溃疡性结肠炎患者外周血T细胞表面CD4+CD45+分子表达的变化及临床意义
2015-03-21李艳荣郭莲怡
李艳荣,郭莲怡
辽宁医学院附属第一医院,辽宁锦州 121001
溃疡性结肠炎患者外周血T细胞表面CD4+CD45+分子表达的变化及临床意义
李艳荣,郭莲怡
辽宁医学院附属第一医院,辽宁锦州 121001
目的探讨溃疡性结肠炎(ulcerative colitis,UC)患者外周血T细胞表面CD4+CD45+分子的表达及临床价值。方法选取2012年3月- 2013年12月于我院消化科住院治疗的UC患者80例,其中轻度活动期27例,中度活动期28例,重度活动期25例,健康对照组80例,用流式细胞仪分别检测各期患者及对照组外周血T细胞表面CD4+CD45+分子的百分含量,并进行比较以判断病情轻重。结果外周血CD4+CD45+T细胞表达含量,UC组为52.93%±3.64%,对照组为41.34%±2.94%,UC组高于对照组(t=-22.159,P<0.05);轻度活动期为50.99%±1.45%,中度活动期为52.66%±1.41%,重度活动期为55.18%±2.18%,中度活动期高于轻度活动期,重度活动期高于中度活动期,各组间比较,F=39.850(P<0.05)。结论外周血CD4+CD45+T细胞表达含量,UC组高于对照组,且随着病情加重而明显增高,对临床诊断及判断病情有指导意义。
溃疡性结肠炎;CD4+CD45+调节性T细胞;流式细胞仪;免疫调节
溃疡性结肠炎(ulcerative colitis,UC)是一种常见肠道炎症性疾病,病变主要累及黏膜和黏膜下层,多位于远端直肠和结肠,但可向近侧延伸,甚至遍布整个结肠,临床上表现为慢性病程,症状为腹泻或便秘,排黏液脓血便,里急后重,伴或不伴腹痛,反复发作。该病的病因和发病机制尚不十分明确[1]。目前认为,其发病是环境、遗传、感染、免疫、精神、肠道菌群及过敏等多种因素共同作用的结果,免疫失调是其发病的关键因素[2]。近年来发现,CD4+CD45+调节性T细胞(regulatory T cells,Tregs)在UC患者发病中起着重要作用[3]。本文研究80例UC患者外周血T细胞表面CD4+CD45+分子的表达,其范围为52.93%±3.64%,明显高于80例健康对照者的范围(41.34%±2.94%),为临床诊断提供了依据。
对象和方法
1 研究对象 随机选取2012年3月- 2013年12月于我院消化科住院治疗的活动期UC患者80例,其中男44例,女36例,年龄32 ~ 66(49±3)岁,诊断标准参照2012年中华医学会消化病学分会炎症性肠病学组于广州达成的我国炎症性肠病诊断与治疗的共识意见(2012年)[4],按照SutherlandDAI[5]分期,其中轻度活动期27例,中度活动期28例,重度活动期25例。健康对照组为正常的健康志愿者80例,其中男41例,女39例,年龄27 ~ 61(47±4)岁,无自身免疫性疾病病史,无家族遗传性疾病史,入选时无腹泻。两组年龄、性别差异有统计学意义。
2 仪器与试剂 人Ficoll淋巴细胞分离液购自上海新睿生物科技有限公司,FITC标记的抗人CD4、PE标记的抗人CD45抗体均购自上海裕平生物科技有限公司,RPMI1640培养液购自GibcoBRL公司,流式细胞仪FACScantoⅡ购自美国Becton-Dickinson(BD)公司。
3 方法 收集对照组受试者和UC组患者清晨空腹条件下静脉血各5 ml,采血管中加入肝素抗凝。血标本采集6 h内应用Ficoll密度梯度离心法,以1 500 r/min的速度离心分离出外周血单个核细胞(peripheral blood mononuelear cell,PBMC),经200目滤布滤过,制成单细胞悬液。用RPMI 1640完全培养基-2洗涤2次,将细胞浓度调整到1×105/ml,加入异硫氰酸荧光素(fluorescein isothiocyanate,FITC)标记的CD4抗体和PE标记的CD45抗体,常温孵育30 min,加入PBS液洗涤2次,速度为2 000 r/min,离心5 min后弃去上清液,准备上机。应用FACScantoⅡ流式细胞仪进行检测,用同型对照做阴性对照消除自发荧光,用空白对照消除非特异荧光,设门划出拟定的淋巴细胞群,根据设定的CD4与CD45单抗分析结合其阳性细胞数及百分比,在双参数图上得到CD4+CD45+细胞的百分比。
4 统计学分析 数据采用-x±s表示,应用SPSS 17.0统计软件处理,采用t检验,组间比较采用方差分析,P<0.05为差异有统计学意义。
结 果
1 外周血CD4+CD45+T细胞表达含量,UC组为52.93%±3.64%,对照组为41.34%±2.94%,UC组高于对照组(t=-22.159,P<0.05),为临床诊断提供了依据。见图1。
2 UC各活动期外周血CD4+CD45+T细胞表达含量UC组轻度活动期27例,中度活动期28例,重度活动期25例,轻度活动期为50.99%±1.45%,中度活动期为52.66%±1.41%,重度活动期为55.18%±2.18%,中度活动期高于轻度活动期,重度活动期高于中度活动期,各组间比较,F=39.850(P<0.05),随着病情加重,外周血CD4+CD45+T细胞表达含量明显升高,对判断病情有指导意义。见图2。

图 1 对照组与UC组外周血CD4+CD45+T细胞表达含量比较(aP<0.05, vs controls)Fig. 1 Comparison of peripheral blood CD4+CD45+T cells expression content between the control group and the UC group (aP<0.05, vs controls)
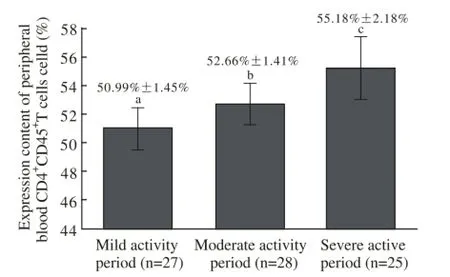
图 2 疾病各活动期患者外周血CD4+CD45+T细胞表达含量比较(aP<0.05,bP<0.05,cP<0.01, vs其他组)Fig. 2 Comparison of peripheral blood CD4+CD45+T cells expression content between different activity periods of diseaseaP<0.05,bP<0.05,cP<0.01, vs other activity periods
讨 论
溃疡性结肠炎是一种常见的直肠和结肠慢性炎症性疾病,国内外学者研究发现,肠道黏膜免疫系统的异常反应是其发病的重要因素,肠黏膜免疫系统失衡可导致免疫防御的破坏[6]。细菌或非细菌的抗原诱导激活肠道黏膜免疫细胞,并分化、成熟后移行到肠黏膜上皮细胞和固有层,产生免疫应答,抵御有害物质的入侵[7-8]。肠黏膜组织内淋巴细胞的异常激活,即为适应性免疫,适应性免疫由许多因素参与完成,其中T细胞是非常重要的免疫活性细胞[9]。
CD4+CD45+T细胞属于调节性T细胞,CD4+在抗原识别受体中能起到辅助作用,CD45+在多种细胞表面均有表达,比如血细胞、肿瘤细胞等,其主要表达于内皮细胞,影响造血干细胞分化和肿瘤细胞的产生及转移[10]。CD4+CD45+T细胞主要辅助于B细胞,其升高可导致B细胞的活化,诱导CD8介导的反应,出现免疫异常而致病[11-14]。
在UC患者中,患者的肠黏膜免疫系统受到有害物质的入侵,破坏免疫平衡,肠黏膜的第一道防线相关淋巴组织就会被激活,即产生免疫应答[15]。T细胞必然会参与免疫反应,调节免疫平衡[16]。T细胞表面分子CD4+CD45+在抗原诱导刺激下,表达活跃,分泌的细胞因子IL-4、IL-5、IL-6、IL-10、IL-13等会大量增加,加速免疫调节[13,17]。当抗原效应大于免疫调节功能时,即产生疾病[18]。
本研究通过流式细胞术分别对80例UC患者及80例健康人进行外周血T细胞CD4+CD45+表面分子检测,结果显示,UC组为52.93%±3.64%,对照组为41.34%±2.94%,比较其检出范围,UC组明显高于对照组,故在UC患者中外周血T细胞CD4+CD45+表面分子表达会异常增高,导致免疫失衡,引起疾病发生,为临床诊断UC提供依据。本研究中分别对UC组轻度活动期27例、中度活动期28例、重度活动期25例进行外周血T细胞CD4+CD45+表面分子检测,结果轻度活动期为50.99%±1.45%,中度活动期为52.66%±1.41%,重度活动期为55.18%±2.18%,随着病情加重明显升高,说明免疫失衡越重,疾病程度越严重,也就是说,UC患者外周血T细胞CD4+CD45+表面分子表达随着疾病严重程度增加而升高,可用于临床判断病情轻重。
综上所述,UC患者外周血T细胞CD4+CD45+表面分子含量高于正常人,即CD4+CD45+T细胞参与了免疫调节过程,且随着病情加重而明显增高,对临床诊断及判断病情轻重有一定指导意义。
1 Kamada N, Hisamatsu T, Okamoto S, et al. Unique CD14 intestinal macrophages contribute to the pathogenesis of Crohn disease via IL-23/IFN-gamma axis[J]. J Clin Invest, 2008, 118(6): 2269-2280.
2 Lord JD, Valliant-Saunders K, Hahn H, et al. Paradoxically increased FOXP3+ T cells in IBD do not preferentially express the isoform of FOXP3 lacking exon 2[J]. Dig Dis Sci, 2012, 57(11):2846-2855.
3 Furihata M, Sawada T, Okada T, et al. Total colectomy improves altered distribution of regulatory T cells in patients with ulcerative colitis[J]. World J Surg, 2006, 30(4): 590-597.
4 中华医学会消化病学分会炎症性肠病学组. 我国炎症性肠病诊断与治疗的共识意见(2012年·广州)[J]. 内科理论与实践,2013, (1): 61-75.
5 De Souza HS, West GA, Rebert N, et al. Increased levels of survivin, via association with heat shock protein 90, in mucosal T cells from patients with Crohn’s disease[J]. Gastroenterology,2012, 143(4): 1017-26.e9.
6 Dahlén R, Strid H, Lundgren A, et al. Infliximab inhibits activation and effector functions of peripheral blood T cells in vitro from patients with clinically active ulcerative colitis[J]. Scand J Immunol, 2013,78(3): 275-284.
7 毛靖伟,王英德.肠黏膜屏障在炎症性肠病中作用机制的研究进展[J].世界华人消化杂志,2010,18(7):695-698.
8 Liu L, Wang ZP, Xu CT, et al. Effects of rheum tanguticum polysaccharide on TNBS -induced colitis and CD4+T cells in rats[J]. World J Gastroenterol, 2003, 9(10): 2284-2288.
9 Radulovic K, Rossini V, Manta C, et al. The early activation marker CD69 regulates the expression of chemokines and CD4 T cell accumulation in intestine[J]. PLoS One, 2013, 8(6): e65413.
10 Liao CM, Zimmer MI, Shanmuganad S, et al. Dysregulation of CD1d-restricted type ii natural killer T cells leads to spontaneous development of colitis in mice[J]. Gastroenterology, 2012, 142(2):326-34.e1.
11 Dias AM, Dourado J, Lago P, et al. Dysregulation of T cell receptor N-glycosylation: a molecular mechanism involved in ulcerative colitis[J]. Hum Mol Genet, 2014, 23(9): 2416-2427.
12 Yamamoto-Furusho JK, Álvarez-León E, Fragoso JM, et al. Protective role of interleukin-19 gene polymorphisms in patients with ulcerative colitis[J]. Hum Immunol, 2011, 72(11):1029-1032.
13 Katsurada T, Kobayashi W, Tomaru U, et al. Decrease of peripheral and intestinal NKG2A-positive T cells in patients with ulcerative colitis[J]. PLoS One, 2012, 7(9): e44113.
14 Lee JC, Lyons PA, Mckinney EF, et al. Gene expression profiling of CD8+ T cells predicts prognosis in patients with Crohn disease and ulcerative colitis[J]. J Clin Invest, 2011, 121(10): 4170-4179.
15 Galitovskiy V, Qian J, Chernyavsky AI, et al. Cytokine-induced alterations of α7 nicotinic receptor in colonic CD4 T cells mediate dichotomous response to nicotine in murine models of Th1/Th17-versus Th2-mediated colitis[J]. J Immunol, 2011, 187(5):2677-2687.
16 Chao K, Zhong BH, Zhang SH, et al. Imbalance of CD4(+) T cell subgroups in ulcerative colitis[J]. Zhonghua Yi Xue Za Zhi, 2011,91(23): 1605-1608.
17 Bamias G, Jia LG, Cominelli F. The tumor necrosis factorlike cytokine 1A/death receptor 3 cytokine system in intestinal inflammation[J]. Curr Opin Gastroenterol, 2013, 29(6): 597-602.
18 Pilarczyk-Zurek M, Chmielarczyk A, Gosiewski T, et al. Possible role of Escherichia coli in propagation and perpetuation of chronic inflammation in ulcerative colitis[J]. BMC Gastroenterol, 2013,13:61.
Expression of peripheral blood CD4+CD45+T cells in patients with ulcerative colitis and its clinical value
LI Yanrong, GUO Lianyi
The First Aff i liated Hospital of Liaoning Medical University, Jinzhou 121001, Liaoning Province, China
GUO Lianyi. Email:angel_gly@163.com
Objective To explore the expression of peripheral blood CD4+CD45+T cells in patients with ulcerative colitis and its clinical value. Methods Eighty patients with UC in the First Aff i liated Hospital of Liaoning Medical University from March 2012 to December 2013 were selected, with mildly active in 27 cases, moderately active in 28 cases and severe active in 25 cases. There were also 80 cases in healthy control group. The fl ow cytometry was used to detect the percentage of molecules in peripheral blood T cell surface CD4+CD45+of patients. And then the percentage of the content was compared to determine the level of severity of illness. Results The expression of CD4+CD45+T cells in peripheral blood was signif i cantly higher than that of control group (52.93%±3.64% in UC group and 41.34%±2.94% in the control group, t=-22.159, P<0.05). The expression of CD4+CD45+T cells in peripheral blood in mild activity period was 50.99%±1.45%, moderate activity period was 52.66%±1.41%, severe active stage was 55.18%±2.18%, which suggested that the moderate activity period was higher than the mild activity period, and the severe active stage was higher than the moderate activity period. Comparison between groups were statistically signif i cant with F=39.850, P<0.05. Conclusion The expression level of peripheral blood CD4+CD45+T cells in UC group is higher than that of control group. With the aggravation of disease, the peripheral blood CD4+CD45+T cells increases signif i cantly, which has guiding sense of clinical diagnosis.
ulcerative colitis; CD4+CD45+regulatory T cells; fl ow cytometry; immune regulation
R574.1
A
2095-5227(2015)02-0127-03
10.3969/j.issn.2095-5227.2015.02.009
时间:2014-11-13 17:17
http://www.cnki.net/kcms/detail/11.3275.R.20141113.1717.003.html
2014-07-07
李艳荣,女,硕士,主治医师。研究方向:溃疡性结肠炎发病机制。Email: 416413064@qq.com
郭莲怡,女,博士,主任医师,主任。Email: angel_gly@ 163.com
