孕期尼古丁暴露所致子2代大鼠神经内分泌代谢编程改变的可遗传效应
2015-03-02郭自景罗瀚文邓梓辛钟卫华
郭自景, 徐 丹,2, 罗瀚文,3, 邓梓辛, 钟卫华, 汪 晖,2
(武汉大学 1. 基础医学院, 2. 发育源性疾病湖北省重点实验室, 3. 中南医院骨科, 湖北 武汉 430071)
孕期尼古丁暴露所致子2代大鼠神经内分泌代谢编程改变的可遗传效应
郭自景1, 徐 丹1,2, 罗瀚文1,3, 邓梓辛1, 钟卫华1, 汪 晖1,2
(武汉大学 1. 基础医学院, 2. 发育源性疾病湖北省重点实验室, 3. 中南医院骨科, 湖北 武汉 430071)
目的 探讨孕期尼古丁暴露所致宫内发育迟缓(IUGR)子代大鼠神经内分泌代谢编程改变的跨代遗传效应。方法 Wistar大鼠孕11 d起每天sc给予尼古丁2 mg·kg-1至分娩。子1代(F1)正常组和尼古丁组IUGR交叉配对而得子2代(F2):CC组(亲氏为正常F1)、CN组(父为正常F1,母为IUGR F1)、NC组(父为IUGR F1,母为正常F1)和NN组(亲氏为IUGR F1)。成年F2给予2周冰水游泳刺激,收集刺激前、后血样,采用放免试剂盒检测血清促肾上腺皮质激素(ACTH)水平,ELISA试剂盒检测皮质酮(CORT)水平,生化试剂盒检测葡萄糖、甘油三酯(TG)和总胆固醇(TCH)水平。结果 慢性刺激前,NN组雄性子代血清CORT较CC组显著降低,为CC组的73.9%(P<0.05),CN和NC组雄性子代血清TG分别升高到CC组的1.43和1.52倍(P<0.05),同时CN, NC和NN组雌性子代血清TG分别升高到CC组的1.71, 1.80和1.81倍(P<0.05);慢性刺激后,CC组雄性子代血清CORT增加率为-1.67%,而NN组雄性子代血清CORT增加率为36.0%,NC组雄性及CN组雌性子代血糖显著升高,分别升高至各自CC对照组的1.61和1.62倍(P<0.01),同时各尼古丁组雌、雄中子代血清TG增加率均较CC组显著降低(P<0.05),具体表现为CN, NC和NN组雄性子代血清TG增加率分别降低至CC组的46.4%, 16.7%和7.7%,而相应雌性子代血清TG增加率分别降低至CC组的20.6%, 4.0%和8.4%。与CC组相比,慢性刺激前,NN组雌、雄性子代血清TCH分别下降40.5%和21.9%(P<0.01);慢性刺激后,雌性子代TCH增长率升高49.7%(P<0.05)。结论 孕期尼古丁暴露致大鼠神经内分泌代谢编程改变具有跨代遗传效应,且具有一定的性别和亲源性差异。
尼古丁; 宫内发育迟缓; 跨代遗传; 糖代谢; 脂代谢
流行病学调查显示,20%~50%的妇女在妊娠期吸烟,而50%不吸烟的孕妇也存在被动吸烟[1-2]。尼古丁是烟草中脂溶性较高的生物碱,可被快速吸收并能透过胎盘在胎儿体内蓄积,被认为是烟草中危害胎儿健康的主要毒性成分[3]。本课题组前期动物实验表明,孕期尼古丁暴露可致胎儿宫内发育迟缓(intrauterine growth retardation, IUGR)和胎鼠母源性糖皮质激素(glucocorticoid, GC)过暴露,后者可介导胎下丘脑-垂体-肾上腺轴(hypothalamic-pituitary-adrenal axis, HPA)相关的宫内神经内分泌代谢编程改变及代谢综合征(metabolic syndrome, MS)易感[4-5],表现为宫内胎HPA轴功能发育抑制、糖脂代谢功能及血表型改变,出生后HPA轴低基础活性和高应激敏感性改变,同时伴随糖脂代谢血表型的GC依赖性改变[6-7]。孕期不良环境对子代的跨代遗传效应已经被证实,但是其潜在的机制仍是近年来研究的热点[8]。研究证实,孕期不良环境可对子代HPA轴功能产生不利影响,并具有跨代遗传效应[9-13],但是孕期尼古丁暴露所致子代HPA轴相关神经内分泌代谢编程改变是否具有遗传效应还未见报道。本研究拟在前期研究基础上,通过观察孕期尼古丁暴露的子2代(F2)大鼠在慢性刺激前、后的HPA轴活性及糖脂代谢改变,探讨尼古丁所致子代生殖发育毒性及神经内分泌代谢编程的跨代遗传效应。
1 材料与方法
1.1 药品和试剂尼古丁购自美国Sigma-Aldrich公司;大鼠促肾上腺皮质激素(adrenocorticotropic hormone, ACTH)放免试剂盒购自北京北方生物技术研究所;血皮质酮(corticosterone, CORT)ELISA试剂盒购自美国Assaypro公司;葡糖氧化酶分析试剂盒购自上海Mind生物工程有限公司;甘油三酯(triglycerides, TG)和总胆固醇(total cholesterol, TCH)分析试剂盒购自上海生工生物工程股份有限公司;异氟烷购自美国巴克斯特医疗保健有限公司;其余化学试剂均为国产分析纯。
1.2 动物和分组实验大鼠由湖北省预防科学院实验动物中心(许可证号:SCXK 2010-2011)提供。相关动物实验均按照中国动物福利委员会的使用原则执行。亲代(P):健康成年Wistar雌性大鼠(180~220 g)和雄性大鼠(260~300 g),适应性喂养1周后,每晚18:00进行合笼交配(雌∶雄=2∶1),次晨进行雌鼠阴道涂片,在显微镜下检见精子时记为受孕0 d(gestational day 0 , GD0)。确定受孕后,将孕鼠随机分为2组。尼古丁组于GD11开始sc给予尼古丁2 mg·kg-1,直至分娩。对照组给予等量生理盐水。给药体积为10 mL·kg-1。子1代(F1):待P的孕鼠自然分娩后,按仔鼠出生日期的不同分批,分别编号并筛选,其筛选原则为:① 在产仔数≥10只孕鼠中挑选;② 对照组保留非IUGR仔鼠,尼古丁组保留IUGR仔鼠;③ 尽量使挑选仔鼠的雌雄比率为1∶1。最终获得的对照组和尼古丁组F1仔鼠各12只,记录出生后每周(postnatal week, PW)体质量变化。F2∶F1的仔鼠出生4个月(postnatal month 4, PM4)时按雌∶雄为2∶1比率进行合笼交配,正常生产得到4组F2,分别为CC组(上一代均为正常F1)、CN组(父为正常F1,母为IUGR F1)、NC组(父为IUGR F1,母为正常F1)和NN组(上一代均为IUGR F1),详见表1。
Tab.1 F1 mating principle and F2 grouping
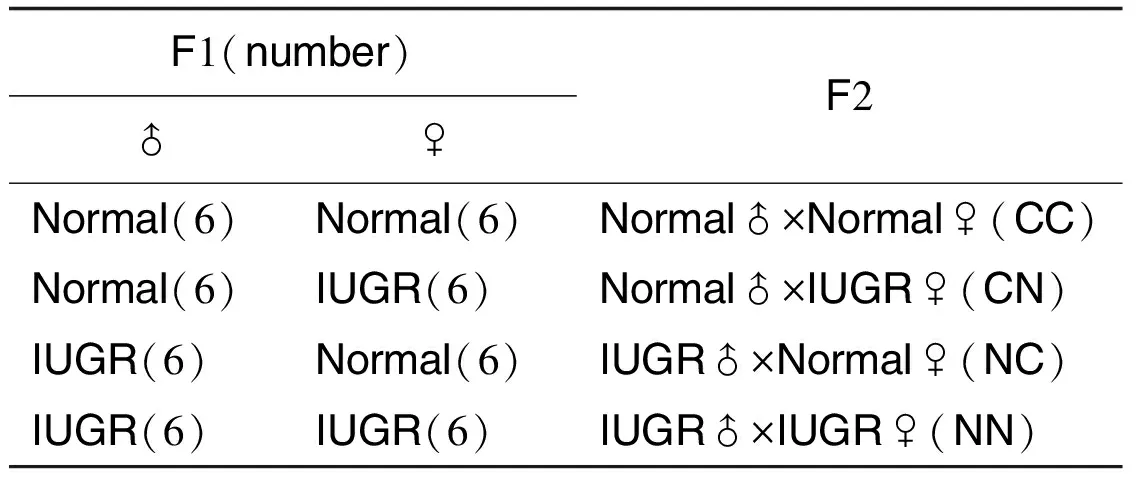
F1(number)♂♀F2Normal(6)Normal(6)Normal♂×Normal♀(CC)Normal(6)IUGR(6)Normal♂×IUGR♀(CN)IUGR(6)Normal(6)IUGR♂×Normal♀(NC)IUGR(6)IUGR(6)IUGR♂×IUGR♀(NN)
IUGR: intrauterine growth retardation.
F1自然分娩后,每窝随机选取1~2只仔鼠,按出生日期不同分批编号,记录其出生后每周体质量变化。F2大鼠于PM4~PM4.5期间每天给予3~5 min冰水游泳慢性刺激。慢性刺激前,分2次对F2 PM4大鼠进行鼠尾取血,每次300 μL,共600 μL;慢性刺激结束后,分2次在游泳完毕后1 h内进行鼠尾取血,每次300 μL,共600 μL。待血液室温凝固后,4℃下16 099×g离心10 min,吸取上层血清,-80℃冻存备用。
1.3 检测指标和方法取-80℃冻存备用的F2大鼠慢性刺激前、后血清,采用放免同位素试剂盒测定大鼠血清ACTH浓度[14],其批间和批内变异系数分别为10%和15%。采用ELISA试剂盒检测大鼠CORT浓度[15],其批间和批内变异系数分别为5.0%和7.2%。血清葡萄糖、TG和TCH浓度检测按照生化分析试剂盒的说明书进行检测。计算血清ACTH、CORT、葡萄糖和TG浓度增长率。浓度增长率(%)=(慢性刺激后浓度- 慢性刺激前浓度)/ 慢性刺激前浓度×100%。
2 结果
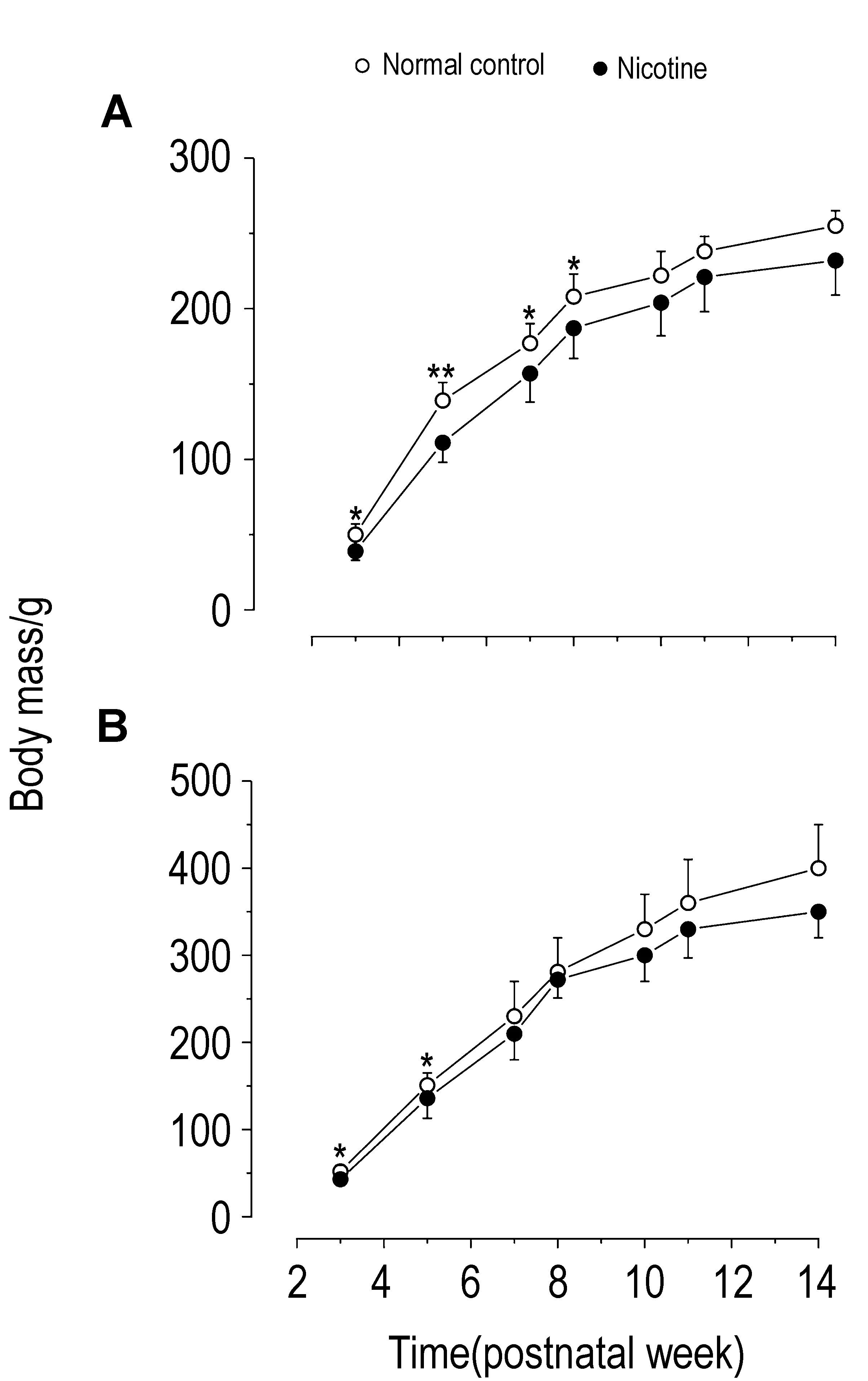
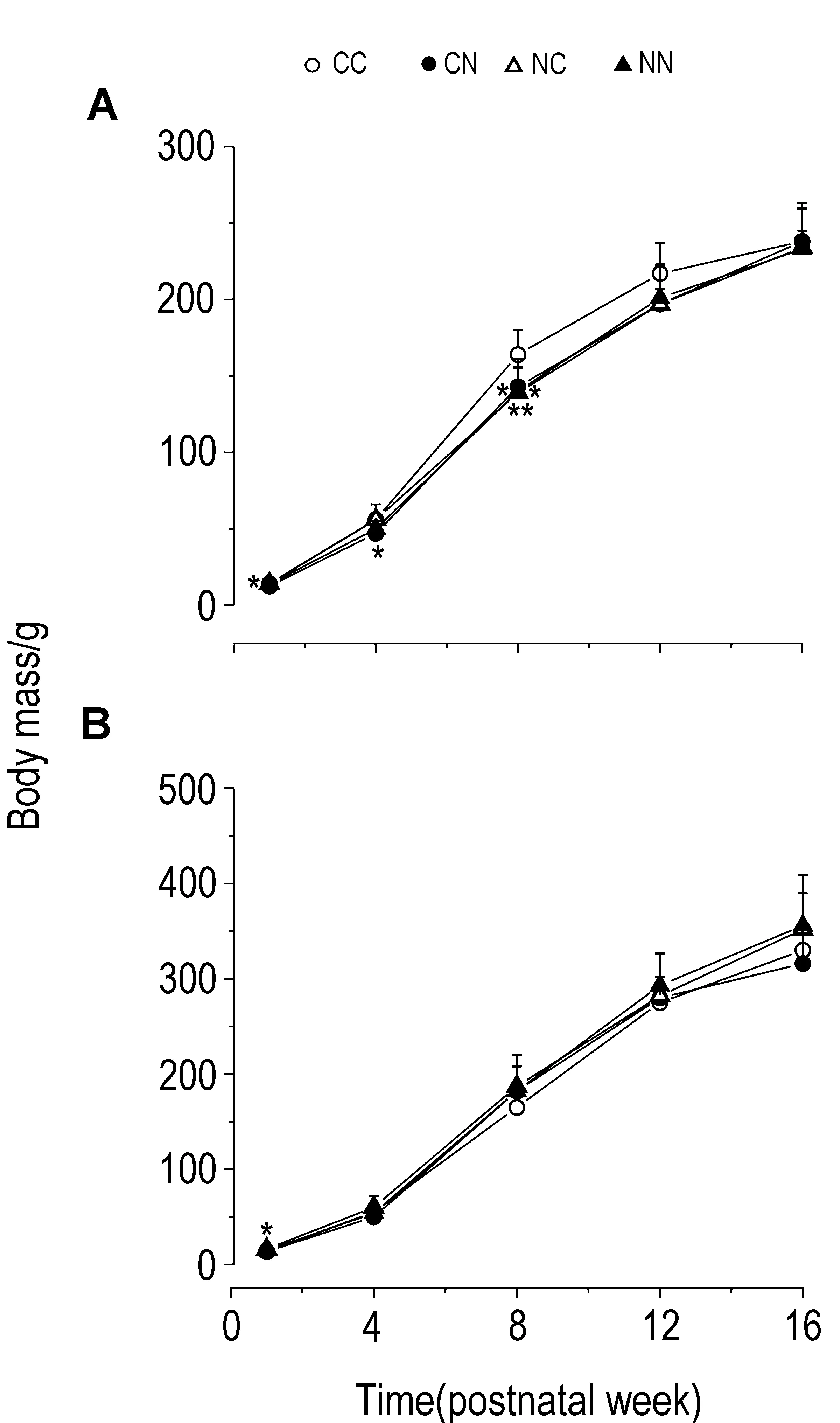
2.1 孕期尼古丁暴露对F1仔鼠体质量的影响如图1所示,与正常对照组相比,尼古丁组F1出生后(PW3~PW14)体质量降低或有降低趋势,雌性F1在PW3~PW8显著降低(图1A,P<0.05),而雄性F1在PW3~PW5显著降低(图1B,P<0.05)。
2.2 孕期尼古丁暴露对F2仔鼠体质量的影响如图2所示,对于雌性F2,与CC组相比,在PW1~PW16内,尼古丁各组体质量均低于CC组(P<0.01),其中CN组在PW1~PW8显著降低(P<0.05),NC组和NN组在PW8显著降低(P<0.05, P<0.01),提示孕期尼古丁暴露可致雌性F2大鼠体质量降低且CN组存在追赶性生长。与CC组相比,雄性F2 NC和NN组体质量显著升高(P<0.05, P<0.01)。
2.3 孕期尼古丁暴露对子代HPA轴神经内分泌代谢编程的影响
2.3.1 血清ACTH和CORT浓度变化如图3所示,对于雌性F2,与CC组相比,慢性
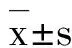
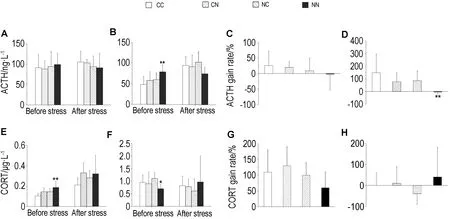
刺激前,血清ACTH浓度在尼古丁各组均无明显变化,血清CORT浓度在NN组显著升高(P<0.01);慢性刺激后,血清ACTH和CORT浓度在尼古丁各组均无显著性改变。对于雄性F2,与CC组相比,慢性刺激前,NN组血清ACTH浓度显著升高(P<0.01),血清CORT浓度显著降低(P<0.05);慢性刺激后,血清ACTH浓度及其增加率在NN组显著降低(P<0.01)或有降低趋势,但血清CORT浓度变化率显示一定的升高趋势。
2.3.2 血糖浓度变化如图4所示,对于雌性F2,与CC组相比,慢性刺激前,尼古丁各组血糖浓度均无显著性改变;慢性刺激后,CN组血糖浓度显著升高(P<0.05)。对于雄性F2,与CC组相比,慢性刺激前,尼古丁各组血糖浓度均无显著性改变;慢性刺激后,NC组血糖浓度及增加率显著升高(P<0.05,P<0.01)。
2.3.3 血脂浓度变化2.3.
3.1 血清TG浓度变化如图5所示,对于雌性F2,与CC组相比,慢性刺激前,尼古丁各组血清TG浓度显著升高(P<0.05, P<0.01),刺激后NC组和NN组TG浓度及尼古丁各组TG增长率显著降低(P<0.05)。对于雄性F2,与CC组相比,CN组和NC组刺激前TG浓度显著升高(P<0.05),刺激后NN组TG浓度显著降低(P<0.01),NC组和NN组TG增加率降低(P<0.05, P<0.01)。2.3.
3.2 血清TCH浓度变化如图6所示,与CC组相比,慢性刺激前,雌、雄F2子代NN组大鼠血清TCH浓度均显著降低(P<0.01),刺激后各组血清TCH浓度均无显著性改 变,NN组雌鼠血清TCH的增加率显著升高(P<0.05)。
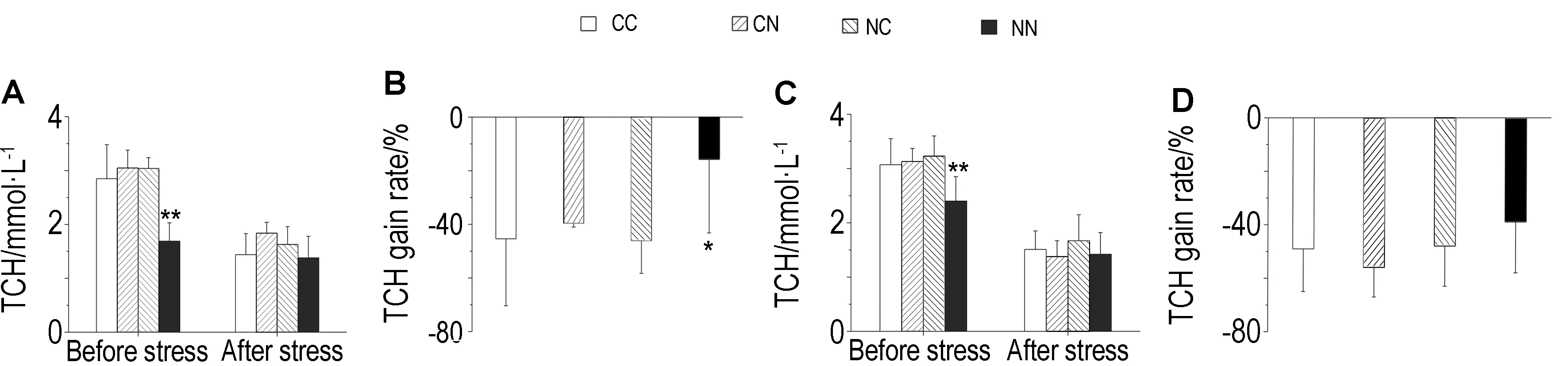
3 讨论
本课题组前期研究发现,孕11 d大鼠sc给予尼古丁后,胎鼠过暴露于母源性GC,从而改变胎鼠生长发育指标(如体质量、身长、尾长以及胎盘重量)和糖脂代谢,且IUGR发生率增加[16-17]。尼古丁暴露的F1成年子代表现为HPA轴低基础活性和高应激敏感性,同时,慢性刺激前血清TG和TCH浓度升高,慢性刺激后血糖增加率升高,血清TG和TCH增加率均降低[6]。本研究发现,F1尼古丁组大鼠体质量降低,同时,F2尼古丁各组雌性大鼠体质量降低,提示孕期尼古丁暴露所致雌性子代的生殖发育毒性具有可遗传性。进一步研究发现,慢性刺激前,F2 NN组雄性大鼠血清CORT显著降低;慢性刺激后,血清CORT变化率有升高趋势。提示F2 NN组雄性大鼠存在HPA轴低基础活性和高应激敏感性。对糖脂水平的检测显示,慢性刺激前,F2尼古丁各组雌性大鼠,CN组及NC组雄性大鼠血清TG均升高;慢性刺激后,F2 CN组雌性大鼠血糖升高,且NC组雄性大鼠血糖及血糖增加率均升高,尼古丁各组大鼠血清TG增加率显著降低或有降低趋势。提示孕期尼古丁暴露所致子代神经内分泌代谢编程改变具有可遗传性。已有报道,孕期不良环境所致子代糖脂代谢改变具有性别差异[18-19];孕期地塞米松暴露所致的跨代遗传效应具有性别差异,且可增加心血管疾病的风险[20]。本研究也发现,孕期尼古丁暴露所致神经内分泌代谢编程改变具有性别差异和亲源性差异:F2尼古丁各组雌性子代体质量降低,而NC组和NN组雄性子代体质量升高,慢性刺激前F2 NN组雌性大鼠血清CORT升高而雄性大鼠血清CORT显著降低;慢性刺激后,父系尼古丁暴露使雄性子代血糖升高,而母系尼古丁暴露使雌性子代血糖升高。提示孕期尼古丁暴露所致子代体质量、血清CORT改变具有性别差异,且血糖改变具有亲源性差异。Drake等[13]研究发现,孕期GC暴露可导致F1大鼠葡萄糖耐受,并增加肝组织中的磷酸烯醇丙酮酸羧激酶,且这些改变可以持续到F2。大量研究均表明,循环中GC可以调控糖脂代谢相关的多个通路[21-25]。本课题组前期研究发现,孕期尼古丁暴露所致胎鼠高水平GC可通过下调胰岛素样生长因子(insulin-like growth factor, IGF)1/胰岛素信号通路,并上调脂联素/瘦素信号通路改变糖脂代谢功能。因此,本研究认为,HPA轴功能改变(GC水平变化)可能是糖脂代谢血表型改变(尤其是TG)的重要原因。Ding等[26]研究发现,妊娠糖尿病所致子代葡萄糖耐受具有跨代遗传效应,可能与生殖细胞中IGF2及其交互印记基因H19的表观遗传修饰改变有关;孕期烟草摄入可改变DNA甲基化水平[27];孕期尼古丁暴露可导致子代印记基因IGF2差异甲基化区甲基化水平升高,且雄性子代甲基化差异更明显[28]。因此初步推测,孕期尼古丁暴露所致大鼠神经内分泌代谢编程的亲源性差异和性别差异可能是由印记基因的表观遗传学改变所致。
[1] Contal M, Masson G, Boyer C, Cazevielle C, Mares P. Neonatal consequences of maternal smoking during pregnancy[J].JGynecolObstetBiolReprod(Paris), 2005, 34(1):3S215-3S222.
[2] Higgins S. Smoking in pregnancy[J].CurrOpinObstetGynecol, 2002, 14(2):145-151.
[3] Yildiz D. Nicotine, its metabolism and an overview of its biological effects[J].Toxicon, 2004, 43(6):619-632.
[4] Chen H, Iglesias MA, Caruso V, Morris MJ. Maternal cigarette smoke exposure contributes to glucose intolerance and decreased brain insulin action in mice offspring independent of maternal diet[J].PLoSOne, 2011, 6(11):e27260.
[5] Oyama M, Nakamura K, Tsuchiya Y, Yamamoto M. Unhealthy maternal lifestyle leads to rapid infant weight gain: prevention of future chronic diseases[J].TohokuJExpMed, 2009, 217(1):67-72.
[6] Liu L, Liu F, Kou H, Zhang BJ, Xu D, Chen B,etal. Prenatal nicotine exposure induced a hypothalamic-pituitary-adrenal axis-associated neuroendocrine metabolic programmed alteration in intrauterine growth retardation offspring rats[J].ToxicolLett, 2012, 214(3):307-313.
[7] Xu D, Liang G, Yan YE, He WW, Liu YS, Chen LB,etal. Nicotine-induced over-exposure to maternal glucocorticoid and activated glucocorticoid metabolism causes hypothalamic-pituitary-adrenal axis-associated neuroendocrine metabolic alterations in fetal rats[J].ToxicolLett, 2012, 209(3):282-290.
[8] Grossniklaus U, Kelly WG, Ferguson-Smith AC, Pembrey M, Lindquist S. Transgenerational epigenetic Kinheritance: how important is it?[J].NatRevGenet, 2013, 14(3):228-235.
[9] Iqbal M, Moisiadis VG, Kostaki A, Matthews SG. Transgenerational effects of prenatal synthetic glucocorticoids on hypothalamic-pituitary-adrenal function[J].Endocrinology, 2012, 153(7):3295-3307.
[10] Govorko D, Bekdash RA, Zhang C, Sarkar DK. Male germline transmits fetal alcohol adverse effect on hypothalamic proopiomelanocortin gene across generations[J].BiolPsychiatry, 2012, 72(5):378-388.
[11] Dunn GA, Bale TL. Maternal high-fat diet promotes body length increases and insulin insensitivity in second-generation mice[J].Endocrinology, 2009, 150(11):4999-5009.
[12] Bertram C, Khan O, Ohri S, Phillips DI, Matthews SG, Hanson MA. Transgenerational effects of prenatal nutrient restriction on cardiovascular and hypothalamic-pituitary-adrenal function[J].JPhysiol, 2008, 586(8):2217-2229.
[13] Drake AJ, Walker BR, Seckl JR. Intergenerational consequences of fetal programming byinuteroexposure to glucocorticoids in rats[J].AmJPhysiolRegulIntegrCompPhysiol, 2005, 288(1):R34-R38.
[14] Morgan C. Plasticity in photoperiodic regulation of adrenal, but not testicular, function in Syrian hamsters[J].GenCompEndocrinol, 2012, 178(3):441-449.
[15] Xu D, Wu Y, Liu F, Liu YS, Shen L, Lei YY,etal. A hypothalamic-pituitary-adrenal axis-associated neuroendocrine metabolic programmed alteration in offspring rats of IUGR induced by prenatal caffeine ingestion[J].ToxicolApplPharmacol, 2012, 264(3):395-403.
[16] Wang T, Chen M, Yan YE, Xiao FQ, Pan XL, Wang H. Growth retardation of fetal rats exposed to nicotineinutero: possible involvement of CYP1A1, CYP2E1, and P-glycoprotein[J].EnvironToxicol, 2009, 24(1):33-42.
[17] Chen M, Wang T, Liao ZX, Pan XL, Feng YH, Wang H. Nicotine-induced prenatal overexposure to maternal glucocorticoid and intrauterine growth retardation in rat[J].ExpToxicolPathol, 2007, 59(3-4):245-251.
[18] Hoile SP, Lillycrop KA, Thomas NA, Hanson MA, Burdge GC. Dietary protein restriction during F0 pregnancy in rats induces transgenerational changes in the hepatic transcriptome in female offspring[J].PLoSOne, 2011, 6(7):e21668.
[19] Tran M, Gallo LA, Jefferies AJ, Moritz KM, Wlodek ME. Transgenerational metabolic outcomes associated with uteroplacental insufficiency[J].JEndocrinol, 2013, 217(1):105-118.
[20] Buchwald U, Teupser D, Kuehnel F, Grohmann J, Schmieder N, Beindorff N,etal. Prenatal stress programs lipid metabolism enhancing cardiovascular risk in the female F1, F2, and F3 generation in the primate model common marmoset (Callithrixjacchus)[J].JMedPrimatol, 2012, 41(4):231-240.
[21] Brabant G, Müller G, Horn R, Anderwald C, Roden M, Nave H. Hepatic leptin signaling in obesity[J].FASEBJ, 2005, 19(8):1048-1050.
[22] Chang L, Chiang SH, Saltiel AR. Insulin signaling and the regulation of glucose transport[J].MolMed, 2004, 10(7-12):65-71.
[23] Hotta K, Funahashi T, Bodkin NL, Ortmeyer HK, Arita Y, Hansen BC,etal. Circulating concentrations of the adipocyte protein adiponectin are decreased in parallel with reduced insulin sensitivity during the progression to type 2 diabetes in rhesus monkeys[J].Diabetes, 2001, 50(5):1126-1133.
[24] López-Bermejo A, Botas P, Funahashi T, Delgado E, Kihara S, Ricart W,etal. Adiponectin, hepatocellular dysfunction and insulin sensitivity[J].ClinEndocrinol(Oxf), 2004, 60(2):256-263.
[26] Ding GL, Wang FF, Shu J, Tian S, Jiang Y, Zhang D,etal. Transgenerational glucose intolerance with Igf2/H19 epigenetic alterations in mouse islet induced by intrauterine hyperglycemia[J].Diabetes, 2012, 61(5):1133-1142.
[27] Breton CV, Byun HM, Wenten M, Pan F, Yang A, Gilliland FD. Prenatal tobacco smoke exposure affects global and gene-specific DNA methylation[J].AmJRespirCritCareMed, 2009, 180(5):462-467.
[28] Murphy SK, Adigun A, Huang Z, Overcash F, Wang F, Jirtle RL,etal. Gender-specific methylation differences in relation to prenatal exposure to cigarette smoke[J].Gene, 2012, 494(1):36-43.
(本文编辑: 乔 虹)
Prenatal nicotine exposure induces transgenerationalneuroendocrine metabolic programming alterationin second-generation rats
GUO Zi-jing1, XU Dan1,2, LUO Han-wen1,3, DENG Zi-xin1, ZHONG Wei-hua1, WANG Hui1,2
(1.BasicMedicalSchool, 2.HubeiProvincialKeyLaboratoryofDevelopmentallyOriginatedDisease,3.DepartmentofOrthopaedics,ZhongnanHospital,WuhanUniversity,Wuhan430071,China)
OBJECTIVE To investigate the transgenerational effect of neuroendocrine metabolic programmed alteration in adult intrauterine growth retardation (IUGR) offspring rats with prenatal nicotine exposure. METHODS Pregnant Wistar rats were administered daily with nicotine (2 mg·kg-1) by sc from gestational day 11 until delivery. F1 offspring was fed with a standard diet before four groups in F2 were set up according to the cross-mating between F1 normal adult rats and nicotine-induced IUGR adult rats. CC group was mated by F1 normal adult rats, CN group by F1 normal adult male rats and IUGR adult female rats, NC group by F1 IUGR adult male rats and normal adult female rats, while NN group was mated by F1 IUGR adult rats. F2 adult rats were subjected to a fortnight ice water swimming stimulus. Blood samples were collected before and after stress and then detected for the levels of adrenocorticotropic hormone (ACTH), corticosterone (CORT), glucose, triglycerides(TG) and total cholesterol (TCH). RESULTS Before stress, the level of serum CORT in F2 male rats of NN group was decreased to 73.9% of that of the CC group (P<0.05),while the level of serum TG in F2 male rats of CN and NC groups was increased to 1.43 and 1.52 times that of the CC group, respectively (P<0.05). Meanwhile, the level of serum TG in F2 female rats of CN, NC and NN groups was increased to 1.71, 1.80 and 1.81 times that of the CC group, respectively (P<0.05). After stress, the serum CORT gain rate in F2 male rats of CC group was -1.67%, but was 36.0% in NN group. The serum glucose level in male NC group and in female CN group was increased to 1.61 and 1.62 times that of the corresponded CC groups, respectively (P<0.01). Furthermore, the serum TG gain rate in F2 rats of each nicotine group was decreased markedly in comparison with their corresponding controls (P<0.05),ie, the serum TG gain rates in F2 male rats of CN, NC and NN groups were decreased to 46.4%, 16.7% and 7.7% of the CC group, while the serum TG gain rates in F2 female rats of these groups were decreased to 20.6%, 4.0% and 8.4% of the CC group, respectively. Compared with CC group, TCH level of females and males in NN group was decreased by 40.5% and 21.9%(P<0.01) before stress, respectively, and the TCH gain rate of females in NN group was increased by 49.7%(P<0.05) after stress. CONCLUSIONThe reproductive and developmental toxicities and the neuroendocrine metabolic programming alterations induced by prenatal nicotine exposure are transgenerated to F2 offspring and these effects exhibit gender and parental differences.Key words: nicotine; intrauterine growth retardation; transgenerational inheritance; glucose metabolism; lipid metabolism
XU Dan, Tel: 15972228956, E-mail: xuyidan70188@whu.edu.cn
国家自然科学基金(81220108026);国家自然科学基金(81430089);国家自然科学基金(81371483)
郭自景,女,硕士研究生,主要从事发育源性疾病研究; 徐 丹,女,副教授,博士,主要从事发育源性疾病研究。
徐 丹, E-mail: xuyidan70188@whu.edu.cn, Tel: 15972228956
Foundation item: The project supported by National Natural Science Foundation of China(81220108026); National Natural Science Foundation of China (81430089); and National Natural Science Foundation of China (81371483)
2015-01-04 接受日期: 2015-03-12)
R394.6
A
1000-3002(2015)02-0277-07
10.3867/j.issn.1000-3002.2015.02.015
--------------------
