans.
5.1 Climate Change and Carbon Dioxide Affect Each Other
Carbon dioxide is a key factor of climate change, which contributes significantly to the warming of the earth’s atmosphere and the oceans. The global climate has drastically changed many times in earth’s history. These changes are in part associated with natural fluctuations of carbon dioxide concentrations in the atmosphere. The drastic increase in carbon dioxide concentrations in the atmosphere by more than 30 percent since the beginning of industrialization is of anthropogenic origin; that is, it is caused by humans. The largest carbon dioxide sources on land are the burning of fossil fuels including natural gas, oil, and coal; changes in land usage; the clearing of forests; the draining of swamps; and the expansion of agricultural areas.
There is a constant exchange of gas between the air and the oceans. If the carbon dioxide levels in the atmosphere rise, the concentrations in the near-surface layers of the ocean increase accordingly. The dissolved carbon dioxide reacts to some extent to form carbonic acid. This reaction releases protons and leads to the acidification of seawater and reduced pH values. It has been demonstrated that the pH value of seawater has, in fact, already fallen to an extent that parallels the increased carbon dioxide in the atmosphere by an average of 0.1 units. This value may decrease by another 0.3 to 0.4 units by the end of this century depending on the future trend of carbon dioxide emissions. This change may appear to be negligible, but it is in fact equivalent to an increased proton concentration of 100 to 150 percent. The total dissolved inorganic carbon of seawater is defined by equation (7), which is in line with the above study.

In this equation, the brackets represent the total concentrations of these constituents in the solution (in mol kg-1), with [CO2*] being the total concentration of all unionized carbon dioxide, which, in this case, is present as carbonic acid or as carbon dioxide.
5.2 Climate Change Impacts the Carbon Cycle in Ocean
The natural carbon cycle transports billions of tons of carbon annually. In a physical sense, the carbon is spatially transported by ocean currents. From a chemical point of view, it changes from one state to another. The foundation for this continuous transport and conversion is made up of a great number of biological, chemical and physical processes that constitute what are also known as carbon pumps. These carbon pumps are driven by climatic factors, or at least strongly influenced by them.
Changes in the carbon cycle are also becoming apparent in another way. That is, the increasing accumulation of carbon dioxide in the oceans leads to the acidification of the oceans or a decrease in the pH value in chemical terms. This process may have a detrimental effect on marine organisms and ecosystems because carbonate-secreting organisms are particularly susceptible to acidifycation. These organisms are susceptible because acidifying environments are less favorable for carbonate production. The topic of ocean acidification is presently being studied in large projects worldwide. Conclusive results relating to the feedback effects between climate and acidification are not yet available. There are many indications for significant feedback effects, but there is currently an insufficient amount of solid knowledge to draw any reasonable quantitative conclusions.
This paper focuses on identifying the impact that global changes will have on the natural carbon cycle in the oceans. It would be naïve to assert that this effect is insignificant and irrelevant for the future climate changes of our planet. On the contrary, our limited knowledge of the relationships between oceanic carbon cycles and climate change should motivate us to study the ocean even more intensely and to develop new methods of observa-tion.
5.3 Equilibrium Constants-Solubility of Carbon Dioxide in Seawater
All the equations for the equilibrium constants presented here use concentrations expressed in moles per kilogram of solution.
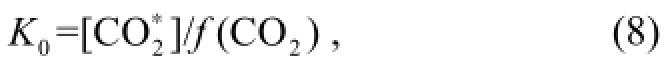
Equation (8) is given by the expression (Weiss, 1974).
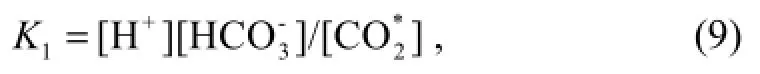
Equation (9) is given by the expression (Luekeret al., 2000).
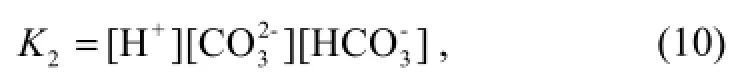
Equation (10) is given by the expression (Luekeret al., 2000).
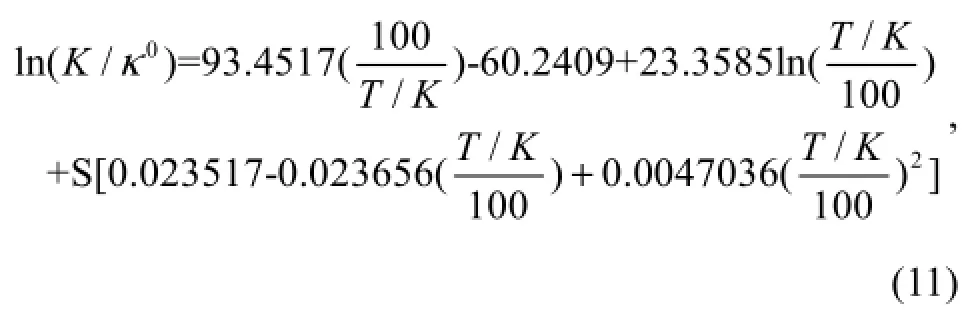
The fugacity of CO2gas is expressed in atom; whereκo= 1molkg-soln-1,S=35, andt=25℃ ( 298.15K), ln(/κ0=-3 .5617).
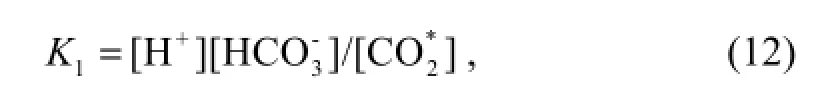
Equation (12) is given by the expression (Luekeret al., 2000)
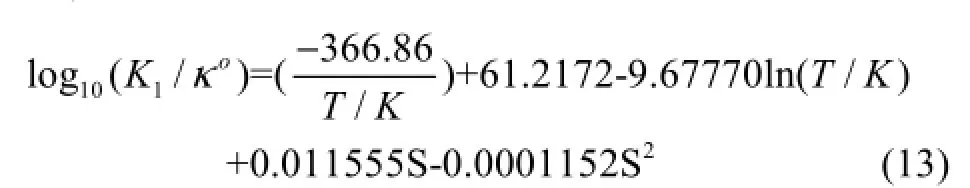
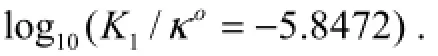
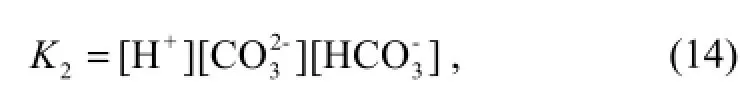
Equation (14) is given by the expression (Luekeret al., 2000)
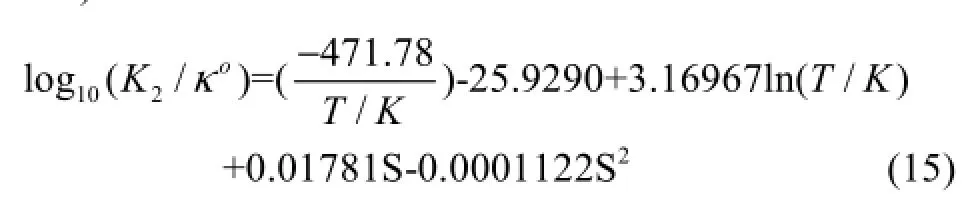
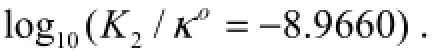
5.4 The Consequences of Ocean Acidification
Climate changes lead not only to the warming of the atmosphere and water but also to an acidification of the oceans. However, it is not clear what the ultimate consequences of this will be for marine organisms and communities, as only a few species have been studied so far. Extensive long-term studies on a large variety of organisms and communities are needed to understand the potential consequences of ocean acidification.
It is possible to compare human-induced and climate-induced atmospheric composition changes on the basis of ice core data (Robert, 1998). For instance, Parreninet al. used the ratio of 15N/14N in nitrogen (N2) because it is enriched in firm sediment due to gravitational settling (Craiget al., 1988). This study indicates that nitrogen concentrations and Antarctic temperatures are tightly coupled throughout the Deglaciation Climate Reversal within a quoted uncertainty of less than 200 years (Fig.7). The paper will show that the anthropogenic effect exceeds the natural fluctuation range in many cases. For example, the present-day atmospheric levels of two major greenhouse gases (carbon dioxide and methane) are unprecedented over the last 400 ka.

Fig.7 Carbon dioxide concentrations and averages of temperature proxy records.
As shown in the image (Fig.7), Pedroet al. used existing and temperature proxy data from coastal Antarctic cores and the temperature anomalies are presented in standard deviation units (the number of standard deviations from the mean of the record) to illustrate the average timing of temperature change. Parreninet alreported the average temperature anomaly for all the records they studied (in °C) relative to modern conditions. Using largely independent methods and data, both studies indicate a very tight coupling between regional Antarctic temperatures and carbon dioxide.
5.4.1 The effect of pH on the metabolism of marine organisms
The currently observed increase in the carbon dioxide concentrations in the oceans is unparalleled in the evolutionary history of the past 20 million years in terms of its magnitude and rate. It is therefore very uncertain to what extent the marine fauna can adapt to these changes over an extended time period. The low pH values in seawater have an adverse effect on the formation of carbonate minerals, which are critical to many invertebrate marine animals with carbonate skeletons such as mussels, coralsand sea urchins (Gazeau, 2007; Guinotte and Fabry, 2008; Hall-Spenceret al., 2008; Kleypas, 2006; Zondervan, 2007). The processes of carbon dioxide concentrations are similar to the dissolution of carbon dioxide in seawater, which further affects the organic tissue of some fragile creatures. Carbon dioxide diffuses through cell membranes into the blood or, in some animals, into the hemolymph, which is analogous to blood. The organism must compensate for this disturbance of its natural pH balance, and some animals do better than others in this respect. Lester Kwiatkowski and his colleagues assessed the drivers of coral growth changes at two sites in the Caribbean between 1880 and 2000 by using observed data and model simulations. The variations of coral growth coincided with fluctuations in sea surface temperatures and incoming sunlight that were, in turn, generated by the emission of anthropogenic aerosols from 1950 onwards. The study concluded that Caribbean coral growth was influenced by anthropogenic aerosol emissions.
Benthic invertebrates (bottom-dwelling animals without a vertebral column) with a limited ability to move great distances, such as mussels, starfish and sea urchins, often cannot accumulate large amounts of bicarbonate in their body fluids to compensate for acidification and the excess protons. Long-term experiments show that some of these species grow more slowly under acidic conditions. However, this protective mechanism could become a disadvantage for the sessile animals when they are exposed to long-term carbon dioxide stress. With the long-term rise in carbon dioxide levels in seawater, the energy-saving behavior and the suppression of metabolism inevitably leads to limited growth, lower levels of activity, and thus a reduced ability to compete within the ecosystem.
In summary, human actions play an indispensable role in the carbon cycle of the oceans.
5.4.2 Threat to the nutrition base in the oceansphytoplankton and acidification
The entire food chain in the oceans is based on the microscopic organisms of the marine phytoplankton, including diatoms (siliceous algae), calcareous algae, and the cyanobacteria (formerly called blue algae), which are responsible for around half of the total global primary productivity through their photosynthetic activity.
Ocean acidification is not the only consequence of raised carbon dioxide levels. Above all, this gas is the elixir of life for plants which take up carbon dioxide from the air or seawater and produce biomass. Despite the acidification problem, raised carbon dioxide levels in sea water should favor the growth of those species with photosynthetic processes that were formerly limited by carbon dioxide. This is also true for certaincoccolithophoressuch asEmilliania huxleyi. However, even forEmilliania, the initially beneficial raise of carbon dioxide levels could become fatal. These species possess a calcareous shell comprising numerous individual plates. There is now evidence that the formation of these plates is impaired by lower pH levels. In contrast, shell formation by diatoms and their photosynthetic activity seem to be only minimally affected by carbon dioxide. However, for diatoms, shifts in species composition have been reported under conditions of increased carbon dioxide concentrations.
According to acidification testing, fossil fuel usage increases the concentrations of carbon dioxide in the atmosphere, while the pH of the oceans is lowered. The resulting equilibrium diminishes the available carbonate in that calcifying organisms precipitate as a calcium salt. This problem has led to a general assumption that calcifying organisms will decrease in abundance as a result of these changes. However, recent research has shown that not all types of organisms will be equally affected or will be affected in the same way. For example, Smith et al. studied how thecoccolithophorespeciesEmiliania huxleyiresponds to seasonal changes in the Bay of Biscay by collecting abundance data and making concurrent measurements of carbonate chemistry over the course of a year. These investigators showed that the more heavily calcified morphotypes are the most abundant in the winter when the oceans are most acidic. This finding is the opposite of what one might predict on the basis of pH changes. Although they cannot attribute this variability to pH-related effects, their work illustrates how difficult it is to predict precisely how specific calcifying process will respond to an acidified ocean environment (Proc, 2012; Ingelngeborg, 2012). Their results are consistent with ours.
5.4.3 Plankton responses to ancient ocean stresses
Phytoplankton, which live exclusively in ocean surface waters, require light for photosynthetic processes and are therefore directly affected by ocean acidification. However, due to global warming, other influencing variables such as temperature, and nutrient availability will also change in the future. These changes will also determine the productivity of autotrophic organisms, primarily bacteria and algae, which purely produce biomass by photosynthesis or the incorporation of chemical compounds. Therefore, it is very difficult to predict which groups of organisms will profit from the changing environmental conditions and which will suffer from them.
A paper published online in Nature Geosciences (Nature, 2013) reported that different species of the same group of marine plankton exhibited varying levels of success during a period of rapid global warming and ocean acidification 56 million years ago. Samantha Gibbs and colleagues used laboratory experiments to monitor the morphology of moderncoccolithophores,which are tiny calcite-producing phytoplankton, during periods of rapid and slow growth. Applying this metric to the fossil record, they found that a species with a prolific evolutionary lineage in the modern oceans continued to grow and reproduce throughout the thermal maximum, whereas the forbear of a species with a more limited range and abundance today does less well. This divergent responseof the different species seems to have influenced their evolutionary success.
In addition, external factors, such as electing public servants who advocate clean energy, ecologically sound land-use and so on can affect the outcome.
The above phenomena are commonplace, and we cannot erase the events that have already occurred. As Edmund Burke said, ‘Nobody makes a greater mistake than he who does nothing because he could do a little’. Oxygen-deprived dead zones in coastal waters around the world have expanded exponentially since the 1960s and are likely to increase further in a warming climate (Sohi, 2012).
6 Conclusions
For dependable climate predictions, it is extremely important to determine exactly how much carbon dioxide is absorbed by the oceans. Therefore, researchers have developed a variety of independent methods to quantify the present role of the oceans in the anthropogenic changes in the carbon cycle. Two procedures in particular have played an important role. The first method (atmosphere-ocean flux) is based on the measurement of carbon dioxide partial-pressure differences between the ocean surface and the atmosphere. The second method attempts the application of rather elaborate geochemical or statistical procedures to calculate how much carbon dioxide of the ocean is derived from natural sources and how much is derived from anthropogenic sources.
Briefly, some conclusions can be drawn from the above study. First, there are several important environmental factors that can impact carbon dioxide dispersal in oceans. The acidification around the release point may be the most important finding of this paper. Impacts around the release point are inevitable. Moreover, the size and severity of the impacted area will depend on the exact method of release. In addition, some experts have addressed zooplankton mortality that may result from exposure to low pH plumes associated with ocean carbon dioxide disposal. Mortality of zooplankton has been shown to correlate with two factors: mass loading per unit area and the travel time to a pH of 7. Finally, analysis of the carbon dioxide cycle has revealed the presence of carbon dioxide reservoirs of the atmosphere, land biomass and ocean. The oceans buffer the concentrations of carbon dioxide and atmospheric trace gases. Certainly, it will take millennia to reach a new equilibrium for carbon dioxide. Therefore, natural processes cannot keep up with the speed at which humans continue to discharge carbon dioxide and other climate-relevant trace gases into the air. The only solution is to save energy and significantly reduce greenhouse gases. The natural ecosystems will remain imbalanced unless human beings stop disturbing them.
Without the oceans and their vast ability to absorb carbon dioxide, Earth would be warming up even faster than it currently is. The oceans take up approximately 9 billion tons of the gas each year — almost one-third of the 30 billion tons emitted globally. Thus, the conclusion that the oceans will function as a large carbon sink without the disturbance of humankind is effectively proven once again.
Acknowledgements
We thank the anonymous reviewer for several helpful comments and advice during the development of this manuscript. Financial support was provided by the National Natural Science Foundation of China (41106094) and the Department of Science and Technology Project (BS2010NY030).
Aalto, R., Maurice-Bourgoin, L., and Dunne, T., 2003. Episodic sediment accumulation on Amazonian flood plains influenced by El Niño/Southern Oscillation.Nature, 425: 493-497.
Algesten, G., Brydsten, L., Jonsson, P., and Kortelainen, P., 2006. Organic carbon budget for the Gulf of Bothnia,Journal of Marine Systems, 63: 155-161.
Anthony, K., Mayorga, E., and Raymond, P. A., 2011. Riverine coupling of biogeochemical cycles between land, oceans, and atmosphere.Frontiers in Ecology and the Environment, 53-60.
Barker, S., and Elderfield, H., 2002. Foraminiferal calcification response to glacial - interglacial changes in atmospheric Carbon dioxide.Science, 297: 833-836.
Bijma, J., Spero, H. J., and Lea, D. W., 1999. Impact of the oceanic carbonate system (experimental results), use of proxies in paleoceanography - examples from the South Atlantic. In:Reassessing Foraminiferal Stable Isotope Geochemistry, Fischer, G.,et al., eds., Springer, Berlin, Heidelberg, 489-512.
Brook, E. J., 2013. Leads and Lags at the end of the last ice age.Science, 339: 1042-1043, DOI: 10.1038/488035a.
Craig, H., Horibe, Y., and Sowers, T., 1988. Gravitational separation of gases and isotopes in polar ice caps.Science, 242:1675-1678.
de Moel, H., Ganssen, G. M., and Peeters, F. J. C., 2009. Planktic foraminiferal shell thinning in the Arabian Sea due to anthro- pogenic ocean acidification?BiogeosciencesDiscussions, 6: 1811-1835.
Fabry, V. J., Seibel, B. A., and Feely, R. A., 2008. Impacts of ocean acidification on marine fauna and ecosystem processes.ICES Journal of Marine Science, 65: 414-432.
Feely, R. A., Sabine, C. L., and Lee, K., 2002. In situ calcium carbonate dissolution in the Pacific Ocean.Global Biogeochemical Cycles, 16: 911-9112, DOI: 10.1029/2002GB001 866.
Feely, R. A., Sabine, C. L., and Lee, K., 2004. Impact of anthropogenic CO2on the CaCO3system in the oceans.Science, 305 (5682): 362-366, DOI: 10.1126/science.1097329.
Gattuso, J. P., and Lavigne, H., 2009. Perturbation experiments to investigate the impact of ocean acidification: approaches and software tools.Biogeosciences Discuss, 6: 4413-4439.
Gattuso, J. P., Frankignoulle, M., and Bourge, I., 1998. Effect of calcium carbonate saturation of seawater on coral calcification.Global Planet. Change, 18: 37-46.
Gattuso, J. P., Pichon, M., and Frankignoulle, M., 1995. Biological control of air-sea CO2fluxes: Effect of photosynthetic and calcifying marine organisms and ecosystems.Marine Ecology-Progress Series, 129 (1-3): 307-312.
Gazeau, F., Quiblier, C., and Jansen, J. M., 2007. Impact of elevated CO2on shellfish calcification.Geophysical Research Letters, 34 (7): 1-5.
Guinotte, J. .M., and Fabry, V. J., 2008. Ocean acidification and its potential effects on marine ecosystems, year in ecology and conservation biology.Annals of the New York Academy of Sciences, 320-342, DOI: 10.1196/annals.1439.013.
Guinotte, J. M., Orr, J., and Cairns, S., 2006. Will human-induced changes in seawater chemistry alter the distribution of deep-sea scleractinian corals?Frontiers in Ecology and the Environment, 4 (3): 141-146.
Guinotte, J. M., Buddemeier, R. W., and Kleypas, J. A., 2003. Future coral reef habitat marginality: temporal and spatial effects of climate change in the Pacific basin.Coral Reefs, 22 (4): 551-558.
Graven, H.D., Gruber, N., and Key, R., 2012. Changing controls on oceanic radiocarbon: New insights on shallow-to-deep ocean exchange and anthropogenic CO2uptake.Journal of Geophysical Research, 117: 1-16.
Hall-Spencer, J. M., Rodolfo-Metalpa, R., and Martin, S., 2008. Volcanic carbon dioxide vents show ecosystem effects of ocean acidification.Nature, 454 (7200): 96-99.
Kleypas, J. A., Buddemeier, R. W., and Archer, D., 1999. Geochemical consequences of increased atmospheric carbon dioxide on coral reefs.Science, 284: 118-120, DOI: 10.1126/ science.284.5411.118.
Kleypas, J. A., Buddemeier, R. W., and Gattuso, J. P., 2001. The future of coral reefs in an age of global change.International Journal of Earth Sciences, 90 (2): 426-437.
Kuss, J., Roeder, W., Wlost, K. P., and DeGrandpre, M. D., 2006. Time series of surface water CO2and oxygen measurements on a platform in the central Arkona Sea (Baltic Sea): Seasonality of uptake and release,Marine Chemistry, 101: 220-232.
Langdon, C., Broecker, W. S., and Hammond, D. E., 2003. Effect of elevated CO2on the community metabolism of an experimental coral reef.Global Biogeochemical Cycles, 17 (1):1-14, DOI: 10.1029/2002GB001941.
Leclercq, N. L., and Gattuso, J. P., 2000. CO2partial pressure controls the calcification rate of a coral community.Global Change Biology, 6 (3): 329-334. DOI: 10.1046/j.1365-2486. 2000.00315.x.
Leclercq, N., Gattuso, J. P., and Jaubert, J., 2002. Primary production, respiration, and calcification of a coral reef mesocosm under increased CO2partial pressure.Limnology and Oceanography, 47 (2): 558-564.
Moy, A. D., Howard, W. R., and Bray, S. G. 2009. Reduced calcification in modern Southern Ocean planktonic foraminifera.Nature Geosci.2: 276-280.
Mucci, A., 1983. The solubility of calcite and aragonite in seawater at various salinities, temperatures, and one atmosphere total pressure.American Journal of Science, 283: 780-799.
Orr, J. C., Fabry, V. J., and Aumount, O., 2005. Anthropogenic ocean acidification over the twenty-first century and its impact on calcifying organisms.Nature, 437: 681-686, DOI:10.1038/nature04095.
Parrenin, F., Masson-Delmotte, V., and Kohler, P., 2013. Synchronous Change of Atmospheric CO2and Antarctic Temperature During the Last Deglacial Warming.Science, 339:1060-1063, DOI: 10.1126/science.1226368.
Pedro, J. B., Rasmussen, S. O., and van Ommen, T. D., 2012. Tightened constraints on the time-lag between Antarctic temperature and CO2during the last deglaciation,Climate of the past, 12-13.
Riebesell, U., Zondervan, I., and Rost, B., 2000.Reduced calcification of marine plankton in response to increased atmospheric CO2.Nature, 407: 364-367, DOI: 10.1038/ 35030078.
Royer, D. L., 2006. CO2-forced climate thresholds during the Phanerozoic.Geochimica et Cosmochimica Acta, 70: 5665-5675.
Sabine, C. L., Feely, R. A., and Gruber, N., 2004. The oceanic sink for anthropogenic CO2.Science305: 367-371, DOI: 10. 1126/science.1097403.
Sohi, S. P., 2012. Carbon storage with benefits.Science, 338:1034, DOI: 10.1126/science.1225987.
Thomas, H., and Schneider, B., 1999. The seasonal cycle of carbon dioxide in Baltic Sea surface waters,Journal of Marine Systems, 22: 53-67.
Wesslander, K., Omstedt, A., and Schneider, B., 2010. Inter-annual and seasonal variations in the air-sea CO2balance in the central Baltic Sea and the Kattegat.Contiental Shelf Research, 30: 1511-1521.
Zondervan, I., 2007. The effects of light, macronutrients, trace metals and CO2on the production of calcium carbonate and organic carbon in coccolithophores - A review.Deep-Sea Research PartII:Topical Studies in Oceanography, 54 (5-7):521-537.
Zondervan, I., Rost, B., and Riebesell, U., 2002. Effect of CO2 concentration on the PIC/POC ratio in the coccolithophore Emiliania huxleyi grown under light-limiting conditions and different daylengths.Journal of Experimental Marine Biology and Ecology, 272 (1): 55-70.
Zondervan, I., Zeebe, R. E., Rost, B., and Riebesell, U., 2001. Decreasing marine biogenic calcification: A negative feedback on rising atmospheric pCO2.Global Biogeochemical Cycles, 15 (2): 507-516.
(Edited by Xie Jun)
(Received November 27, 2013; revised May 22, 2014; accepted June 9, 2014)
© Ocean University of China, Science Press and Springer-Verlag Berlin Heidelberg 2015
* Corresponding author. E-mail: ykmch@hotmail.com
杂志排行
Journal of Ocean University of China的其它文章
- Impacts of the Two Types of El Niño on Pacific Tropical Cyclone Activity
- Numerical Simulation of Typhoon Muifa (2011) Using a Coupled Ocean-Atmosphere-Wave-Sediment Transport (COAWST) Modeling System
- Estimating the Turbulence Characteristics in the Bottom Boundary Layer of Monterey Canyon
- Composition and Origin of Ferromanganese Crusts from Equatorial Western Pacific Seamounts
- Hydroelastic Analysis of a Very Large Floating Structure Edged with a Pair of Submerged Horizontal Plates
- A Storm Surge Intensity Classification Based on Extreme Water Level and Concomitant Wave Height
