Chemometric study of the retention mechanism on butyl column: effect and relation of pH and pKa
2015-03-24KOUSKOURAMariaMITANIConstantinaMARKOPOULOUCatherine
KOUSKOURA Maria G, MITANI Constantina V, MARKOPOULOU Catherine K
(Laboratory of Pharmaceutical Analysis, Department of Pharmaceutical Technology, School of Pharmacy,Faculty of Health Sciences, Aristotle University of Thessaloniki, 54124 Thessaloniki, Greece)
Abstract: Reversed phase chromatographic separations are optimized for analytes containing ionizable groups by adjustment of pH of mobile phases. As it seems the pKa values of compounds affect their retention because of the variety in their solvation. However, it is of stressful need to predict their behavior taking into account also a series of other parameters. This work focuses on the development of ten different models, using partial least squares regression, which will identify and quantify the impact of several factors in the chromatographic behavior of 104 analytes. The combined effect of their numerous characteristics is obvious since along with pH (at 2.3 and 6.2), factors such as lipophilicity, molecular volume, polar surface area and the presence of specific moieties in their structures are not diminished. On the contrary, they work increasing or counterbalancing several effects on the retention time. The models compiled can be applied to predict with reliability (R2>0.865 and Q2>0.777) the behavior of unknown drugs.
Article
Chemometric study of the retention mechanism on butyl column: effect and relation of pH and pKa
KOUSKOURA Maria G, MITANI Constantina V, MARKOPOULOU Catherine K*
(LaboratoryofPharmaceuticalAnalysis,DepartmentofPharmaceuticalTechnology,SchoolofPharmacy,FacultyofHealthSciences,AristotleUniversityofThessaloniki, 54124Thessaloniki,Greece)
Abstract: Reversed phase chromatographic separations are optimized for analytes containing ionizable groups by adjustment of pH of mobile phases. As it seems the pKavalues of compounds affect their retention because of the variety in their solvation. However, it is of stressful need to predict their behavior taking into account also a series of other parameters. This work focuses on the development of ten different models, using partial least squares regression, which will identify and quantify the impact of several factors in the chromatographic behavior of 104 analytes. The combined effect of their numerous characteristics is obvious since along with pH (at 2.3 and 6.2), factors such as lipophilicity, molecular volume, polar surface area and the presence of specific moieties in their structures are not diminished. On the contrary, they work increasing or counterbalancing several effects on the retention time. The models compiled can be applied to predict with reliability (R2>0.865 andQ2>0.777) the behavior of unknown drugs.
partial least squares regression; ionization percentage; butyl column; pKa
Reversed phase chromatography (RPC) is a widely used analytical method accounting a substantial majority of several assays as it is considered reliable, robust and versatile. Several types of appropriate columns determine the kind of separation and in combination with the mobile phase are the main conducting tools of an HPLC procedure [1-3]. More specifically, based on the theory of chromatography, the retention of an analyte is related with its competitive dispersive interactions with both stationary and mobile phases [4]. Generally, when such interaction mechanisms are referring to dissimilar groups of compounds, their behavior may vary.
A simple rule in reversed phase HPLC is that the more hydrophobic an analyte is, the more it will be retained. Of course, this observation is only valid when the mobile phase consists of solvents that cannot induce ion formation of the analyte molecules. However, it is commonplace that using mobile phases adjusted at different pH values, compounds bearing acidic or basic groups will ionize to a different extent. Indeed, according to the equation expressed by Hendesron-Hasselbach, the pH value of the mobile phase and the pKaof an analyte affect its ionization [5]. Therefore, depending on the pH, analytes will be found in partially or fully ionized form and their retention might have a considerable shift [6].
Although pH is a primary tool for controlling selectivity via the change of the analyte form (neutral or ionized), still there are certain drawbacks in the retention of analytes. The presence of both ionized and unionized forms may result in some cases in peak tailing or fronting. This fact led to difficulties in the retention time determination and the information provided is not always accurate [7]. According to the literature, all ionizable compounds may have specific hydrophobic interactions with reversed stationary phases [8-10]. These phenomena are additionally affected by the nature of the organic modifiers in the mobile phase [11]. Thus, in some cases a column packing with an intermediate polarity such as butyl, is considered as the first choice for the separation of both neutral and ionic samples. These columns are capable of providing the information necessary concerning the mechanism on reversed phase chromatography with relatively low retention times for the analytes.
A realistic approach of the retention mechanism should include the study of all interactions taking place. These are determined by the physicochemical properties and structural characteristics of the analytes and also the nature of the solvents used as mobile phases. In order to take into account all these parameters the phenomenon should be dealt as a multidimensional system. For this purpose quantitative structure retention relationships models (QSRR) [12,13] are employed and, with the aid of special technique such as principal component analysis (PCA) [14-18] or multiple linear regression (MLR) analysis [19] is tried to give answers to related problems.
Partial least squares (PLS) to latent structures is also considered one of them and could be used since a large number ofXvariables (descriptors) can be correlated to variableY(retention time). Though, PLS reports referring to chromatographic characterization of analytes are rare [20-22], scientists tried to model the retention of small groups of compounds (with similar structures) on reversed phase columns using QSRR [23], MLR and PLS techniques [19]. Therefore, separations of methyl and unsubstituted polycyclic aromatic hydrocarbons were examined on an octadecyl column using PLS analysis [24]. Nonetheless, it is necessary to further clarify the interaction forces occurring as well as the degree of influence of the pH of the mobile phase on the retention of ionizable probes.
The current work focuses on answering these questions employing a butyl column, at two different mobile phases in terms of pH (2.3 and 6.2) and PLS methodology. The retention behaviour of 104 analytes used as probes is clarified considering both their physicochemical properties and structural characteristics. In order to run the models and for their corresponding interpretation it was considered necessary to divide these compounds into subgroups based on their ionization. Verification of whether structural characteristics related with ionization (e. g. -COOH, -NH2,-NH-) or the pKa(of acidic and basic groups) are indeed determining for the chromatographic behavior of the analytes, and it can be achieved using the PLS methodology.
The novelty of this work also lies on the fact that the data was fully exploited in order to bring out and interpret the slight changes that occur in the behavior of analytes on the column due to the pH variation. Therefore, this research proves that combining mathematical models with chromatography cannot only correlate the chromatographic mechanism with different parameters of the analytes, but it also has the potential to interpret and at the same time to point out those slight details that affect this phenomenon.
1 Experimental
1.1 Chemicals and reagents
The experiments were carried out with 104 highly different compounds (Table 1 and Table 2) and analytical grade reagents. The analytes were United States Pharmacopoeia-grade and obtained by i. Carlo Erbra (Milano, Italy), ii. Panreac Company (Controla, Thessaloniki, Greece), iii. Sigma-Aldrich (Life Science Chemilab).
Methanol and water were gradient grade for liquid chromatography and were obtained from Panreac. The pH value of the mobile phase was adjusted by the addition of aqueous solution of NH4OH (>25%) or HCl (37%), obtained by Fluka.
1.2 Apparatus
The experimental part of the procedure was carried out on an HPLC (Shimadzu) instrument, equipped with two LC-20AD pumps, a SIL-10AD auto-sampler and a UV-diode array detector. LC Solution software was used for the detection of the chromatographic peaks. A column oven (Shimadzu) was employed to maintain the temperature of the analysis at 40 ℃. The stationary phase was a butyl column donated by ACE, with dimensions of 150 mm×4.6 mm and particle size of 5 μm.
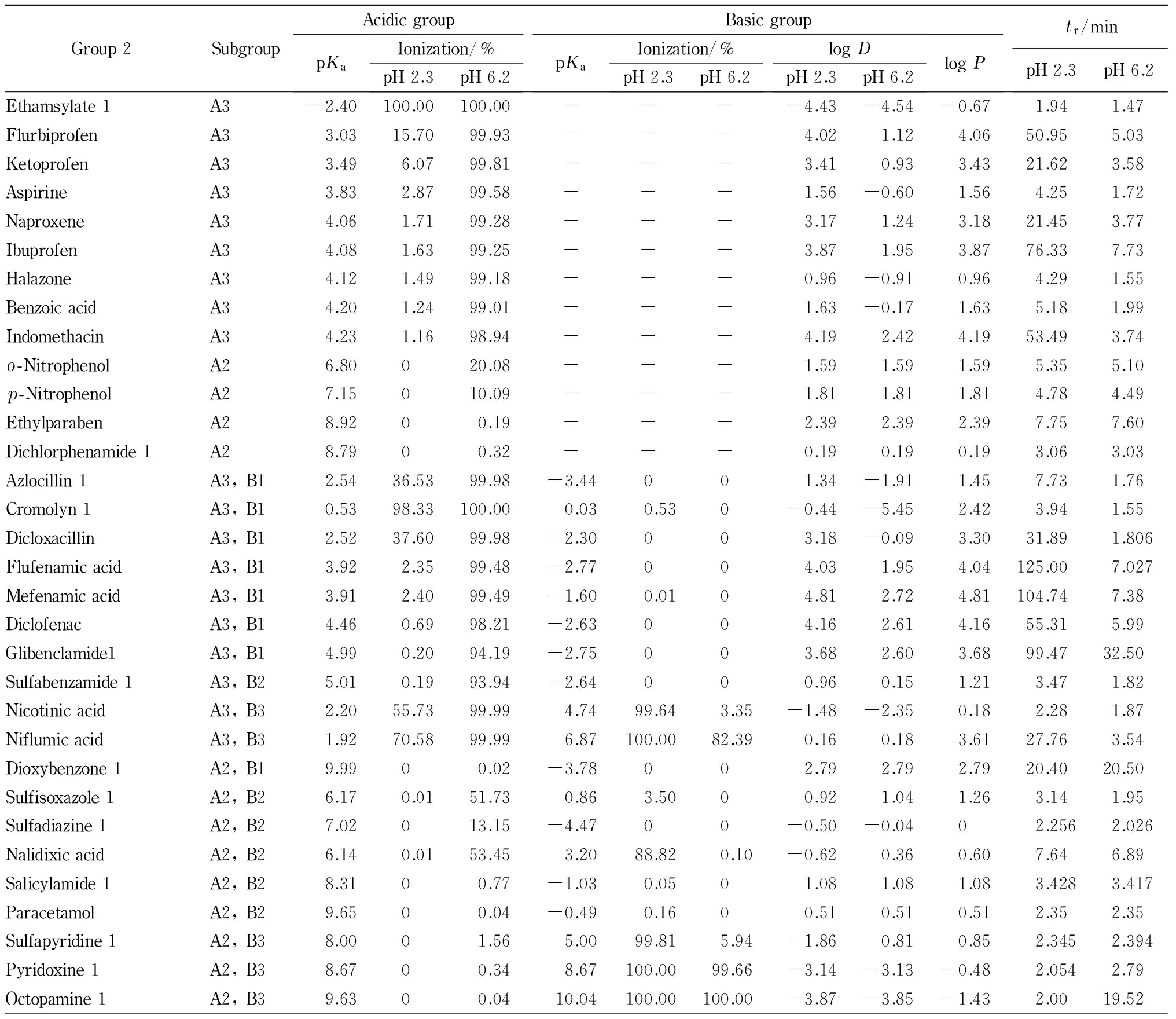
Table 2 Retention times (tr), log P, log D, pKa values and ionization percentages for the remaining analyte compounds of Group 2

Table 2 (Continued)
Chromatographic characterization of the probes was studied on the basis of the pH difference of the mobile phases. The solvent mixtures used as mobile phases are 40% (v/v) MeOH/H2O adjusted at two pH values (2.3 and 6.2) using aqueous solutions of HCl and NH3respectively. The analytes under investigation, after being diluted with methanol to final concentrations of 40-50 μg/mL, were injected into the chromatograph. Samples of 20 μL injection volume were eluted on C4column with a flow rate of 1 mL/min and their retention times were determined by the mean of at least duplicate measurements. The retention time (tr) of naphthol was checked at frequent time intervals in order to control the stability of chromatographic system.
1.3 Partial least squares
The software used to develop and evaluate the PLS models is an independent modeling of class analogies Simca-P (version 9; Umetrics, Upsala, Sweden).
The first column of each dataset (Table 3) corresponds to the observations (analytes studied). The second part contains physicochemical properties (37) and structural characteristics (28) of the compounds. TheYvariable refers to the last column of the dataset corresponding to the retention time of the analytes expressed as logK(logK=log [(tr-to)/to]), wheretrandtoare their retention times of the analyte and the solvent front respectively).
The structural features are encountered in the constitutional parameters and were outlined to decode the chemical structure of all analytes on the same basis. For this purpose, integer numbers and zero were used to indicate the presence, the multiplicity or the absence of a structural characteristic. Some of the molecules are rather complex and they were chosen in an attempt to determine the factor which prevails on the retention mechanism or to reach a conclusion concerning the combined effect of numerous descriptors.
Apart from the structural characteristics of the analytes, the descriptors (Xvariables) used in the implementation of the models include lipophilicity parameters, topological, geometrical, electronic and drug relevant properties (Table 3). The data for the physicochemical properties were derived by five different databases Advanced Chemistry Development/Labs (ACD/Labs Inc.) or calculation platforms Dragon (Todeschini R, Consonni V, Pavan M, ed Talete srl), Osiris (Sander T, Osiris Properties Explorer, openmolecules org), Hyperchem (Professional, Hypercube Inc), Pallas (Compudrug Chemistry Ltd, Sedona). In order to determine specific properties (e. g. LUMO (lowest unoccupied molecular orbital), HOMO (highest occupied molecular orbital), surface area), it was necessary to optimize the 3D structures of the analytes. The method used for 3D structure optimization (using Hyperchem software) was a semi-empirical method based on the Polak-Ribiere Conjugate Gradient Algorithm.
For practical reasons, the observations studied (Total group of 104 analytes) were divided in two large groups (Fig. 1). The first (Group 1) consists of chemical compounds, including drugs, which lack acidic or basic moieties in their structures. The second (Group 2) contains molecules that are affected by the pH of the mobile phase. In order to separate the analytes in subgroups, it was also considered necessary to emphasize on their pKavalue which is pH dependent while unavoidably the distribution coefficient (logD) is influenced. Given the fact that logDindicates the distribution of the neutral or ionic form of a molecule in water against the non-ionized species in octanol, there was also a need to calculate the corresponding logDvalues of the analytes studied (Table 1 and 2). The tendency of a molecule to dissociate (logD) can be estimated using equation logD=logP-log (1+10pH-pKa) for acids and logD=logP-log (1+10pKa-pH) for bases, where it is obvious that both logPand logDvalues depend on its dissociation constant (pKa) and the pH of the solvent.

Fig. 1 Division of the analytes into groups
Since the ionization of the analytes (for acids or bases) depends on their pKavalues, they are additionally divided into subgroups. Group A (Fig. 1) includes compounds bearing at least one acidic moiety; Group B includes compounds bearing at least one basic; and Group C contains molecules with both acidic and basic moieties in their structures (Fig. 1). Finally, based on the variety in ionization percentage, Groups A and B were further divided to three subgroups respectively using the Henderson-Hasselbalch equations [5].
Therefore, datasets were separately designed for each group or subgroup, in order to compile the respective PLS models. The first two models (called Total) included all probes (104 observations) and were analyzed at two pH values (2.3 and 6.2). Furthermore, eight models were developed containing the four different subgroups (Group 1, A, B, C), at the two pH values.
2 Results and discussion
2.1 General theoretical aspects for the analytes studied
Chromatographic peaks for almost the majority of analytes appear to be symmetrical with relatively good peak width and no distortion at both 2.3 and 6.2 pH values. Since selectivity of ionic samples primarily depends on the pH of the mobile phase, it is of stressful need to explore factors that may determine their behavior on a butyl column. Useful information about the priority of such descriptors are provided through the Variable’s Importance in the Projection (VIP) values and the PLS models.
Table 4 presents two sides data about the VIP values of the total groups of substances, at pH 2.3 and 6.2 respectively. More specifically, the first column of Table 3 contains the VIP values in descending order. The second describes the positive (+ sign) or negative (- sign) effect of each descriptor on the retention, taking into account the w*c[1] plots (loadings plots).
Therefore, based on the VIP values, it is proved that the ability of a molecule to be retained longer on the surface of a non-polar stationary phase is mainly determined by its lipophilicity (logP, logD) and the number of benzene rings. Indeed, these two coefficients are the measure of partition (logP) or distribution (logD) of a non-ionized or an ionized solute in octanol-water mean. A molecule that is highly lipophilic seems to have stronger interaction with a non-polar stationary phase and it is retained longer. On the contrary, there are structural characteristics or physicochemical properties related with the high polarity (e. g. water solubility, polar surface area, dipole moment, hydrogen bond donor (HBD)) of a molecule which are responsible for faster elution. The volume of a molecule as well as the relative physicochemical properties (refractivity, polarizability, molecular weight (M. W.), presence of condensed rings) also increases its retention.

Table 4 (Continued)
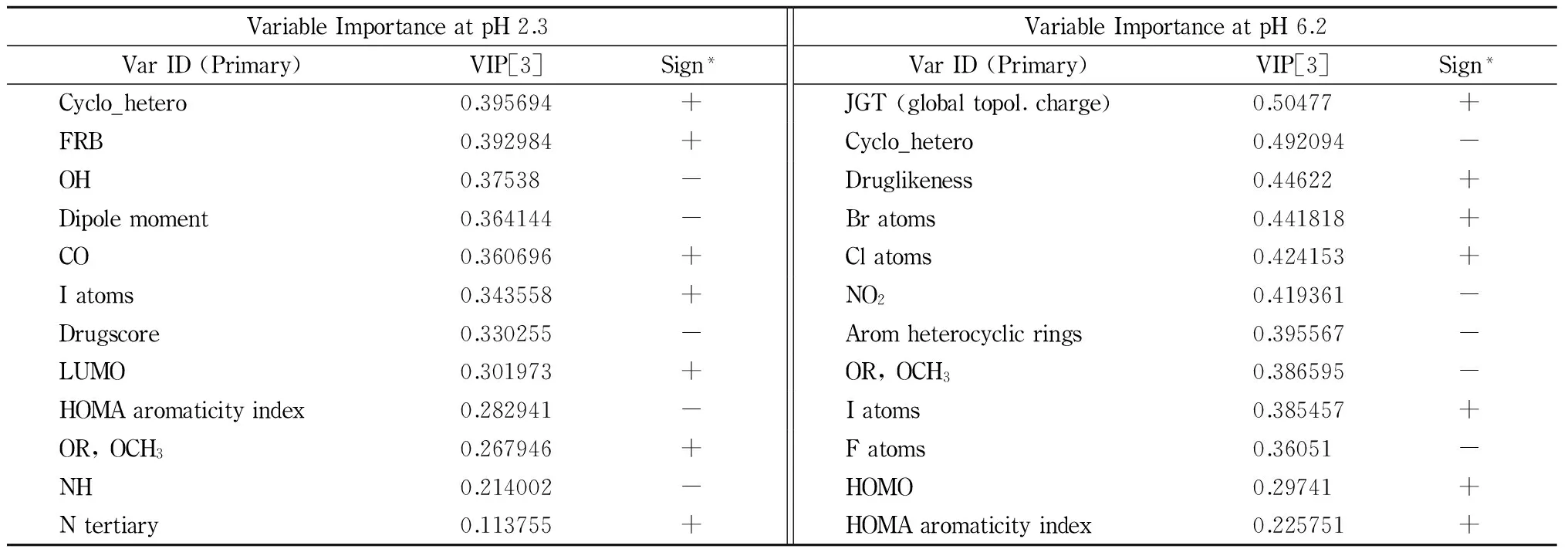
VariableImportanceatpH2.3VarID(Primary)VIP[3]Sign*VariableImportanceatpH6.2VarID(Primary)VIP[3]Sign*Cyclo_hetero0.395694+JGT(globaltopol.charge)0.50477+FRB0.392984+Cyclo_hetero0.492094-OH0.37538-Druglikeness0.44622+Dipolemoment0.364144-Bratoms0.441818+CO0.360696+Clatoms0.424153+Iatoms0.343558+NO20.419361-Drugscore0.330255-Aromheterocyclicrings0.395567-LUMO0.301973+OR,OCH30.386595-HOMAaromaticityindex0.282941-Iatoms0.385457+OR,OCH30.267946+Fatoms0.36051-NH0.214002-HOMO0.29741+Ntertiary0.113755+HOMAaromaticityindex0.225751+
* Positive (+) or negative (-) effect.
Molecular volume is the volume occupied by one mole, generally equal to the M. W. divided by the density. If we used the Van Deemter equation to explain the effect of this factor [4] then we would expect an opposite behavior, since small molecules can enter the stationary phase pores more easily. Therefore they can be retained more based on the stagnant mobile and stationary phase mass transfer mechanism. Nonetheless, it seems that the first term of the Van Deemter equation, which describes the transfer of compounds though central flow-streams, is more important and comes first in this process. According to the theory, sample molecules take different paths through the packed bed, depending on which flow-streams they follow. Therefore, analytes move faster in wide paths and slower in narrow paths, while molecules with high volume are generally impended to move. Additionally we should say that there is a cross-correlation between molecule’s volume, refractivity and polarizability. Molar refractivity (Rf) can be considered as the sum of either atom or bond retroactivities. This sum, which can be obtained directly from the compound’s structure, should equal the value given by the Lorenz-Lorenz equation when the measured values of density (d), M. W. and refractive index (μ) have been inserted [26]:
(1)
At the same time, molar refractivity is also defined as a measure of the total polarizability (p) of one mole of a substance and is given by the following equation [27]:
(2)
whereNA≈6.022×1023is the Avogadro Number.
Summarizing we could say that the volume is the factor of the dispersive interactions that occur between the analyte and the lipophilic stationary phase [28] and such interactions are also related to the refractivity index and polarizability. The lower the refractivity index or polarizability of a substance is, the weaker the dispersive interactions.
The information provided by the VIP values (Table 4) is in accordance with the data presented at Table 1. Thus, based on the retention time of the analytes, it is obvious that the presence of halogen atoms or an increase in the number of rings (all rings, benzene rings) cause a delayed elution (e. g. chlorobenzene ~20 min, chloronaphthalene ~37 min). The presence of halogen atoms seems to be crucial and its effect is more intense, especially at acidic conditions (Table 4, pH 2.3).
Moreover, it is important to mention that, in all PLS models developed, a parameter that has a negative effect (with varying intensity) on the retention time of a compound is the descriptorUi(unsaturation index). This happens due to the fact that Uiis an indirect indicator of a molecule to developπ-πbonds with the stationary phase. Hence, the negative effect is logical since butyl column does not have such ability, compared to other stationary phases (e. g. phenyl).
Moreover, the overall comparison of the VIP values of the total models (Table 4) showed that there are certain similarities as well as differences between the two pH studies. This happens because when all observations are included, their behavior is governed mainly by the principal rules that control retention. However, when the analytes are examined in groups the models compiled are satisfactory in terms of their statistical parameters but their corresponding VIP values are diverged depending on the pH. Thus, it was considered necessary to study the effect of the two pH values separately for all subgroups, since there are molecules that dissociate with different mechanism. The consequence of such influence is different in their elution time.
2.2 Chromatographic behavior
2.2.1 Group 1
Based on the retention time of the analytes, it is easy to say that there are molecules that do not appear to be affected by the change in the pH value. These compounds are molecules that lack acidic or basic moieties and they belong to Group 1. As shown in Table 1, their logDvalue is the same regardless the pH and it coincides with their logP. This behavior also imprinted in both VIP plots at pH 2.3 and 6.2 which is almost identical (Sup. Inf. 2; http://www.chrom-China.com/UserFiles/File/Supplementary%20File%20(sup.%20inf.).doc).
Comparing the VIP values of the Total group with those of Group 1, we can identify that the most important difference between them is that in the case of Group 1 there are descriptors structural characteristics and the parameters Ui and HOMA (harmonic oscillator model of aromaticity) that have a negligible effect on the retention time.
2.2.2 Group 2
The rest of the compounds (Group 2) have a diverse character and were separately evaluated (Group A, B, C) based on their ionization percentage, their logPand logDvalues, as well as the w*c[1] and VIP loadings’ plots. As mentioned earlier, distribution coefficient and related structural features prevail on the retention mechanism. An example of w*c[1] loadings’ plot shows that for Group A, logD2.3value overrides logD6.2when the pH of the mobile phase is 2.3 and vice versa (Fig. 2). In Group B, the behavior is opposite due to the presence of basic moieties.
Concerning the impact of -NH2or -COOH moieties in Group A, it is noteworthy that they both cause a decrease in the retention at pH 6.2. While the effect of acidic moieties is expected, the presence of amine groups (-NH2) which may coexist in some molecules of Group A, should not affect the retention. Of course this would be valid only if the pKof the basic moieties is lower than 6.2. However, in this case the pKvalues are >6.2 and therefore such groups are also ionized, decreasing the retention of an analyte on a lipophilic column. The same phenomenon but to a limited extent, due to lower pKvalues, is observed in the case of secondary amine groups (-NH-). On the other hand, at pH 2.3 the effect of -NH2or -COOH is the opposite, with the carboxylic group being partly responsible for the delayed elution of analytes. This observation concerning amine and carboxylic groups was opposite in Group B.
Concerning the presence of a sulfur atom in a compound, it was shown that it caused a decrease on the retention time on a lipophilic column and this effect was more intense in the models developed at pH 6.2. This happens because, in basic pH, a sulfur atom makes a molecule more acidic, facilitating its deprotonation (e. g. sulfonamides, alkyl-thiols, sulfonimides).
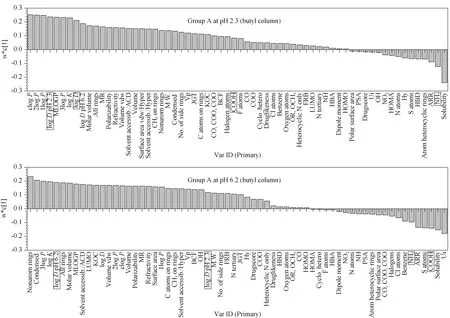
Fig. 2 w*c[1] scatter plot for Group A on butyl column
Apart from the compounds of Group 1, there are analytes in Group 2 that also have similar retention times at both mobile phases. However, major differences in the ionization percentage and behavior of some analytes, made it necessary to further split Group A into Subgroups A1, A2 and A3 and Group B into Subgroups B1, B2 and B3. Members of Subgroup A1 although bearing acidic groups are not ionized at pH 6.2 and behave as weak acids (pKa>9.5) since their ionization percentage is negligible. The fact that such molecules are practically not ionized was also verified chromatographically since there was only a slight shift in their retention time at different pH values which is of no importance (Sup. Inf. 4; http://www.chrom-China.com/UserFiles/File/Supplementary%20File%20(sup.%20inf.).doc). The logDvalues of Subgroup A1 and B1 at both pH values (2.3 and 6.2) are the same and they coincide with their logP. The above is a confirmation that the ionization percentage is <0.01% (Table 5).

Table 5 Analytes’ tr depending on pKa, ionization percentage and log P, log D values
On the other hand, molecules of Group A3 (e. g. thiabendazole) and B3 (e. g. flurbiprofen) are fully ionized (51.7%, by deprotonation or protonation respectively) at the corresponding pH values and change their retention times as shown in Table 2 (Sup. Inf. 5; http://www.chrom-China.com/UserFiles/File/Supplementary%20File%20(sup.%20inf.).doc).
The retention mechanism prevailing in the case of such molecules can be explained using as an example ibuprofen, which at pH 6.2 is found in fully ionized form. Ibuprofen is more hydrophobic in its neutral form while in its ionized it behaves as a hydrophilic molecule. Thus, the ionized ibuprofen has less interaction with a hydrophobic stationary phase since it becomes more susceptible to solvate with water molecules, primarily by the formation of hydrogen bonds (Sup. Inf. 6; http://www.chrom-China.com/UserFiles/File/Supplementary%20File%20(sup.%20inf.).doc). The effect of this property on the behavior of the analytes was indirectly studied using the PLS models via the descriptor solubility in water (solubility). Hence, according to VIP and w*c[1] scatter plot (Table 4), solubility is also an important factor determining the decrease in the retention time. Of course, apart from water, the organic modifier in the mobile phase can also participate in the analyte solvation in reversed phase chromatography. Though, this can only happen when the organic modifier is methanol (not acetonitrile).
On the contrary, analytes belonging to Group B3 and bearing basic groups (e. g. phenylephrine) in their molecules, are fully ionized and highly solvated at pH 2.3. Hence, the retention time of phenylephrine decreases at low pH. Yet, there are compounds such as isoniazid, nicotinamide, metronidazole whose logDvalues at pH 2.3 are different from their logP(Fig. 3). Differences in their logDvalues can be explained as these analytes have both basic and acidic moieties in their molecule (Group C). It is rather odd though that their retention time remained intact, probably because their ionization is insufficient (e. g. metronidazole) or false.
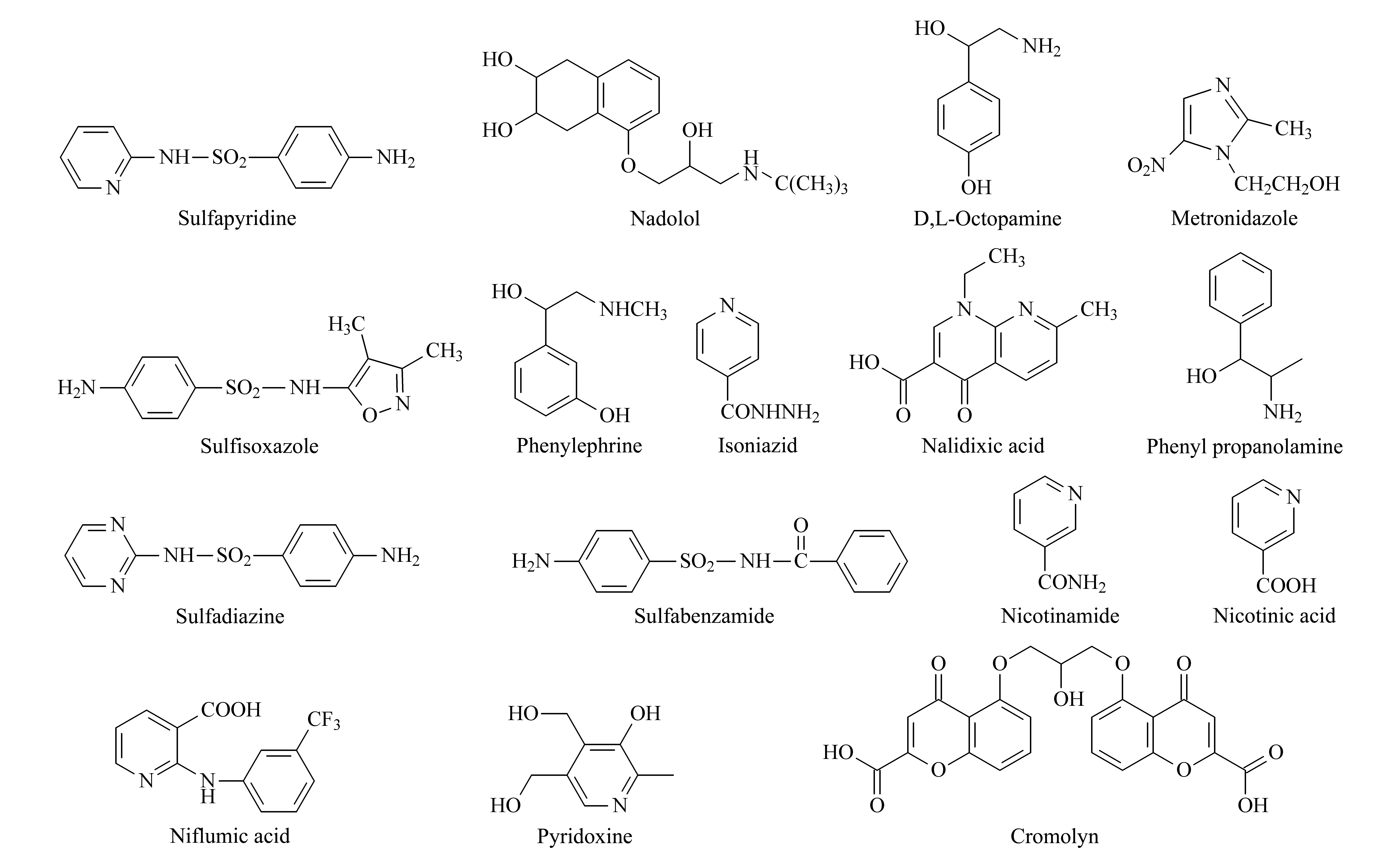
Fig. 3 Chemical structures of selected molecules bearing acidic and basic groups
Subgroup A2 includes analytes in which ionization of the acidic group at pH 6.2 ranges from 0.01% to 51.7%. Still, their ionization (pKa6.17-9.46) affects neither their retention nor their logPand logDvalues (Table 5). For example, the ionization percentage of the hydroxyl group ino- andp- nitrophenol is 20% and 10% respectively, but their retention times are slightly different. On the other hand there is a number of analytes as pyridoxine, sulfapyridine, sulfadiazine whose logPdoes not coincide with logD2.3. This can be explained due to the ionization of the coexisting basic group in the molecule although it is not confirmed by their retention time or due to false estimation of the logDvalue by the software.
Ionization of analytes that belong to Subgroup A3 is chromatographically verified by a decrease in the retention time. An exception to this rule is observed when the ionization percentage of the acidic group does not change at any of the pH values (e. g. ethamsylate).
In regard to the logD-logPrelations, logD6.2is always lower than the corresponding logD2.3(Table 5) and both of them are lower or equal to logP. Given the fact that ionization of the acidic moieties increases at pH 6.2 and therefore the ionized form of the analyte is distributed more easily in water (denominator’s increase) the decrease of logD6.2can be explained. However, the presence of a basic moiety in their molecule might interfere in the above relation (e. g. nicotinic acid, sulfisoxazole, sulfabenzamide, niflumic acid, nalidixic acid, cromolyn).
Concerning compounds containing at least one acidic moiety in their molecules the retention times (Table 5) at pH 2.3 and 6.2 are different only when their ionization percentage of the acidic group is ≥51.7% (pKa<6.17).
Based on ionization (%), almost 90% of the members of Group B have higher logDandtrvalues at pH 6.2 than at 2.3. This result is indeed expected for such compounds keeping in mind their pKavalues. Notwithstanding, even in the case of probes whose ionization percentage is >90% the retention time might not change (e. g. allopurinol).
Evaluation of the statistic coefficients proved that the correlation of the PLS model for Group B was improved (Sup. Inf. 1c; http://www.chrom-China.com/UserFiles/File/Supplementary%20File%20(sup.%20inf.).doc) when molecules with coexisting acidic groups were excluded. The corresponding loadings’ column plot verifies: a) the importance of logD2.3and the presence of an amino group at pH 2.3 and b) the importance of logD6.2and of the presence of a carboxylic group at pH 6.2 respectively. It is though important that based on the chromatographic behavior the retention time of these compounds is affected only when the ionization degree of the basic group(s) exceeds 58% (pKa>2.45) (Table 5).
Analytes that belong to Group C (Table 5) might ionize both their acidic and basic groups at pH 2.3 or 6.2. The ionization percentage which is responsible for the logDvalue is the criteria which will affect the chromatographic behavior depending on whether the acidic or basic character will prevail. For example based on the chromatographic behavior of nicotinic acid the acidic character prevails since itstrvalue at pH 2.3 is higher and considering its logDvalue, nicotinic acid should behave mostly as an acid. Another example is sulfisoxazole which, based on the logDvalues (0.92 at pH 2.3 and 1.04 at pH 6.2) could be considered to have basic character and be retained longer on the column. However, this behavior is not verified chromatographically. Faster elution of sulfixazole at pH 6.2 is justified based on the fact that the ionization degree of the acidic group (51.7%) is higher compared to the ionization degree of its basic group at pH 2.3 (35.9%). Consequently, since the ionization degree of the analyte is higher at pH 6.2, elution at this pH is faster. However, in the case of nalidixic acid, although the ionization percentage of the basic group is 88.8%, its retention time implies that it is the acidic character that prevails (ionization percentage 53.4%).
However, there are compounds whose behavior (tr) does not agree with their ionization degree (e. g. nicotinamide, isoniazid). Although the chromatographic behavior of phenylpropanolamine, phenylephrine, octopamine, nadolol, implies that their basic character is the predominant aspect, still the above seem to disagree with their ionization percentage and their logDand logPvalues; such results would require pKa≤6.3 for the basic groups of the analytes. Cromolyn, niflumic and nalidixic acid based ontrbehave as acids. Still, in order to come along with their ionization percentage the pKaof the basic group of nalidixic acid should be ≤2.1, the pKaof the basic group for niflumic acid should be ≤2.1 and for the acidic group ≥3, and the pKaof the two acidic groups for cromolyn should be ≥2.2. To end with, although sulfapyridine and nicotinamide should count among the compounds with basic character still, their retention time implies that they are not affected by the pH of the mobile phase.
3 Conclusions
The effect of pH variations in the retention in reversed phase chromatography is crucial especially in ionizable compounds. This is related not only to the fact that ionization of analytes is different but also due to the fact that solvation of neutral and ionized species occurs at a different extend in the mobile phase. Thus, their retention is determined by alteration of their affinity to the mobile and the stationary phase respectively. The behaviour of molecules with similar characteristics, like the presence of groups that are easily ionized is well characterized and described with accuracy by corresponding PLS models. Therefore, descriptors as lipohilicity, molar volume, refractivity, polarizability, increase their retention while on the other hand, polar surface area, solubility, HBD and hydrogen bond acceptor (HBA), favour faster elution.
Still, for a highly variegated gathering of compounds these descriptors are not prevailing. On the contrary, they work counterbalancing the effect of the general characteristics that determine the chromatographic behaviour. There lies the importance of this work since these models take into account numerous parameters describing a large number of analytes.
[1] Zisi C, Fasoula S, Pappa-Louisi A, et al. J Chromatogr A, 2013, 1314: 138
[2] Tan L C, Carr P W. J Chromatogr A, 1997, 775: 1
[3] Nikitas P, Pappa-Louisi A. J Chromatogr A, 2009, 1216: 1737
[4] Snyder L R, Kirkland J, Dolan J W. Introduction to Modern Liquid Chromatography. 3rd ed. New York: Willey Interscience, 2005
[5] Po H N, Senozan N M. J Chem Educ, 2001, 78: 1499
[6] Pellett J, Lukulay P, Mao Y, et al. J Chromatogr A, 2006, 1101: 122
[7] Buckenmaier S M, McCalley D V, Euerby M R. Anal Chem, 2002, 74: 4672
[8] Lewis J A, Lommen D C, Raddatz W D, et al. J Chromatogr A, 1992, 592: 183
[9] Wan Q H, Davies M C, Shaw P N, et al. Anal Chem, 1996, 68: 437
[10] Schoenmakers P J, Tijssen R. J Chromatogr A, 1993, 656: 577
[11] Marchand D H, Snyder L R, Dolan J W. J Chromatogr A, 2008, 1191: 1
[12] Baczek T, Kaliszan R, Novotna K, et al. J Chromatogr A, 2005, 1075: 109
[13] Mazza C B, Sukumar N, Breneman C M, et al. 2001, 73: 5457
[14] Euerby M R, Petersson P. J Chromatogr A, 2005, 1088: 1
[15] Buszewski B, Kowalska S, Kowalkowski T, et al, J Chromatogr B, 2007, 845: 253
[16] Visky D, Vander Heyden Y, Ivanyi T, et al. J Chromatogr A, 2003, 1012: 11
[17] Brereton R, Mccalley D. Analyst, 1998, 123, 1175
[18] Ivanyi T, Vander Heyden Y, Visky D, et al. J Chromatogr A, 2002, 954: 99
[19] Silva M F, Chipre L F, Raba J, et al. Chromatographia, 2001, 53, 392
[20] Markopoulou C K, Kouskoura M G, Koundourellis J E. J Sep Sci, 2011, 34: 1489
[21] Hansson G, Ahnoff M. J Chromatogr A, 1994, 666: 505
[22] Robertsson G, Andersson G, Kaufmann P. Chromatographia, 1998, 47: 643
[23] Montana M P, Pappano N B, Debatista N B, et al. Chromatographia, 2000, 51: 727
[24] Lippa K A, Sander L C, Wise S A. Anal Bioanal Chem, 2004, 378: 365
[25] Haaland D M, Thomas E V. Anal Chem, 1988, 60: 1193
[26] Glasstone S. Textbook of Physical Chemistry. New York: D. Van Nostrand Company, 1946
[27] Padrón J A, Carrasco R, Pellón R. J Pharm Pharm Sci, 2002, 5: 258
[28] Markopoulou C K, Watson D G, Tweedlie T, et al. Chromatogr, 2009, 70: 705
10.3724/SP.J.1123.2015.06037
O658 Document code: A Article IC:1000-8713(2015)12-1274-13
* Corresponding author. Tel:+30-2310-997665, Fax:+30-2310-997652, E-mail: amarkopo@pharm.auth.gr.
Received date: 2015-06-23
