C2对称手性四氮配体的合成、结构及催化苯乙酮不对称氢转移反应研究
2015-03-20俞黄琴刘训高
俞黄琴,刘训高,沈 良
(杭州师范大学材料与化学化工学院,浙江 杭州310036)
由于催化反应易操作及氢源易得的优点,酮的不对称转移氢化已成为手性仲醇的一种高效合成方法[1-3].日本化学家野依良治的TsDPEN 是不对称转移氢化反应中一个非常重要的配体,表现出了优异的对映选择性和催化活性[4-6].
近年来,手性氮杂配体备受关注,因为它们不仅价格便宜、合成简单,而且比磷烷配体在空气中更加稳定[7].此外,可以通过结构修饰来调控配体及其金属配合物的空间效应和电子效应[8].手性氮杂配体已成功用作许多不对称催化反应的手性辅助剂,如不对称烯丙基烷基化反应[9-10]、不对称硅氢化反应[11-12]、不对称硅腈化反应[13-14]以及二烷基化反应[15-16].然而,在用于潜手性酮的不对称转移氢化时,催化性能却表现得并不令人满意[17-18].本文报道4 个手性四氮配体的合成、表征及在异丙醇/KOH 体系中与[Ru(p-cymene)Cl2]2原位催化苯乙酮的不对称氢转移反应,获得了较好的效果.
1 实验部分
1.1 主要仪器
所使用试剂和原料如无特殊说明,均为购置无需进一步处理.柱层析硅胶(200~300目,青岛海洋化工厂分厂),硅胶板(厚度:0.20~0.25 mm,青岛海洋化工厂分厂).X-5熔点仪器(上海精细科学仪器有限公司)、薄层层析用紫外灯(254 nm)、旋光仪(Perkin Elmer 341)、红外光谱仪(KBr压片,Bruker Tensor 27)、核磁共振仪(TMS 内标,Brucker公司,400 MHz)、质谱仪(Agilent 5979)、气相色谱仪(Shimadzu GC-2014)、高效液相色谱法(Elite P230,大赛璐CHIRALCEL OZ-H 柱).
1.2 手性四氮配体(2a-d)的制备
如图1,按文献方法[19]合成(1S,2S)-(+)-N-对甲苯磺酰基-1,2-二苯基乙二胺[(S,S)-TsDPEN].白色固体,熔点:123.2~126.9 ℃=+67.5°(c 0.4,CH2Cl2);Anal.Calcd.(%)for C21H22N2SO2:C,68.82;H,6.05;N,7.64%;Found(%):C,68.41;H,6.287;N,7.519%.Selected IR,ν(cm-1):3344.19,3286.73(N—H),3084.91,2863.55,1595.07,1492.11,1455.14(benzene C=C),1329.21(—SO2—),1154.95,1085.24,1055.13,810.85,768.70,699.07.1H NMR(CDCl3,400 MHz),δ(ppm):7.320,7.300[d,2H,J=8.0 Hz],7.164~7.128[m,10H],6.979,6.959[d,2H,J=8.0 Hz],4.399,4.386[d,2 H,J=5.2 Hz],4.166,4.153[d,2 H,J=5.2 Hz],2.318[s,3H,-CH3],EI-MSm/z:367[M+H]+,260,155,106,91,79,65,51.

图1 C2对称手性四氮配体(2a-d)的合成Fig.1 The synthesis of C2-symmetric chiral tetraaza ligands(2a-d)
1.2.1 (S,S,S,S)-(+)-N,N′-2[2-(对-甲苯磺酰基)氨基-1,2-二苯基乙基]乙二胺(2a)的制备
将(S,S)-TsDPEN(366.5 mg,1.0 mmol)和1,2-二溴乙烷(94.0 mg,0.5 mmol)置于一个密封玻璃容器中,加热至130 ℃,反应过夜.将粗产物溶于二氯甲烷(15 m L)中,并用20%NaOH 溶液(20 m L)洗涤.加入NaOH 溶液,沉淀出白色固体.该化合物用二氯甲烷(3×20 m L)萃取,Na2SO4干燥,蒸发溶剂得到粗产物.粗产物用硅胶柱层析(33%→67%v/v乙酸乙酯己烷)纯化,得到产物为白色固体.m.p:167.5~169.2 ℃;=+15.97°(c 0.4,CH2Cl2);Anal.Calcd.(%)for C44H46N4S2O4:C,69.63;H,6.11;N,7.38%;Found(%):C,69.28;H,6.14;N,7.36%.Selected IR,υ(cm-1):3327.45,3030.49,2919.17,1598.86,1494.51,1452.05,1329.45,811.52,773.01,770.03,672.90.1H NMR(CDCl3,400 MHz),δ(ppm):7.453,7.433[d,4 H,J=8.0 Hz],7.149~6.978[m,20H],6.855,6.835[d,4 H,J=8.0 Hz],4.424,4.403[d,2H,J=8.4 Hz],3.751,3.730[d,2 H,J=8.4 Hz],2.616,2.596[d,2 H,J=8.0 Hz],2.333,2.313[d,2H,J=8.0 Hz],2.312[s,6H].EI-MSm/z:759[M+H]+,498,379,350,327,294,260,237,196,155.
1.2.2 (S,S,S,S)-(+)-N,N′-2[2-(对-甲苯磺酰基)氨基-1,2-二苯基乙基]丙二胺(2b)的制备
将(S,S)-TsDPEN(366.5 mg,1.0 mmol)和1,3-二溴丙烷(101.0 mg,0.5 mmol)置于一个密封玻璃容器中,加热至130 ℃,反应过夜.将粗产物溶于二氯甲烷(15 m L)中,并用20%NaOH 溶液(20 m L)洗涤.加入NaOH 溶液,沉淀出白色固体.该化合物用二氯甲烷(3×20 m L)萃取,Na2SO4干燥,旋转蒸干得到粗产物.粗产物用硅胶柱层析(25%→50%v/v乙酸乙酯己烷)纯化,得到产物为白色固体.m.p:75.2~76.8 ℃;=+18.63°(c 0.4,CH2Cl2);Anal.Calcd.(%)for C45H48N4S2O4:C,69.95;H,6.22;N,7.25%;Found(%):C,70.23;H,6.48;N,6.7%.Selected IR,υ(cm-1):3260.01,3029.81,2924.02,1599.23,1494.55,1454.77,1326.44,1158.68,1092.69,812.62,767.73,699.95,668.38.1H NMR(CDCl3,400 MHz),δ(ppm):7.435,7.415[d,4 H,J=8.0 Hz],7.073~6.844[m,20H],6.781,6.761[d,4 H,J=8.0 Hz],4.375,4.352[d,2H,J=7.2 Hz],3.824,3.812[d,2 H,J=4.8 Hz],2.696[m,2 H],2.561[m,2 H],2.253[s,6 H],1.667[m,2 H].EI-MSm/z:773[M+H]+,512,393,350,251,196,155.
1.2.3 (S,S,S,S)-(+)-N,N′-2[2-(对-甲苯磺酰基)氨基-1,2-二苯基乙基]-1,3-苯二甲胺(2c)的制备
(S,S)-TsDPEN(366.5 mg,1.0 mmol)和间-二溴甲基苯(132.0 mg,0.5 mmol)置于一个密封玻璃容器中,并加热至130 ℃过夜.将粗产物溶于二氯甲烷(15 m L)中,并用20%NaOH 溶液(20 m L)洗涤.加入NaOH 溶液,沉淀出白色固体.该化合物用二氯甲烷(3×20 m L)萃取,Na2SO4干燥,蒸发溶剂得到粗产物.粗产物用硅胶柱色谱法(30%→60%v/v乙酸乙酯/己烷)纯化,得到产物为白色固体.m.p:139.9~141.5 ℃;=+61.62°(c 0.4,CH2Cl2);Anal.Calcd.(%)for C50H50N4S2O4:C,71.94;H,5.99;N,6.71%;Found(%):C,71.39;H,6.081;N,6.464%.Selected IR,υ(cm-1):3248.73,3059.78,3027.73,2926.55,1736.29,1599.61,1492.76,1454.45,1333.33,1184.42,806.52,699.53,670.73.1H NMR(CDCl3,400 MHz),δ(ppm):7.391,7.370[d,4 H,J=8.4 Hz],7.158~7.143[m,8 H],7.048~7.003[m,8 H],6.976~6.942[m,8 H],6.861,6.840[d,4 H,J=8.4 Hz],4.341,4.321[d,2H,J=8.0 Hz],3.789,3.769[d,2H,J=8.0 Hz],3.665,3.632[d,2H,J=13.2 Hz],2.433,2.300[d,2H,J=13.2 Hz],2.283[s,6H].EI-MSm/z:574,379,313,290,260,235,209,155,130,105,65.1.2.4 (R,R,S,S,S,S)-1,3-二[4,5-二苯基-1-(对甲苯磺酰基)-2-咪唑烷]苯(2d)的制备
(S,S)-TsDPEN(366.5 mg,1.0 mmol),1,3-苯二甲醛(67 mg,0.5 mmol)和2-丙醇(8 m L)置于圆底烧瓶中.将反应混合物加热回流并搅拌直至溶液中出现白色固体,并通过过滤分离,用热乙醇洗涤并真空干燥.乙醇中挥发析出白色晶体(R,R,S,S,S,S)-2d,适于用X 射线进行衍射.m.p:178.7~179.5℃;=+117.83°(c 0.4,CH2Cl2);Selected IR,υ(cm-1):3291.33,3027.58,2919.59,1598.64,1492.72,1449.35,347.82,1167.60,1091.72,1044.48,770.56,699.72.1H NMR(CDCl3,400 MHz),δ(ppm):8.333[s,1H],7.976~7.953[m,1H],7.868~7.887[m,2H],7.751,7.730[d,4 H,J=8.4 Hz],7.227~7.172[m,12H],6.981~6.971[m,8 H],6.895,6.874[d,4 H,J=8.4 Hz],4.584,4.565[d,2 H,J=7.6 Hz],4.214,4.195[d,2 H,J=7.6 Hz],2.389[s,6 H,-CH3].EI-MSm/z:416,377,284,260,207,179,155,91,65,50.
1.3 晶体结构测定
选取尺寸为0.49 mm×0.28 mm×0.17 mm 的单晶(2d),将其置于Rigaku RAXIS-RAPID射线衍射仪上,采用石墨单色化的MoKα射线(λ=0.71073Å),在296(1)K,以ω扫描方式,在(2θ)max=54.8°的范围内收集到8049个独立衍射点,其中可观测衍射点[I>2σ(I)]4477个,这些数据用于结构解析和修正.数据经Lp和经验吸收校正.晶体结构解析用SHELX 97程序[20],用直接法求得结构参数,经全矩阵最小二乘法修正.所有非氢原子采用各向异性热参数修正,氢原子理论加氢.各向异性的修正包括所有非氢原子收敛于一致的因子R=0.0518,Rw=0.1096,最终差值密度图上最高峰为0.253 e·Å-3.(R,R,S,S,S,S)-2d的晶体学数据见表1.
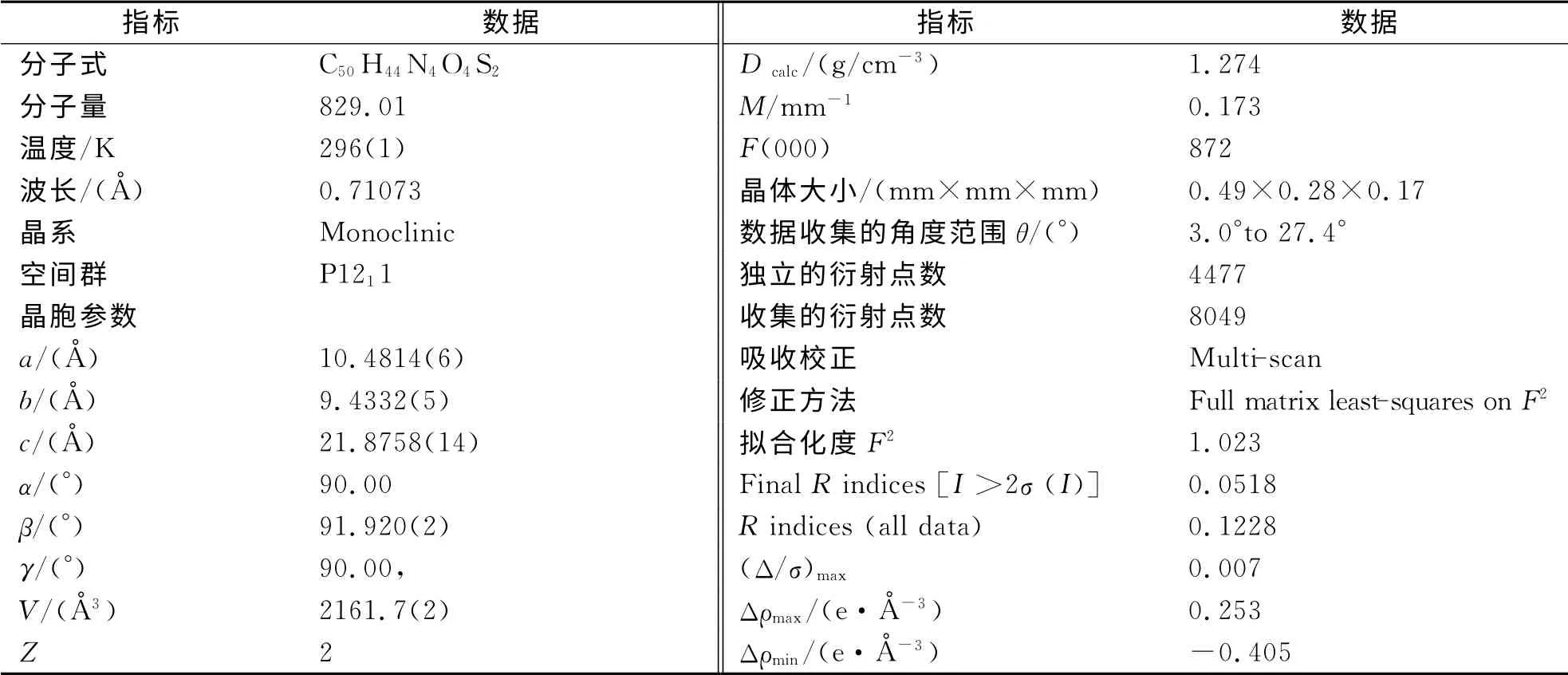
表1 (R,R,S,S,S,S)-2d的晶体学数据Tab.1 The crystallographic data of(R,R,S,S,S,S)-2d
1.4 苯乙酮的不对称氢转移反应研究
将金属前体[Ru(p-cymene)Cl2]2(0.02 mmol)和手性配体2a-2d(0.042 mmol)加入到新蒸馏的2-丙醇中,在氮气保护下80℃搅拌2 h.加入氢氧化钾的2-丙醇溶液,继续搅拌3 h.然后加入苯乙酮(4 mmol),在给定温度时间下进行(TCL监测).反应完成后,用氯化钠的饱和水溶液洗涤,用HCl溶液(C=2 mmol/L)中和,用Et2O(3×10 m L)萃取.将有机相合并,用无水硫酸钠干燥,过滤,减压浓缩,并通过快速柱层析纯化.用HPLC法测定对映体过量(ee),GC测定转化率[21-22].催化反应的转化率和ee值数据列于表2.

表2 在异丙醇的KOH 体系中,用Ru(II)-配体(2a-d)原位催化苯乙酮不对称氢转移反应的转化率和ee值Tab.2 The conversations and ee values of Ru(II)-Ligand(2a-2d)catalysts for the asymmetric transfer hydrogenation of acetophenone by 2-propanol in the presence of KOH
反应条件:催化剂,2.0×10-5mol;配体,4.4×10-5mol;苯乙酮,4.0×10-3mol;IPA,25 m L S/M/L/base(S=Substrate,M=Metal,L=Ligand,base=KOH).
采用气相色谱法测定转化率;S和R 构型由高效液相色谱法确定.
2 结果与讨论
2.1 结构描述

图1 (R,R,S,S,S,S)-2d的分子结构Fig.1 Molecular structure of(R,R,S,S,S,S)-2d
如图2所示,配体(R,R,S,S,S,S)-2d包含6个手性中心.在合成反应中,形成2个咪唑环和2个R 构型的手性中心.此外,TsDPEN 的S构型在反应中并没有发生变化.
咪唑环的C9—C10和C30—C31键长分别为1.561(6)和1.548(6)Å,明显长于C—Cσ单键键长[1.498(6)~1.530(4)Å].而咪唑环的C—N 键长为1.454(5)~1.512(5)Å,S1—N1 和S2—N3的键长分别为1.629(3)和1.626(3)Å.S—O 平均距离为1.427(4)Å,最小的N—S—O 键角为105.6(2)°,最大N—S—O 键角为107.0(2)°,与文献[23]中报道的N—S—O 键角比较接近.
2.2 催化研究
2.2.1 配体对催化活性的影响
根据表2的实验结果,催化产物1-苯乙醇的绝对构型高度依赖于手性配体的构型.在本实验条件下,当手性配体为(S)构型对映体时,可以获得(R)构型的(R)-1-苯乙醇对映体.
手性配体2a-2d均表现出良好的催化活性(转化率),但与配体2a-2c相比,2d显示出明显高的对映选择性(ee值)(编号4,10,13,16).结合晶体结构,我们认为配体2d中两个新生成的手性中心在对映体过量中发挥了重要作用,而配体2a-2c中C—C单键的存在减小了分子骨架的刚性,从而导致催化反应对映选择性的降低,这个结果与Noyori提出的金属-配体-底物形成的六元环过渡态理论相一致[24].此外,配体2b比配体2a和2c表现出较高的对映体性(编号5,12),我们推测,活性中心间的距离可能会影响催化反应的对映选择性[25].
2.2.2 碱量对催化活性的影响
催化剂与碱的量比对不对称氢转移反应的催化活性有非常重要的影响,同时碱量对对映选择性也有重要的影响[26].对于2d配体,很少的碱量就能获得较高的ee值和转化率(编号16-18).对于配体2a-2c,转化率随着碱量的增加而缓慢下降,ee值在底物/金属/配体/碱(S/M/L/Base)比为100∶1∶1.1∶25时给出了最好的结果(编号10-12,13-15).
2.2.3 温度对催化活性的影响
根据表2数据,反应温度从60 ℃到80 ℃时,转化率和ee值分别明显增加(编号1-3,7-9).温度对催化反应的研究结果表明,本催化体系中苯乙酮不对称转移氢化的合适温度为80 ℃.
3 结论
本文首次以C2对称手性四氮配体(2a-d)和[RuCl2(p-cymene)]2为催化剂,原位催化苯乙酮的不对称氢转移.这些配体的制备简单易行,在苯乙酮的不对称氢化中表现出较好的催化活性,催化反应的转化率最高达98%,对映选择性最高达60%.
[1]Furii A,Hashiguchi S,Uematsu N,etal.Ruthenium(II)-catalyzed asymmetric transfer hydrogenation of ketones using a formic acid-triethylamine mixture[J].J Am Chem Soc,1996,118(10):2521-2522.
[2]Murata K,Ikariya T.New chiral rhodium and iridium complexes with chiral diamine ligands for asymmetric transfer hydrogenation of aromatic ketones[J].J Org Chem,1999,64(8):2186-2187.
[3]Ikariya T,Blacker A J.Asymmetric transfer hydrogenation of ketones with bifunctional transition metal-based molecular catalysts[J].Acc Chem Res,2007,40(12):1300-1308.
[4]Hashiguchi S,Fujii A,Takehara J,etal.Asymmetric transfer hydrogenation of aromatic ketones catalyzed by chiral ruthenium(II)complexes[J].J Am Chem Soc,1995,117(28):7562-7563.
[5]Everaere K,Mortreux A,Bulliard M,etal.β-Amino alcohol(arene)ruthenium(II)-catalyzed asymmetric transfer hydrogenation of functionalized ketones-scope,isolation of the catalytic intermediates,and deactivation processes[J].Eur J Org Chem,2001,2001(2):275-291.
[6]Mikhailine A,Lough A J,Morris R H.Efficient asymmetric transfer hydrogenation of ketones catalyzed by an iron complex containing a P-N-N-P tetradentate ligand formed by template synthesis[J].J Am Chem Soc,2009,131(4):1394-1395.
[7]Fonseca M H,Konig B.Chiral tetraaza ligands in asymmetric catalysis:recent progress[J].Adv Synth Catal,2003,345(11):1173-1185.
[8]Haung H,Okuno T,Tsuda K,etal.Enantioselective hydrogenation of aromatic ketones catalyzed by Ru Complexes of Goodwin-Lionstype sp2N/sp3N hybrid ligands R-BINAN-R-Py[J].J Am Chem Soc,2006,128(27):8716-8717.
[9]Albano V G,Bandini M,Monari M,etal.Synthesis and crystallographic characterization of chiral bis-oxazoline-amides fine-tunable ligands for Pd-catalyzed asymmetric alkylations[J].J Org Chem,2006,71(17):6451-6458.
[10]Trost B M,Dogra K,Hachiya I,etal.Designed ligands as probes for the catalytic binding mode in Mo-catalyzed asymmetric allylic alkylation[J].Angew Chem Int Ed,2002,41(21):1929-1932.
[11]Nishiyama H,Yamaguchi S,Park S B,etal.New chiral bis(oxazolinyl)bipyridine ligand(bipymox):enantioselection in the asymmetric hydrosilylation of ketones[J].Tetrahedron:Asymmetry,1993,4(1):143-150.
[12]Lee S G,Lim C W,Song C E,etal.Synthesis of new 2-symmetric bioxazoles and application as chiral ligands in asymmetric hydrosilylation[J].Tetrahedron:Asymmetry,1997,8(17):2927-2932.
[13]Liu Y L,Liu X H,Xin J G,etal.Asymmetric cyanosilylation of aldehydes catalyzed by novel chiral tetraaza-titanium complexes[J].Synlet,2006,7:1085-1089.
[14]Xiong Y,Huang X,Gou S H,etal.Enantioselective cyanosilylation of ketones catalyzed by a nitrogen-containing bifunctional catalyst[J].Adv Synth Catal,2006,348(4/5):538-544.
[15]Dangel B D,Polt R.Catalysis by amino acid-derived tetracoordinate complexes:enantioselective addition of dialkylzincs to aliphatic and aromatic aldehydes[J].Org Lett,2000,2(19):3003-3006.
[16]Pastor I M,Adolfsson H.Novel highly modular C2-symmetric oxazoline ligands—application in titanium-catalyzed diethylzinc additions to aldehydes[J].Tetrahedron Lett,2002,43(9):1743-1746.
[17]Marson C M,Schwarz I.Amide catalysts with tetradentate ligands and the asymmetric transfer hydrogenation of carbonyl compounds[J].Tetrahedron Lett,2000,41(46):8999-9003.
[18]Shen W Y,Zhang H,Zhang H L,etal.Novel chiral tetraaza ligands:synthesis and application in asymmetric transfer hydrogenation of ketones[J].Tetrahedron:Asymmetry,2007,18(6):729-733.
[19]Xue D,Chen Y C,Cui X,etal.Transfer hydrogenation of activated C=C bonds catalyzed by ruthenium amido complexes:reaction scope,limitation,and enantioselectivity[J].J Org Chem,2005,70(9):3584-3591.
[20]Sheldrick G M.SHELXS-97,program for the solution of crystal structures[M].Göttingen:University of Göttingen,1997.
[21]He W,Liu P,Zhang B L,etal.Effcient iridium and rhodium-catalyzed asymmetric transfer hydrogenation using 9-amino(9-deoxy)cinchona alkaloids as chiral ligands[J].Appl Organometal Chem,2006,20(5):328-334.
[22]Shen Y S,Chen Q,Lou L L,etal.Asymmetric transfer hydrogenation of aromatic ketones catalyzed by SBA-15 supported Ir(I)complex under mild conditions[J].Cata Lett,2010,137(1):104-109.
[23]Pritchett S,Gantzel P,Walsh P J.Synthesis and structural study of titanium bis(sulfonamido)bis(amide)complexes[J].Organometallics,1999,18(5):823-831.
[24]Yamakawa M,Ito H,Noyori R.The metal-ligand bifunctional catalysis:a theoretical study on the ruthenium(II)-catalyzed hydrogen transfer between alcohols and carbonyl compounds[J].J Am Chem Soc,2000,122(7):1466-1478.
[25]Cortez N A,Aguirre G,Hake M P,etal.New heterogenized C2-symmetric bis(sulfonamide)cyclohexane-1,2-diamine-RhIIICp*complexes and their application in the asymmetric transfer hydrogenation(ATH)of ketones in water[J].Tetrahedron Lett,2009,50(19):2228-2231.
[26]Diez C,Nagel U.Chiral iridium(I)bis(NHC)complexes as catalysts for asymmetric transfer hydrogenation[J].Appl Organometal Chem,2010,24(7):509-516.
