Absorption of CO2in a novel ternary deep eutectic solvent
2015-04-01LIGuihuaSHANHaifangAINingDENGDongshun
LI Guihua, SHAN Haifang, AI Ning, DENG Dongshun
(Zhejiang Province Key Laboratory of Biofuel,College of Chemical Engineering,Zhejiang University of Technology,Hangzhou310014,Zhejiang,China)
Introduction
As main greenhouse gas, the increasing concentration of CO2from the industrial expansion tends to raise the temperature of earth surface[1].Numerous technologies were utilized to control andreduce CO2emissions, especially aqueous alkanolamine solutions absorption[2-3]. However, the inherit disadvantages including equipment corrosion,intensive energy consumption and secondary pollution made it necessary to explore more environmental alternatives.
Ionic liquids (ILs), another class of solvent, have been selected to overcome obstacles of traditional absorbents due to their non-volatility, thermal stability,and tunable physiochemical properties[4-9]. Many efforts were focused on measuring solubility of CO2in various ILs because of these favorable characteristics[10-16]. On the other hand, the high production cost and uncertain toxicity limited their industrial applications[17-19].
Recently, a new type of ionic liquids named deep eutectic solvents (DESs) have been widely used in CO2absorption owing to their low cost and interesting properties consistent with conventional ILs such as negligible vapor pressure, wide liquid range, high thermal and chemical stabilities, non-flammability[20].DESs are made by mixing a substituted quaternary ammonium salt and a metal halide, amide or carboxylic acid. In previous work, Liet al.[21]reported the solubilities of CO2in choline chloride-urea mixture at temperature ranging from 313.15 K to 333.15 K under pressures up to 13 MPa. Leronet al.[22-23]compared the absorption capacity of CO2in DESs combined choline chloride with ethylene glycol or glycerol. In the ways of carboxylic acids, Franciscoet al.[24]prepared a new low transition temperature mixture (LTTM) formed by choline chloride + lactic acid and studied the solubility of CO2in LTTM. In our previous study, we discussed the effects of DESs synthesized by choline chloride and dihydric alcohols in CO2absorption[25]. That work investigated the ability of different kinds of dihydric DESs in the separation of CO2. Researches of CO2in ternary deep eutectic solvents (TDESs) are absent, although Liuet al.[26]founded lower freezing point TDESs formed by alkylimidazolium halides ([BMIM]X, X = Cl, Br),zinc halides (ZnX2, X = Cl, Br) and amides in appropriate molar ratios, which can be applied in organic reactions as solvents or catalyst.
In present work, we developed a series of novel low price TDESs containingN,N-dimethyl acetamide (DMA), choline chloride (CC) and ethylene glycol or glycerol with different mole ratios (nDMA:nCC:nethyleneglycol=1:1:3,1:1:4,nDMA:nCC:nglycerol=1:1:3,1:1:4). Experiment measurements were completed by isochoric saturation method in the temperature rangeT=293.15—323.15 K with 10℃intervals under pressures ranging from 0 to 600.0 kPa.Henry constants were determined from solubility data.Thermodynamics of CO2absorption including enthalpy, entropy, Gibbs free energy were also calculated.
1 Experimental
1.1 Materials
The TDESs were synthized byN,N-dimethyl acetamide (DMA), choline chloride (CC) and ethylene glycol or glycerol with different ratios(nDMA:nCC:nethyleneglycol=1:1:3, 1:1:4,nDMA:nCC:nglycerol=1:1:3, 1:1:4) atT=363.15 K. And then, they were used after drying for 24 h under vacuum at 350 K to remove volatile impurities. Choline chloride (>98.5%)was produced by Jinan Hualing Pharmaceutical Co.,Ltd.N,N-dimethyl acetamide (DMA) (≥99.0%),ethylene glycol (≥99.0%) and glycerol (≥99.0%)were all produced by Sinopharm Chemical Reagent Co., Ltd. Densities of the TDESs at atmospheric pressure were carefully measured using a(5.567±0.004)cm3pycnometer in the temperature range 293.15 K to 323.15 K at 10℃ intervals. The pycnometer was immersed in an oil-bath and previously calibrated using double distilled water at 303.15 K. CO2with the mass fraction of more than 0.99995 was supplied by Jingong Special Gas Co., Ltd.The summary of the chemical used, their purities, and sources were listed in Table 1.
1.2 Apparatus
The stainless apparatus was an upgrade version on the basis of our previous glass apparatus[27]and illustrated in Fig.1. It was mainly composed of a CO2cylinder (1), two water baths (2, 5), CO2gas equilibrium cell (EC, 4) with magnetic stirrer, gas reservoir (GR, 3) and pressure transmitters (6,7). Thevolumes of EC and GR were determined using the previous method[28]with the results of 141.61 cm3and 370.99 cm3, respectively. The temperatures of water baths were carefully controlled with a precision of±0.05 K. The pressures were monitored using pressure transmitter (Fujian WIDEPLUS Precision Instruments Co., Ltd., WIDEPLUS-8, 0 to 600.0 k Pa,with an accuracy of 0.1 % full scale).

Table 1 Description of chemicals used in this study

Fig. 1 Schematic diagram of CO2solubility apparatus
1.3 Methods
The measurement of CO2solubility was performed using isochoric saturation method[28]. The mass of TDES was measured using electronic balance(Mettler-Toledo AL204) with an uncertainty of 2×10-4g. During experiments, temperatures were controlled by two water-bathes. About 15—40 g TDES dried 24 h under vacuum at 350 K were loaded into EC and degassed under vacuum at 343.15 K while stirring for 1 h. After cooling, the whole system was controlled at a specified oven temperature with water-bathes and evacuated to pressuresp1for 1 h.And then, CO2was fed from gas cylinder into GR until the pressure reached scheduled valuep2.When the valve was opened between EC and GR, CO2was brought into EC and was absorbed by TDESs. A magnetic stirring was used to facilitate the CO2absorption. It was assumed that the equilibrium was reached until the pressure of EC constant for 4 h. The final pressures of EC and GR were recorded asp3,p4,respectively. The amounts of absorbed gas were calculated from a difference between the total amount of gas in GR and residual amount in GR as well as in EC. The next measurement at the same equilibrium temperature was carried out by introducing further amount CO2into the EC from GR with the similar procedure. The measurement was repeated until the pressure between GR and EC was equal.
2 Results and discussion
2.1 Density
Density measurements of TDESs at atmospheric pressure were carefully made using a (5.567±0.004)cm3pycnometer in the temperature range 293.15—323.15 K at 10℃ intervals. The pycnometer was immersed in an oil-bath and previously calibrated using double distilled water at 303.15 K. As shown in Table 2, densities increased with decreasing of temperatures.

Table 2 Densities of TDESs
2.2 Solubility data of CO2in TDESs
In Table 3, solubility data of CO2in TDESs under pressures ranging from 0 to 600.0 kPa at temperatures(T=293.15 K, 303.15 K, 313.15 K, 323.15 K) were summed up.
The calculation of CO2solubilities are based on mole fractionxCO2and molalitymCO2of CO2in liquids phase as well as gas phase CO2equilibrium pressurepabove the liquid absorbent. The mole fraction and molality of CO2were obtained by the following equations


Table 3 Experimental CO2mole fraction (xCO2) and molality (mCO2) in solutions at different temperature and equilibrium pressure

nTDESsis the mole quantity of absorbent which was obtained according to the mass (wTDESs) used and molar mass of TDESs (MW).
The gas phase composition was regarded as pure CO2because of the negligibly low vapor pressure of TDESs. The amount of CO2absorbed in TDESs can be calculated by the following equation

WherenCO2is the amount of CO2absorbed by TDESs.nandn1are the initial and residual amounts of CO2in GR, respectively.n2represents the amount of gaseous CO2in EC at equilibrium. The various amounts of CO2can be calculated from experimental PVT data at different conditions using Soave-Redlich-Kwong (SRK) equation. The volume of liquid solution in EC is directly obtained from the mass and density of TDESs at different temperatures.The volume expansion of the liquid in EC because of the dissolution of CO2is very small.
The dependence of the solubility on temperatures and pressures were shown in Fig.2 and Fig.3, respectively. It can be known that the solubility of CO2in the liquids increased linearly with increasing pressure and decreased with increasing temperature at all the pressures.Moreover, it is clear that the solubility of CO2in TDESs (nDMA:nCC:nethyleneglycol=1:1:3) is higher than others at 303.15 K and present lower molality value with increasing temperature. Such solubility phenomena may indicate that the capture of CO2is physical dissolution process[29].
2.3 Henry’s constants
Henry’s constants of TDESs fitted from solubility data of CO2were listed in Table 4. Henry’s constants includingHxbased on mole fraction andHmbased on molarity. Henry’s constants are key physical property that means the solubility of solute in the absorbent[30]. To evaluate Henry’s constants, it is necessary to estimate the fugacity coefficient of CO2in the TDESs at the system temperature and pressure.In our experiments, the gaseous phase can be assumed to be pure CO2and the fugacity of gas was approximately equal to equilibrium pressure of CO2[31-32]because of the relative low equilibrium pressure and non-volatility of TDESs. As shown in Fig.2, the isothermal CO2solubility in TDESs at temperatureT=303.15 K increased with increasing pressure. Thus, the Henry’s constantsHx(Hm) were determined from the slope of the isotherm created from linear fit of CO2mole fraction (molarity)versusequilibrium pressure.

Table 4 Experimentally determined Henry’s constants of CO2in solutions at various temperatures

Fig.2 CO2solubility as a function of CO2equilibrium pressure(p) atT=303.15 K

Fig.3 Solubilities of CO2in TDES(nDMA:nCC:nethyleneglycol=1:1:3)
With the increasing of temperature, the Henry’s constantsHx(Hm) increased and the solubility of CO2decreased for all systems. We can find that solubility of CO2in TDES obtained from DMA, CC and ethylene glycol with mole ratio 1:1:3 showed the lowest value of 2.174 MPa·kg·mol-1at 293.15 K,which is consistent with results in Fig.2 and Fig.3.Moreover, our work can compare with existing binary systems by stainless apparatus using isochoric saturation method. As listed in Table 5, it is noted that the solubility of CO2in TDESs(nDMA:nCC:nethyleneglycol=1:1:3) based onHmis higher than DESs composed of CC and urea or glycols but lower than DESs combined from CC and lactic acid or phenol.
2.4 Thermodynamics
The behavior of Henry’s constants as a function of temperature was correlated using an empirical equation as following

The optimized coefficients,Bi, obtained using a linear regression of multiple-variables calculation, were listed in Table 6. Thermodynamic properties of dissolutions of CO2in TDESs can be calculated by relating to the Henry’s constants as follows
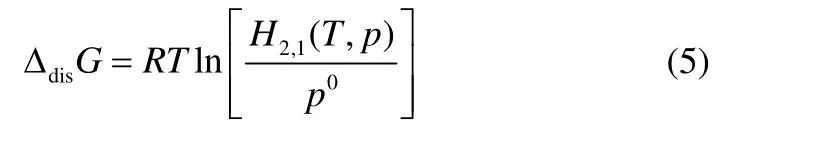

Table 5 Hmof CO2in different solutions at 313.15 K

Table 6 Values of coefficientsB0,B1, andB2for equation
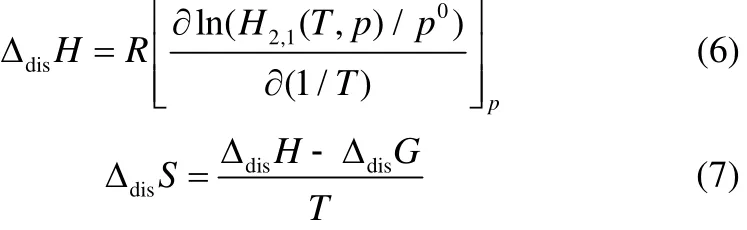
In which ΔdisG, ΔdisH, ΔdisSare the standard Gibbs free energy, enthalpy and entropy changes of CO2solution at the standard state pressure ofp0=0.1 MPa,respectively. The enthalpy of solution is an important parameter of the system because it is related with strength of interaction between the liquids and the gas,and ΔdisSshows the degree of ordering of the liquid/gas mixture. The thermodynamic properties changes at 303.15 K and 0.1 MPa were shown in Table 7.
At all the conditions, the negative value of ΔdisHindicates that the process is exothermic, which means that the dissolution of CO2in TDESs is favorable enthalpically. The absolute value of ΔdisHbased onHxof TDES (nDMA:nCC:nethyleneglycol=1:1:3) is the largestat 293.15 K and indicates stronger TDES /CO2interactions. From the molecular points, the ΔdisSis largely related to the TDESs organization surrounding the soluble CO2[34]. A larger negative entropy of TDES/ CO2indicates stronger interactions, while stronger order in the TDES /CO2mixture correlates to larger negative value of the entropy. As a result, the ΔdisGshows positive value.
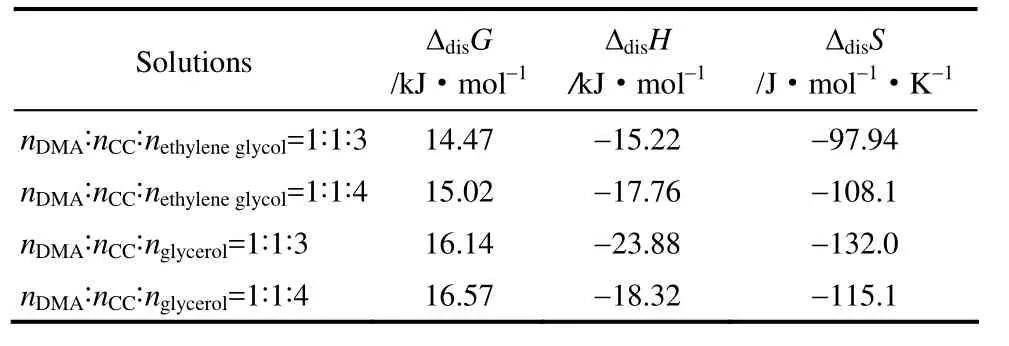
Table 7 Calculated Gibbs free energy, enthalpy and entropy of solution at 0.1 MPa and 303.15K
3 Conclusions
In this work, solubility data of CO2in different TDESs were determined at temperatures (T=293.15 K,303.15 K, 313.15 K, 323.15 K) under pressures ranging from 0 to 600.0 kPa using the isochoric saturation method. The results replied that the solubility of CO2in the liquids increased linearly with increasing pressure and decreased with increasing temperature. Moreover, it was found that solubility of CO2in TDESs (nDMA:nCC:nethyleneglycol=1:1:3) based onHmis higher than DESs composed of CC and urea or glycols but lower than DESs combined from CC and lactic acid or phenol at the same conditions. Henry’s constants as a function of temperature were also calculated and TDESs obtained from DMA, CC and ethylene glycol with mole ratio 1:1:3 showing the lowest value 2.174 MPa·kg·mol-1at 293.15 K.Thermodynamics of CO2absorption including enthalpy, entropy, Gibbs free energy were also reported. The negative enthalpy demonstrated that the process was exothermic.
[1] Paul S, Ghoshal A K, Mandal B. Theoretical studies on separation of CO2by single and blended aqueous alkanolamine solvents in flat sheet membrane contactor (FSMC) [J].Chemical Engineering Journal, 2008, 144(3):352-360.
[2] Jamal A, Meisen A, Lim C J. Kinetics of carbon dioxide absorption and desorption in aqueous alkanolamine solutions using a novelhemispherical contactor(Ⅱ):Experimental results and parameter estimation [J].Chemical Engineering Science, 2006, 61(19):6590-6603.
[3] Kim I, Hoff K A, Hessen E T, Warberg T H, Svendsen H F. Enthalpy of absorption of CO2with alkanolamine solutions predicted from reaction equilibrium constants [J].Chemical Engineering Science,2009, 64(9):2027-2038.
[4] Aparicio S, Atilhan M. Computational study of hexamethylguanidinium lactate ionic liquid:a candidate for natural gas sweetening [J].Energy Fuels, 2010, 24(9):4989-5001.
[5] Revelli A L, Mutelet F, Jaubert J N. Prediction of partition coefficients of organic compounds in ionic liquids:use of a linear solvation energy relationship with parameters calculated through a group contribution method [J].Ind. Eng. Chem. Res., 2010, 49(8):3883-3892.
[6] Mutelet F, Revelli A L, Jaubert J N, Sprunger L M, Acree W E, Baker G A. Partition coefficients of organic compounds in new imidazolium and tetralkylammonium based ionic liquids using inverse gas chromatography [J].J. Chem. Eng. Data, 2010, 55(1):234-242.
[7] Revelli A L, Mutelet F, Jaubert J N, Martinez M G, Sprunger L M,Acree W E, Baker G A. Study of ether-, alcohol-, or cyano-functionalized ionic liquids using inverse gas chromatography[J].J. Chem. Eng. Data, 2010, 55(7):2434-2443.
[8] Revelli A L, Mutelet F, Jaubert J N. High carbon dioxide solubilities in imidazolium-based ionic liquids and in poly(ethylene glycol) dimethyl ether [J].J. Phys. Chem. B, 2010,114(40):12908-12913.
[9] Bara J E, Carlisle T K, Gabriel C J, Camper D, Finotello A, Gin D L,Noble R D. Guide to CO2separations in imidazolium-based roomtemperature ionic liquids [J].Ind. Eng. Chem. Res., 2009, 48(6):2739-2751.
[10] Pennline H W, Luebke D R, Jones K L, Myers C R, Morsi B I, Heintz Y J, Ilconich J B. Progress in carbon dioxide capture and separation research for gasification-based power generation point sources [J].Fuel Process Technol., 2008, 89(9):897-907.
[11] Goodrich B F, Fuente J C, Gurkan B E, Zadigian D J, Price E A,Huang Y, Brennecke J F. Experimental measurements of amine-functionalized anion-tethered ionic liquids with carbon dioxide[J].Ind. Eng. Chem. Res., 2011, 50(1):111-118.
[12] Bates E D, Mayton R D, Ntai I, Davis J H. CO2capture by a task-specific ionic liquid [J].J. Am. Chem. Soc., 2002, 124(6):926-927.
[13] Cui Y H, Chen Y F, Deng D S, Ai N, Zhao Y. Difference for the absorption of SO2and CO2on [Pnnnm][Tetz] (n=1,m=2, and 4) ionic liquids:a density functional theory investigation [J].Journal of Molecular Liquids,2014, 199:7-14.
[14] Makino T, Kanakubo M, Masuda Y, Umecky T, Suzuki A. CO2absorption properties, densities, viscosities, and electrical conductivities of ethylimidazolium and 1-ethyl-3- methylimidazoliumionic liquids [J].Fluid Phase Equilibria, 2014, 362:300- 306.
[15] Kumełan J, Tuma D, Kamps A P, Maurer G. Solubility of CO2in the ionic liquids [bmim][CH3SO4] and [bmim][PF6] [J].J. Chem. Eng.Data, 2006, 51(5):1802-1807.
[16] Wang M, Zhang L Q, Liu H, Zhang J Y, Zheng C G. Studies on CO2absorption performance by imidazole-based ionic liquid mixtures [J].J. Fuel Chem. Technol., 2012, 40(10):1264-1268.
[17] Bernot R J, Kennedy E A, Lamberti G A. Effects of ionic liquids on the survival, movement, and feeding behavior of the freshwater snail [J].Environ. Toxicol. Chem., 2005, 24(7):1759-1765.
[18] Latala A, Stepnowski P, Nedzi M, Mrozik W. Marine toxicity assessment of imidazolium ionic liquids:acute effects on the baltic algaeo ocystis submarina and cyclotella meneghiniana Aquat [J].Toxicol., 2005, 73(1):91-98.
[19] Couling D J, Bernot R J, Docherty K M, Dixon J K, Maginn E J.Assessing the factors responsible for ionic liquid toxicity to aquatic organismsviaquantitative structure-property relationship modeling[J].Green Chem., 2006, 8(1):82-90.
[20] Siongco K R, Leron R B, Li M H. Densities, refractive indices, and viscosities ofN,N-diethylethanol ammonium chloride- glycerol or-ethylene glycol deep eutectic solvents and their aqueous solutions[J]. J. Chem. Thermodynamics, 2013, 65:65-72.
[21] Li X Y, Hou M Q, Han B X, Wang X L, Zou L Z. Solubility of CO2in a choline chloride + urea eutectic mixture [J].J. Chem. Eng. Data,2008, 53(2):548-550.
[22] Leron R B, Li M H. Solubility of carbon dioxide in a choline chloride-ethylene glycol based deep eutectic solvent [J].Thermochimica Acta, 2013, 551:14-19.
[23] Leron R B, Li M H. Solubility of carbon dioxide in a eutectic mixture of choline chloride and glycerol at moderate pressures [J].J. Chem.Thermodynamics, 2013, 57:131-136.
[24] María F, Adriaan V D B, Lawien F Z, Cor J P, Maaike C K. A new low transition temperature mixture (LTTM) formed by choline chloride + lactic acid:characterization as solvent for CO2capture [J].Fluid Phase Equilibria, 2013, 340:77-84.
[25] Chen Y F, Ai N, Li G H, Shan H F, Cui Y H, Deng D S.Solubilities of carbon dioxide in eutectic mixtures of choline chloride and dihydric alcohols [J].J. Chem. Eng. Data, 2014,59(4):1247-1253.
[26] Liu Y T, Chen Y A, Xing Y J. Synthesis and characterization of novel ternary deep eutectic solvents [J].Chinese Chemical Letters, 2014,25(1):104-106.
[27] Deng D S, Cui Y H, Chen D, Ai N. Solubility of CO2in amide-based brønsted acidic ionic liquids [J].J. Chem. Thermodyn., 2013, 57:355-359.
[28] Jacquemin J, Costa Gomes M F, Husson P, Majer V. Solubility of carbon dioxide, ethane, methane, oxygen, nitrogen, hydrogen, argon,and carbon monoxide in 1-butyl-3-methylimidazolium tetrafluoroborate between temperatures 283 K and 343 K and at pressures close to atmospheric [J].J. Chem. Thermodyn., 2006, 38(4):490-502.
[29] Hasib-ur-Rahman M, Siaj M, Larachi F. Ionic liquids for CO2capture-development and progress [J].Chem. Eng. Process., 2010,49(4):313-322.
[30] Zhang N, Zhang J B, Zhang Y F, Bai J, Wei X. H. Solubility and Henry’s law constant of sulfur dioxide in aqueous polyethylene glycol 300 solution at different temperatures and pressures [J].Fluid Phase Equilibria, 2013, 348:9-16.
[31] Cadena C, Anthony J L, Shah J K, Morrow T I, Brennecke J F,Maginn E J. Why is CO2so soluble in imidazolium-based ionic liquids? [J].J. Am. Chem. Soc., 2004, 126(16):5300-5308.
[32] Smith J M, van Ness H C, Abbott M M. Introduction to themical engineering thermodynamics (seventh edition) [R]. New York:Mc-Graw Hill, 2005.
[33] Li G H, Deng D S, Chen Y F, Shan H F, Ai N. Solubilities and thermodynamic properties of CO2in choline-chloride based deep eutectic solvents [J].J. Chem. Thermodynamics, 2014, 75:58-62.
[34] Anthony J L, Maginn E J, Brennecke J F. Solubilities and thermodynamic properties of gases in the ionic liquid 1-n-butyl-3-methylimidazolium hexafluorophosphate [J].J. Phys.Chem. B, 2002, 106(29):7315-7320.
