贝达喹啉治疗结核病的研究进展
2015-03-17黎友伦
彭 蔚,黎友伦,彭 丽
贝达喹啉治疗结核病的研究进展
彭 蔚,黎友伦,彭 丽
结核病依然是全球重大公共卫生问题。耐多药和广泛耐药结核病的出现与蔓延是结核病控制面临的一大挑战。目前WHO推荐用于治疗耐药结核病的化疗药物存在种类少、疗效欠佳、不良反应多、疗程长等不足,因此,开发新型作用机制的抗结核药势在必行。贝达喹啉,靶点为分枝杆菌ATP合成酶,具有作用机制新颖、耐药率、半衰期长、特异性高、抗菌活性强、缩短耐多药结核病疗程,安全性良好等优点,但是同时也存在难以解释的高致死率等缺陷。本文主要从抗菌及耐药机制、药代动力学、抗菌活性、临床试验、安全性方面综述贝达喹啉治疗结核病的研究进展。
结核病;贝达喹啉;耐药结核病
WHO在2013结核病报告中指出2012年约860万结核病例,而由结核病引起的死亡人数达130万。抗结核资源有限、DOTS策略执行不当及病人依从性差等是结核病控制的屏障。自1963年以来,几乎没有新的抗结核药物用于结核病治疗。贝达喹啉(bedaquiline,BDQ)[1-3],又称TMC207、R207910、J,商品名:斯耐瑞(Sirturo),作为联合方案中的一部分,用于其他治疗手段无效的成人耐多药肺结核(multidrug resistance pulmonary tuberculosis,MDR-PTB),在我国也适用于成人(前期)广泛耐药肺结核(pre-)extensively drug resistance pulmonary tuberculosis , (pre-)XDR-PTB),是到目前为止最有前途的抗结核病新药之一。本文就近年来国内外贝达喹啉治疗结核病的研究进展作一综述。
1 作用机制
贝达喹啉属于二芳基喹啉类化合物,与分枝杆菌ATP合成酶c亚基结合,影响ATP合成酶质子泵生物学功能,导致ATP耗竭和内环境稳态失衡,从而达到抑菌和(或)杀菌效果[4]。
2 耐药机制
体外研究提示贝达喹啉致结核分枝杆菌(Mycobacterium tuberculosis,MTB)耐药率为1×10-7~1×10-8[4]。
2.1 基因突变 Petrella等[5]指出MTB编码ATP合成酶c亚基的atpE基因Ala63Pro(A63P)和(或)Ile66Met(I66M)点突变阻止其与贝达喹啉结合,导致MTB天然或获得性耐药。
2.2 外排泵 Gupta等[6]指出外排泵抑制剂异搏定使贝达喹啉针对MTB的最低抑菌浓度(minimal inhibitory concentration,MIC)降低8-16倍。Andries等[7]指出MTB MmpS5-MmpL5外排泵过表达使贝达喹啉针对MTB的MIC增加4倍,而异搏定使贝达喹啉针对MTB的MIC降低4倍。
2.3 交叉耐药 体内研究未发现贝达喹啉与异烟肼(isoniazid,INH)、利福平(rifampicin,RIF)、吡嗪酰胺(pyrazinamide,PZA)、阿米卡星(amikacin,AMK)、莫西沙星(moxifloxacin,Mfx)等发生交叉耐药[8]。贝达喹啉与氯法齐明(clofazimine,Cfz)存在交叉耐药,Andries等[7]指出其可能与两者均是MmpL5外排泵的底物有关。
3 药代动力学
贝达喹啉口服吸收良好,与食物同食时生物利用度是空腹的2倍,5 h达到血药浓度峰值,与人血浆蛋白结合率超过99.9%,血浆半衰期173小时,组织中分布广泛,稳态时分布容积超过10 000 L,清除率低,终末消除半衰期达5.5月[8,9]。贝达喹啉主要经CYP3A4,部分经CYP2C8、CYP2C19脱甲基化形成代谢产物1~8(metabolites 1~8,M1~8),其中最主要的是去甲基产物M2。M2生物活性仅为贝达喹啉的1/3~1/6,但具有更强细胞毒性且更易形成药物诱导的磷脂质病(drug-induced phospholipidosis,DIP)[9]。贝达喹啉及代谢产物绝大部分经粪便排泄,只有1%~4%经尿液排出[8]。贝达喹啉药代动力学与年龄、性别、体重、种族、是否合并HIV感染无关[8]。贝达喹啉治疗成人MDR-PTB的给药方法和剂量为与食物同服,400 mg每天1次共2周接着200 mg每周3次共22周[8]。
4 抗菌活性
4.1 体外抗菌活性 Nacer等[10]在3种不同培养基条件下检测到贝达喹啉针对增殖期MTB 标准株的MIC值分别为:7H11琼脂培养基--MIC=0.03 μg/ml,接种5%牛血清蛋白的7H1l琼脂培养基—MIC=1 μg/ml,改良罗氏培养基--MIC=14.33 μg/ml。贝达喹啉对体外增殖期药敏(drug-susceptible,DS)和耐多药(multidrug-resistant,MDR)MTB表现出几乎同等强度的抗菌活性[4,11]。Koul等[12]指出贝达喹啉作用于静止期MTB同样有效,且静止期生物活性更强,可能与静止期代谢缓慢有关。贝达喹啉对大多数非结核分枝杆菌也有效,但至少有蟾分枝杆菌(Mycobacteriumxenopi)、石氏分枝杆菌(Mycobacteriumshimoidei)和新卡城分枝杆菌(Mycobacteriumnovocastrense)天然耐药[4,11]。贝达喹啉具有分枝杆菌特异性,对其他革兰阳性和革兰阴性菌无效[4]。贝达喹啉的抗菌谱及MIC值见表1[2,4,11]。
4.2 体内抗菌活性 在小鼠模型中,单用贝达喹啉(25 mg/kg,每周5次)与WHO推荐方案治疗DS/MDR-PTB疗效相当,但单药易致耐药,所以采用替换和(或)添加推荐方案中任何一种药物的方法[4,13]。BDQ-PZA-INH/RIF、BDQ-PZA-AMK-Mfx±乙硫异烟胺(ethionamide,ETH) 治疗1月与推荐方案治疗2月杀菌效果相当,治疗2月使肺脏或(和)脾脏培养阴性,提示含贝达喹啉的方案可能使DS/MDR-PTB疗程缩短一半。Makarov等[14]指出贝达喹啉与含哌嗪的苯并噻唑169(piperazine-containing benzothiazinones 169,PBTZ169)具有协同作用,在小鼠模型中BDQ- PBTZ169-PZA疗效优于WHO推荐方案。Ibrahim等[15]指出贝达喹啉联合推荐方案治疗小鼠模型4月与单纯推荐方案治疗6月复发率相同,提示贝达喹啉可能降低PTB复发率。Shang等[16]指出贝达喹啉在豚鼠模型中也有相似疗效。贝达喹啉既能杀菌也能降低复发率,但两者并不完全一致。Andries等[17]指出BDQ-PZA-Mfx治疗小鼠模型4周肺培养阴性,治疗5月复发率与推荐方案治疗6月相当;而BDQ-PZA-利福喷丁(rifapentine,RFT)治疗4周肺培养并未完全转阴,而治疗3月复发率与推荐方案治疗6月相当。Ibrahim等[18]指出BDQ与PZA具有协同杀菌作用,Veziris等[19]采用每周1次BDQ-PZA-RFT联合方案治疗小鼠模型,其疗效优于每周5次的推荐方案,且优于任何已知周用药方案,提示此三联方案可能成为候选抗结核间歇方案。
在少菌的潜伏结核杆菌感染(latent TB infection, LTBI)小鼠模型中,zhang等[20-21]采用每周5次BDQ-RFT-PZA联合方案治疗1月肺培养阴性、复发率7%,提示此三联方案可能使DS-LTBI疗程缩短到1月;单用BDQ与INH-RIF治疗3、4月复
表1 贝达喹啉针对结核分枝杆菌、非结核分枝杆菌、革兰阳性菌和革兰阴性菌的MIC值范围和MIC50值发率水平相当,提示BDQ可能使DR-LTBI疗程缩短到3~4月。
Tab.1 Range and median minimal inhibitory concentration of bedaquiline forMycobacteriumtuberculosis, nontuberculous mycobacteria and relevant Gram-positive and Gram-negative bacteria
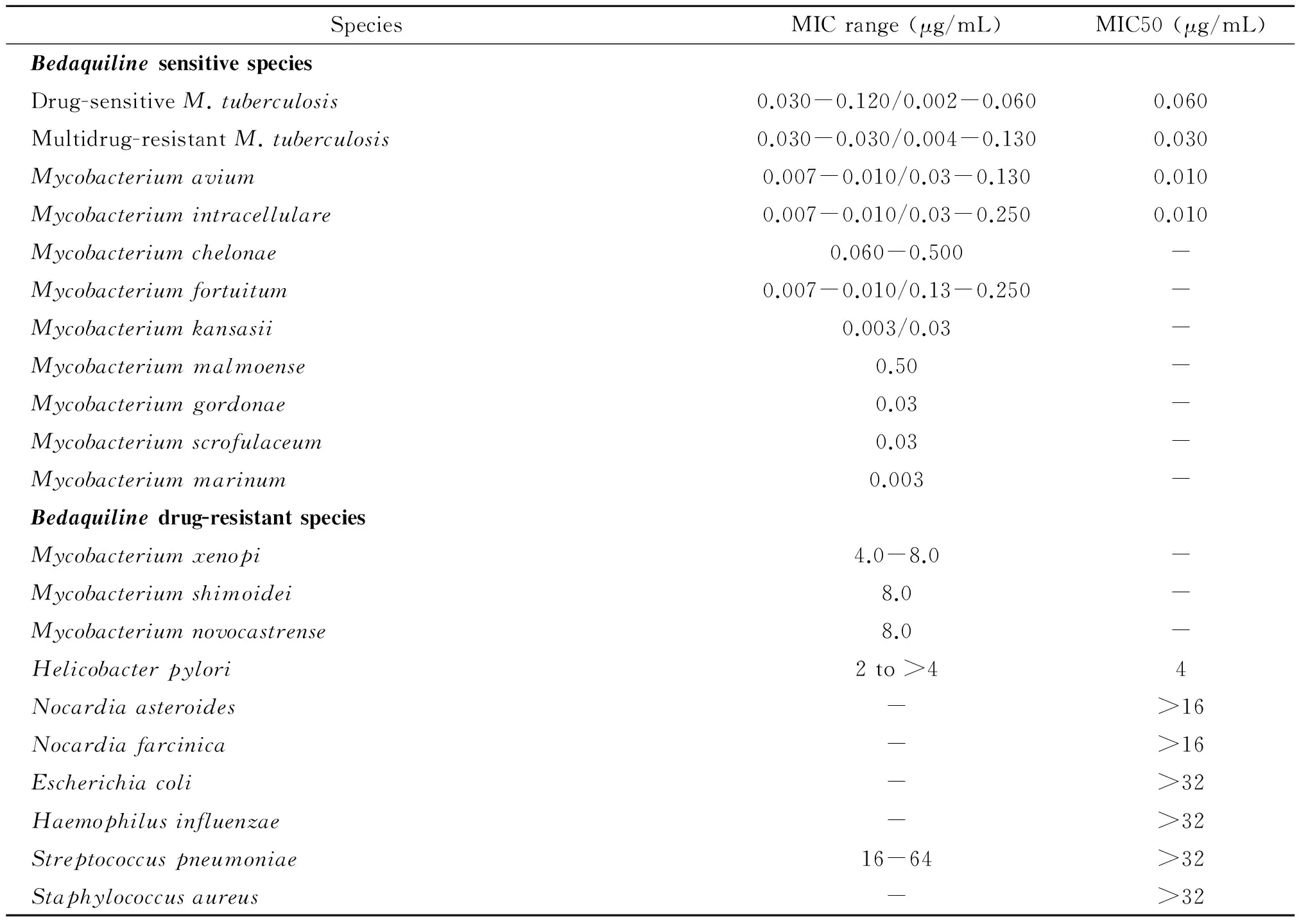
SpeciesMICrange(μg/mL)MIC50(μg/mL)BedaquilinesensitivespeciesDrug-sensitiveM.tuberculosis0.030-0.120/0.002-0.0600.060Multidrug-resistantM.tuberculosis0.030-0.030/0.004-0.1300.030Mycobacteriumavium0.007-0.010/0.03-0.1300.010Mycobacteriumintracellulare0.007-0.010/0.03-0.2500.010Mycobacteriumchelonae0.060-0.500-Mycobacteriumfortuitum0.007-0.010/0.13-0.250-Mycobacteriumkansasii0.003/0.03-Mycobacteriummalmoense0.50-Mycobacteriumgordonae0.03-Mycobacteriumscrofulaceum0.03-Mycobacteriummarinum0.003-Bedaquilinedrug-resistantspeciesMycobacteriumxenopi4.0-8.0-Mycobacteriumshimoidei8.0-Mycobacteriumnovocastrense8.0-Helicobacterpylori2to>44Nocardiaasteroides->16Nocardiafarcinica->16Escherichiacoli->32Haemophilusinfluenzae->32Streptococcuspneumoniae16-64>32Staphylococcusaureus->32
5 临床试验
早期杀菌活性(early bactericidal activity, EBA)试验[22-23]将75例痰涂片阳性初治PTB病人随机每天口服贝达喹啉25 mg、100 mg、400 mg和WHO推荐方案治疗7天,结果表明每天口服400 mg贝达喹啉从第4天开始到研究终点其疗效与推荐方案相当,延迟的杀菌效应与贝达喹啉诱导MTB重塑新陈代谢有关。Ⅱb期[8]C208试验将208例痰涂片阳性初治MDR-PTB病人(大部分HIV阴性)随机分为贝达喹啉组和对照组,前者按照贝达喹啉说明书给予贝达喹啉,后者给予安慰剂,分别联合背景方案治疗8或24周,之后背景方案继续治疗96周。C208第1阶段(贝达喹啉 8周组)主要终点为第8周时贝达喹啉组痰培养中位阴转时间缩短,痰阴转率升高;次要终点为第24周时贝达喹啉组痰培养中位阴转时间仍然缩短,第24、72周时两组痰培养阴转率差异无统计学意义。C208第2阶段(贝达喹啉 24周组)主要终点为第24周时贝达喹啉组痰培养中位阴转时间缩短,痰阴转率升高;次要终点为第72周时贝达喹啉组痰培养中位阴转持续缩短,而痰培养阴转率两组差异无统计学意义;次要终点第120周贝达喹啉组痰培养阴转率升高。 C209试验将233例MDR/XDR-PTB病人(大部分HIV阴性)采用贝达喹啉联合个体化背景方案治疗24周,第25周末终止试验,主要终点为第24周时痰培养中位阴转时间57天和痰阴转率79.5%。贝达喹啉C208和C209临床试验结果见表2。
6 安全性
C208试验[8]指出贝达喹啉组不良反应大多为轻至中度,发生率依次为恶心(35.3%)、关节痛(29.4%)、头痛(23.5%)、高尿酸血症(22.5%)、呕吐(20.6%)、咯血(16.7%)、嗜睡(12.7%)、耳聋(11.8%)、瘙痒(11.8%)、失眠(10.8%)等,而除了前三项外贝达喹啉组与安慰剂组差异无统计学意义。C209试验[8]贝达喹啉组不良反应发生率依次为高尿酸血症(13.7%)、关节痛(11.6%)、恶心(10.7%)、呕吐(8.6%)、头痛(8.6%)、腹泻(7.7%)、血尿酸升高(6.9%)、低钾血症(6.0%)、瘙痒(6.0%)、注射部位疼痛(5.6%)等。贝达喹啉组与安慰剂组3级或4级严重不良发应发生率差异无统计学意义,贝达喹啉组主要表现为转氨酶升高和心电图QTc延长[8]。值得注意的是,C208第2阶段试验发现的死亡率贝达喹啉组(12.7%)高于安慰剂组(2.5%),但具体原因尚不清楚。
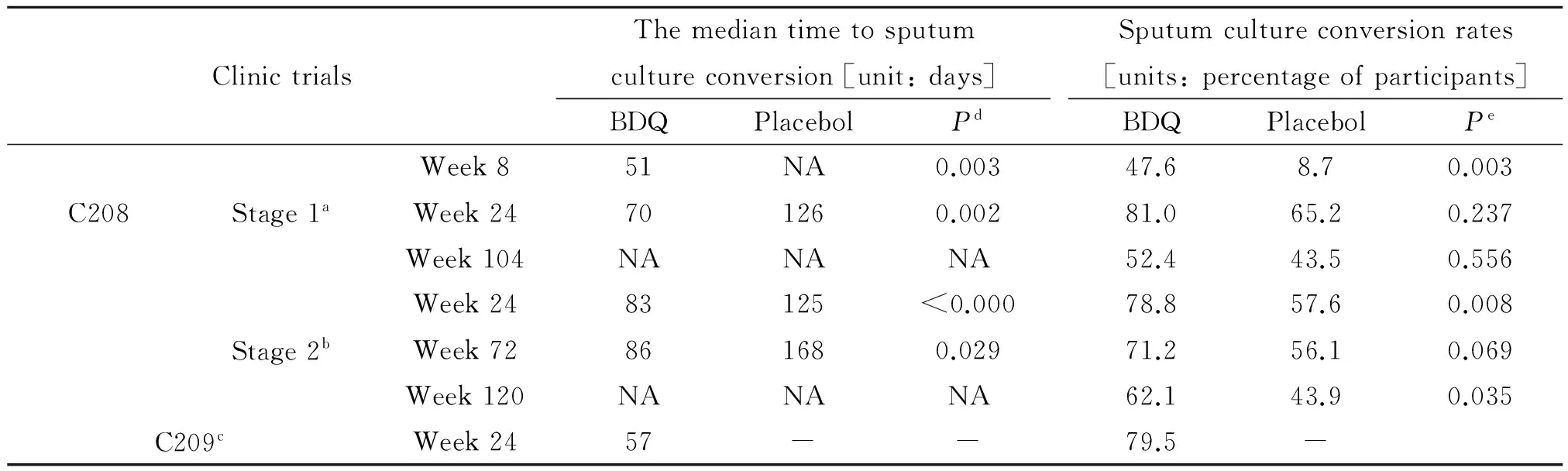
表2 贝达喹啉C208和C209临床试验结果
Note: NA--not analysed;aThe number of participants analyzed consists of 21 subjects in the BDQ group and 23 subjects in placebo groups;bThe number of participants analyzed consists of 66 subjects in the BDQ group and 66 subjects in placebo groups;cThe number of participants analyzed consists of 205 subjects in the BDQ group;dBased on a Cox proportional hazards model with treatment as covariate;eBased on a logistic regression model with treatment as covariate.
贝达喹啉引起转氨酶升高多为可逆性,与背景方案中转氨酶升高药物联用可能增加药物性肝损风险。贝达喹啉引起的QTc延长多为可逆性,联用QTc延长药物(如唑类抗真菌药)和(或)结核病合并某些心律失常(如尖端扭转型室性心动过速)时QTc延长风险增加,当出现有临床意义的室性心律失常或QTc>500 ms(经过重复心电图确定)时应立即停用贝达喹啉[8]。
贝达喹啉与CYP3A4激动剂(如RIF)联用时可能使贝达喹啉浓度降低而影响杀菌效应,而与拮抗剂(如蛋白激酶抑制剂)联用时可能使贝达喹啉累积而增加不良反应[8]。在人体内利福平与贝达喹啉同服时贝达喹啉血浆浓度降低50%,而在小鼠模型中发现两者联用并没有影响贝达喹啉杀菌效应[24]。Dooley等[25]指出贝达喹啉与抗逆转录药物依法韦伦序贯口服并没有相互影响其疗效。因此,贝达喹啉血浆浓度可能与临床效应无关。
贝达喹啉及其主要代谢产物M2属于阳离子两亲性药物(cationic amphiphilic drugs,CAD),易引起细胞内磷脂质聚积形成DIP。DIP具有可逆性,可逆程度取决于CAD与磷脂质的分离率和在组织中的消除率[26]。在某些CAD(如胺碘酮)中,DIP与临床毒性相关,但这种观点与其他CAD是否一致尚有争议。在临床试验中贝达喹啉与氯法齐明联用时心脏毒性增加,可能与DIP有关。
[1]Sotqiu G, Migliori GB. Facing multi-drug resistant tuberculosis[J]. Pulm Pharmacol Ther, 2014:1-5. DOI: 10.1016/j.pupt.2014.04.006
[2]Matteelli A, Carvalho AC, Dooley KE, et al. TMC207: the first compound of a new class of potent anti-tuberculosis drugs[J]. Future Microbiol, 2010, 5(6): 849-858. DOI: 10.2217/fmb.10.50
[3]Xiao HP. Bedaquiline for MDR-TB: WHO interim guidance and appliacation in China[C]. The 80th anniversary of antituberculosis association and 2013 national academic meeting, 2013: 17-21. (in Chinese) 肖和平. 贝达喹啉治疗MDR-TB WHO暂行指导原则和中国的应用[C]. 中国防痨协会80周年纪念暨2013年全国学术大会论文集, 2013:17-21.
[4]Andries K, Verhasselt P, Guillemont J, et al. A diarylquinoline drug active on the ATP synthase of mycobacterium tuberculosis[J]. Science, 2005, 307: 223 -227.
[5]Petrella S, Cambau E, Chauffour A, et al. Genetic basis for natural and acquired resistance of the diaryquinoline R207910 in mycobacteria[J]. Antimicrob Agents Chemother, 2006, 50(8): 2853-2856. DOI: 10.1128/AAC.00244-06
[6]Gupta S, Cohen KA, Winglee K, et al. Efflux inhibition with verapamil potentiates bedaquiline in mycobacterium tuberculosis[J]. Antimicrob Agents Chemother, 2014, 58: 574-576. DOI: 10.1128/ AAC.01462-13
[7]Andries K, Villellas C, Coeck N, et al. Acquired resistance ofMycobacteriumtuberculosisto Bedaquiline[J]. PLoS One, 2014, 9(7): e102135. DOI: 10.1371/journal.pone.0102135
[8]US Food and Drug Administration. Briefing Package: NDA 204-384: Sirturo. 2012[EB/OL]. http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/Anti-InfectiveDrugsAdvisoryCommittee/UCM 329258.pdf, 2014-08-16/2014-10-04
[9]Liu K, Li F, Lu J, et al. Bedaquiline metabolism: enzymes and novel metabolites[J]. Drug Metab Dispos, 2014, 42(5): 863-866. DOI: 10.1124/dmd.113.056119
[10]Lounis N, Gevers T, Van Den Berg J, et al. Prevention of drug carryover effects in studies assessing antimycobacterial efficacy of TMC207[J]. J Clin Microbiol, 2008, 46(7): 2212-2215. DOI: 10.1128/ JCM.00177-08
[11]Huitric E, Verhasselt P, Andries K, et al. In vitro antimycobacterial spectrum of a diarylquinoline ATP-synthase inhibitor[J]. Antimicrob Agents Chemother, 2007, 51(11): 4202-4204. DOI: 10.1128/AAC.00181-07
[12]Koul A, Vranckx L, Dendouga N, et al. Diarylquinolines are bactericidal for dormant mycobacteria as a result of disturbed ATP homeostasis[J]. J Biol Chem, 2008, 283(37): 25273-25280. DOI: 10.1074/jbc.M803899200
[13]Lounis N, Veziris N, Chauffour A, et al. Combinations of R207910 with drugs used to treat multidrug-resistant tuberculosis have the potential to shorten treatment duration[J]. Antimicrob Agents Chemother, 2006, 50(11): 3543-3547. DOI: 10.1128/AAC.00766-06
[14]Makarov V, Lechartier B, Zhang M, et al. Towards a new combination therapy for tuberculosis with next generation benzothiazinones[J]. EMBO Mol Med Mar, 2014, 6(3): 372-383. DOI: 10.1002/ emmm.201303575
[15]Ibrahim M, Truffot-Pernot C, Andries K, et al. Sterilizing activity of R207910 (TMC207)-containing regimens in the murine model of tuberculosis[J]. Am J ResPir Crit Care Med, 2009, 180(6): 553-557. DOI: 10.1164/rccm.200807-1152OC
[16]Shang SB, Shanley CA, Caraway ML, et al. Activities of TMC207,rifampin,and pyrazinamide against mycobacterium tuberculosis infection in guinea pigs[J]. Antimicrob Agents Chemother, 2011, 55(1): 124-131. DOI: 10.1128/AAC.00978-10
[17]Andries K, Gevers T, Lounis N. Bactericidal potencies of new regimens are not predictive of their sterilizing potencies in a murine model of tuberculosis[J]. Antimicrob Agents Chemother, 2010, 54(11): 4540-4544. DOI: 10.1128/AAC.00934-10
[18]Ibrahim M, Andries K, Lounis N, et al. Synergistic activity of R207910 combined with pyrazinamide against murine tuberculosis[J]. Antimicrob Agents Chemother, 2007, 51(3): 1011-1015. DOI: 10.1128/AAC.00898-06
[19]Veziris N, Ibrahim M, Lounis N, et al. A once weekly R207910-containing regimen exceeds activity of the standard daily regimen in murine tuberculosis[J]. Am J Respir Crit Care Med, 2009, 179(1): 75-79. DOI: 10.1164/rccm.200711-1736OC
[20]Zhang T, Zhang M, Rosenthal IM, et al. Short-course therapy with daily rifapentine in a murine model of latent tuberculosis infection[J]. Am J Respir Crit Care Med, 2009, 180(11): 1151-1157. DOI: 10.1164/rccm.200905-0795OC
[21]Zhang T, Li SY, Williams KN, et al. Short-course chemotherapy with TMC207 and rifapentine in a murine model of latent tuberculosis infection[J]. Am J Respir Crit Care Med, 2011, 184(6): 732-737. DOI: 10.1164/rccm.201103-0397OC
[22]Rustomjee R, Diacon AH, Allen J, et al. Early bactericidal activity and pharmacokinetics of the dairylquinoline TMC207 in treatment of pulmonary tuberculosis[J]. Antimicrob Agents Chemother, 2008, 52(8): 2831-2835. DOI: 10.1128/AAC.01204-07
[23] Koul A, Vranckx L, Dhar N, et al. Delayed bactericidal response of Mycobacterium tuberculosis to bedaquiline involves remodelling of bacterial metabolism[J]. Nat Commun, 2014, 5: 3369. DOI: 10.1038/ncomms4369
[24]Lounis N, Gevers T, Berg JVD, et al. Impact of the interaction of R207910 with rifampin on the treatment of tuberculosis studied in the mouse model[J]. Antimicrob Agents Chemother, 2008, 52(10): 3568-3572. DOI: 10.1128/AAC.00566-08
[25]Dooley KE, Park JG, Swindells S, et al. Safety, tolerability, and pharmacokinetic interactions of the antituberculous agent TMC207 (bedaquiline) with efavirenz in healthy volunteers: AIDS Clinical Trials Group study A5267[J]. J Acquir Immune Defic Syndr, 2012, 59(5): 455-462. DOI: 10.1097/ QAI.0b013e3182410503
[26]Diacon AH, Donald PR, Pym A, et al. Randomized pilot trial of eight weeks of bedaquiline (TMC207) treatment for multidrug-resistant tubercolosis: longterm outcome, tolerability, and effect on emergence of drug resistance[J]. Antimicrob Agents Chemother, 2012, 56(6): 3271-3276. DOI: 10.1128 /AAC.06126-11
Bedaquiline for tuberculosis
PENG Wei,LI You-lun,PENG Li
(DepartmenofRespiratoryMedicine,theFirstAffiliatedHospitalofChongqingMedicalUniversity,Chongqing400016,China)
Tuberculosis is remaining a global public health problem. The apperance and spread of multidrug-resistant and extensively drug-resistant tuberculosis is a big challenge for tuberculosis control. Antituberculosis drugs which were recommended by World Health Organization for treatment of drug-resistant tuberculosis have many shortcomings, such as less kinds, poor curative effects, serious adverse events and long treatment course. Therefore, it is urgent to develop drugs with new action. Bedaquiline, which is target ATP synthase of mycobacterium, is possessed of merits with unique mechanism, low resistance rate, long haf-life, high specificity, strong antibacterial activity, reduced treatment duration for multidrug-resistant tuberculosis, well safety and so on. At the same time, it has defects with high fatality rate which is diffcut to explain and the like. The artical mainly focus on the research progress of bedaquiline about its action and resistance mechanism, pharmacokinetics, antibacteria activity, clinic trials and safety in antituberculosis.
tuberculosis; bedaquiline; drug-resistant tuberculosis
Li You-lun, Email: liyoulun83@163.com
国家临床重点专科建设项目(No.2012-649)资助
黎友伦,Email:Liyoulun83@163.com
重庆医科大学附属第一医院呼吸内科,重庆 400016
10.3969/cjz.j.issn.1002-2694.2015.02.017
R378
A
1002-2694(2015)02-0174-05
2014-07-14;
2014-11-11

Supported by the State Key Clinical Specialty Construction Project (No. WeiBanYi ZhengHan[2012]No.649)
