连花清瘟胶囊对脂多糖致急性肺损伤小鼠炎症因子和连接蛋白表达的影响
2015-03-02崔雯雯张彦芬王宏涛何奇龙
崔雯雯, 金 鑫, 张彦芬, 王宏涛,2, 秘 尧, 何奇龙
(1. 黑龙江中医药大学药学院, 黑龙江 哈尔滨 150040; 2. 河北以岭医药研究院, 河北 石家庄 050035;3. 河北省络病重点实验室, 河北 石家庄 050035; 4. 国家中医药管理局心脑血管络病重点研究室,河北 石家庄 050035)
连花清瘟胶囊对脂多糖致急性肺损伤小鼠炎症因子和连接蛋白表达的影响
崔雯雯1, 金 鑫1, 张彦芬2,3, 王宏涛1,2, 秘 尧2,4, 何奇龙2,4
(1. 黑龙江中医药大学药学院, 黑龙江 哈尔滨 150040; 2. 河北以岭医药研究院, 河北 石家庄 050035;3. 河北省络病重点实验室, 河北 石家庄 050035; 4. 国家中医药管理局心脑血管络病重点研究室,河北 石家庄 050035)
目的 研究连花清瘟胶囊(LHQW)对脂多糖(LPS)致急性肺损伤小鼠肺组织连接蛋白表达的影响。方法 雄性KM小鼠随机分为6组,正常对照组、模型组、模型+地塞米松5 mg·kg-1组、模型+LHQW 2, 4和8 g·kg-1组,每组20只。地塞米松和LHQW均ig给药,每天1次,共7 d。末次给药24 h后,除正常对照组外其余5组气管内滴注LPS溶液,制备小鼠急性肺损伤模型。造模24 h后,处死小鼠。光镜下观察肺组织病理形态变化,透射电镜下观察肺泡上皮超微结构,流式细胞术检测外周血T细胞中肿瘤坏死因子α(TNF-α)阳性表达细胞百分率,免疫组化法检测肺组织间隙连接蛋白43(Cx43)、闭锁蛋白和闭锁小带蛋白(ZO-1)的表达。结果 光镜下观察发现,模型组小鼠肺内出现大量炎症细胞浸润,肺泡壁增厚;模型+地塞米松组、模型+LHQW 2, 4和8 g·kg-1组较模型组炎症细胞浸润减少,肺泡壁增厚减轻。电镜下可见,模型组肺泡上皮细胞出现损伤,模型+地塞米松组、模型+LHQW 2, 4和8 g·kg-1组较模型组均不同程度减轻。正常对照组、模型组、模型+地塞米松组、模型+ LHQW 2, 4和8 g·kg-1组外周血T细胞中TNF-α阳性表达细胞百分率分别为(3.6±0.9)%,(6.4±0.8)%,(2.8±0.7)%,(4.7±1.6)%,(4.0±1.5)%和(3.6±1.2)%,模型组明显高于正常对照组(P<0.01),其余各组均低于模型组(P<0.05,P<0.01)。模型组肺组织中Cx43、闭锁蛋白和ZO-1表达均低于正常对照组(P<0.01),模型+地塞米松、模型+LHQW 4和8 g·kg-1组3种蛋白表达均高于模型组(P<0.05)。结论 LHQW可能通过抑制炎症细胞浸润,改善肺泡上皮细胞和肺血管内皮细胞连接蛋白的表达,缓解LPS导致的急性肺损伤。
连花清瘟胶囊; 脂多糖; 急性肺损伤; 连接蛋白
急性肺损伤(acute lung injury, ALI)是由肺内外多种致病因素引起全身失控性炎症反应导致的急性、进行性呼吸衰竭[1],临床表现为呼吸困难、肺水肿和低氧血症等[2],其主要病理特征表现为肺泡上皮细胞和肺毛细血管内皮细胞发生损伤、通透性增加和细胞间隙增宽等[3]。已有研究表明,脂多糖(lipopolysaccharides,LPS)可以引起肺泡上皮细胞和毛细血管内皮细胞发生损伤,改变细胞间隙和通透性,引发肺水肿[1,4]。紧密连接广泛存在于肺泡上皮细胞和肺毛细血管内皮细胞间,对维持肺泡组织机械屏障的完整性和通透性起着重要作用[5]。闭锁蛋白(occludin)和闭锁小带蛋白-1(zonula occludens protein-1,ZO-1)是肺泡上皮和血管内皮紧密连接的重要成分[6]。缝隙连接介导的细胞间信号传递对维持细胞内稳态有重要意义,缝隙连接蛋白43(connexin 43,Cx43)在肺泡上皮细胞和肺毛细血管内皮细胞均有表达,对肺部炎症反应有重要的调控作用[7-8]。连花清瘟胶囊(Lianhuaqingwencapsules,LHQW)是具有广谱抗病毒、天然抗菌和抗炎等作用的中药复方制剂,能够明显抑制肺炎链球菌并减轻肺组织炎症反应[9]。本研究采用气管内滴注LPS导致小鼠ALI,以探讨LHQW对ALI小鼠连接蛋白的表达影响。
1 材料与方法
1.1 药物、试剂和仪器LHQW,石家庄以岭药业股份有限公司提供,批号120434;地塞米松片,天津天药药业股份有限公司,批号130211;LPS,德国Sigma公司;兔抗小鼠Cx43多克隆抗体,美国SAB公司;兔抗小鼠闭锁蛋白多克隆抗体,美国Abcam公司;兔抗小鼠ZO-1多克隆抗体,美国Abcam公司;SP检测试剂盒和DAB显色剂盒,中国中杉金桥生物技术有限公司;FITC标记抗小鼠CD3、PE标记抗小鼠肿瘤坏死因子α(tumor necrosis factor-α,TNF-α)单抗,美国Promega 公司。Leica RM2015切片机,德国Leica公司;Olympus IX71荧光倒置显微镜,日本Olympus公司;ABI 7300流式细胞仪,美国Beckman Coulter公司;H-7500型透射电镜,日本日立公司。
1.2 动物、动物分组和给药120只SPF级雄性KM小鼠,6周龄,体质量18~20 g,由中国食品药品检定研究所提供,合格证号:SCXK(京)2009-0017。实验前适应性喂养4 d后,随机分成6组,正常对照组,模型组,模型+地塞米松组,模型+LHQW 2, 4和8 g·kg-1组,每组20只。LHQW和地塞米松片采用0.5%羧甲纤维素钠配制成混悬液,模型+地塞米松组ig给予地塞米松5 mg·kg-1,模型+LHQW 2, 4和8 g·kg-1组分别ig给予LHQW 2, 4和8 g·kg-1,正常对照组和模型组ig给予等剂量的0.5%羧甲纤维素钠,每天1次,连续7 d。末次给药24 h后,除正常对照组外,其他各组小鼠用10%水合氯醛3.5 mg·kg-1腹腔麻醉后,将小鼠仰卧位固定在手术板上,颈部消毒,纵向切开颈部皮肤,剥离皮下组织,暴露气管,用1 mL注射器吸取LPS生理盐水溶液(5 mg·kg-1),从气管两气管环间快速刺入注射,2 mg·kg-1,滴入后立即直立小鼠,上下轻度晃动数次,使药物均匀分布于肺部,缝合伤口,让小鼠自然清醒。
1.3 光镜下观察肺组织病理变化气管滴注LPS溶液24 h后,将小鼠采用眼球取血的方式处死,开胸取肺。取左肺上叶组织块,放入4%甲醛溶液固定48 h,石蜡包埋,切片(片厚5 μm),每组中随机抽取3张切片,HE染色,光镜下观察肺组织病理变化。
1.4 透射电镜观察肺泡上皮超微结构每组中随机3只小鼠,取左肺下叶组织块(2 mm×2 mm×2 mm),放入2.5%戊二醛溶液中固定48 h,二甲砷酸缓冲液冲洗2遍,四氧化锇固定再经缓冲液冲洗,逐级丙酮脱水,环氧树脂浸透包埋,超薄切片,醋酸铀-枸橼酸铅染色,透射电镜观察肺组织超微结构的变化。
1.5 流式细胞术法检测外周血T细胞中TNF-α表达阳性细胞率取出小鼠全血,用肝素抗凝,分离外周血单个核细胞,调整细胞密度。然后采用佛波酯和离子霉素刺激淋巴细胞,收集单个核细胞,红细胞裂解液裂解,对照管加入同型对照,TNF管加入FITC-抗CD3单抗 3.5 μL,室温避光孵育30 min,加入破膜剂1号和2号,放置5 min,加入PE标记的抗TNF-α单抗2 μL,放置30 min,离心,洗去未结合抗体,应用流式细胞仪检测细胞。Exp32软件获取分析细胞,每个检测获取10 000个细胞,采用前向角和侧向角散射光散点图设门区分淋巴细胞,分析胞内TNF-α表达,计算TNF-α表达阳性细胞百分率。
1.6 免疫组化法检测肺组织中Cx43、闭锁蛋白和ZO-1的表达取左肺上叶组织块,放入4%甲醛溶液固定48 h,石蜡包埋,切片(片厚5 μm),于切片中任意抽取其中3片,脱蜡, 水化,PBS冲洗,加入一抗(兔抗小鼠Cx43,闭锁蛋白和ZO-1多克隆抗体),4℃过夜,PBS冲洗;滴加生物素标记的羊抗兔IgG二抗,37℃孵育30 min,PBS冲洗;滴加辣根酶链霉卵白素工作液,37℃孵育30 min,PBS冲洗;DAB显色,蒸馏水洗,复染(苏木素染色,1%盐酸乙醇分化,自来水洗),脱水,透明,封片,光镜(×400)下观察。在相同放大倍数下,每张切片取3个不同的视野采集图像,经过Image Pro Plus 6.0图像分析系统进行半定量分析。
2 结果
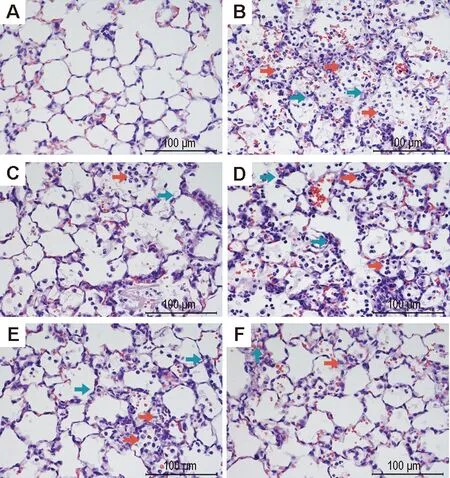
2.1 LHQW对LPS致ALI小鼠肺组织病理损伤的影响正常对照组小鼠肺泡结构完整,肺泡壁无增厚,无炎症细胞浸润,无充血,未见明显病理改变(图1A)。模型组肺泡腔内可见大量中性粒细胞、少量淋巴细胞和巨噬细胞的渗出物,大量红细胞渗出,肺泡壁薄厚不均,肺泡间质增生、肺泡壁水肿(图1B);模型+地塞米松组较模型组,中性粒细胞等炎症细胞明显减少,红细胞渗出减少,肺泡间质增生及肺泡壁水肿明显减轻(图1C)。与模型组比较,模型+ LHQW 3个剂量组肺组织病理形态损伤均有改善,模型+LHQW 2 g·kg-1组虽仍可见大量中性粒细胞、巨噬细胞和红细胞等渗出,肺泡壁薄厚不均,但较模型组减轻(图1D);模型+LHQW 4 g·kg-1组可见肺内炎症细胞浸润、红细胞渗出和间质增生等现象均有减轻(图1E);模型+LHQW 8 g·kg-1组可见少量中性粒细胞和红细胞等渗出物,肺泡壁水肿和肺泡间质增生等现象明显改善(图1F)。
Fig.1 Effect of Lianhuaqingwen capsules (LHQW) on lung pathological damage of acute lung injury (ALI) mice induced by lipopolysaccharides(LPS) (HE staining). LHQW and dexamethasone were ig given once a day for 7 d, and LPS 5 mg·kg-1was intratracheally given 24 h after the final administration of drugs. 24 h later, the mice were sacrificed and lung pathological damage was observed. A:normal control; B: model; C:model+dexamethasone 5 mg·kg-1; D, E and F: model+LHQW 2, 4 and 8 g·kg-1, respectively. The red arrows show inflammatory cell aggregation,and the green arrows show alveolar wall of uneven thickness.
2.2 LHQW对LPS致ALI小鼠肺组织超微结构变化的影响正常对照组肺组织结构基本正常,肺泡Ⅱ型上皮细胞微绒毛排列整齐,胞浆内有大量板层小体和线粒体,板层小体结构清晰,基膜完整(图2A);模型组Ⅱ型上皮细胞损伤,纤毛轻微断裂、融合,板层小体空泡化,嗜锇性板层小体排空(图2B);模型+地塞米松组和模型+LHQW 8 g·kg-1组病变明显减轻,可见大量板层小体和线粒体,板层小体结构轻微混乱(图2C,F);模型+LHQW 2 g·kg-1组可见线粒体和板层小体均空泡化,Ⅱ型上皮细胞损伤(图2D);模型+LHQW 4 g·kg-1组线粒体出现空泡化,板层小体轻微空泡化(图2E)。

Fig.2 Effect of LHQW on lung ultrastructure changes of ALI mice induced by LPS (transmission electron microscope, ×20 000 ). See Fig.1 for the mouse treatment. A: normal control; B: model; C:model+dexamethasone 5 mg·kg-1; D, E and F: model+LHQW 2, 4 and 8 g·kg-1, respectively. The red arrows show lamellar body structure confusion or vacuolization, and the green arrows show cilia or mitochondrial fracture.
2.3 LHQW胶囊对LPS致ALI小鼠外周血中TNF-α阳性表达细胞百分率的影响与正常对照组比,模型组全血中TNF-α阳性表达细胞百分率明显升高(P<0.01),提示LPS造模后炎症因子表达升高;与模型组相比,模型+LHQW 2,4和8 g·kg-1组均明显下降(P<0.05,P<0.01),说明LHQW能够不同程度降低炎症因子的表达(表1)。
Tab.1 Effect of LHQW on cell percentage of tumor necrosis factor-α (TNF-α) positive expression in peripheral blood of ALI mice induced by LPS

GroupDose/g·kg-1TNF⁃αpositiveexpressioncell/%Normalcontrol3.6±0.9Model6.4±0.9∗∗Model+dexamethasone2.8±0.7##Model+LHQW24.7±1.6#44.0±1.5##83.6±1.2##
2.4 LHQW对LPS致ALI小鼠肺组织Cx43、闭锁蛋白、ZO-1表达的影响免疫组化染色结果(图3, 4和5,表2)表明,正常对照组小鼠肺组织Cx43(图3A)和ZO-1(图5A)在肺泡上皮细胞和血管内皮细胞胞浆内有大量阳性表达,呈棕褐色颗粒状,闭锁蛋白(图4A)在肺泡上皮细胞和血管内皮细胞的胞膜和胞浆均有阳性表达;与正常对照组相比,模型组小鼠肺内Cx43(图3B)、闭锁蛋白(图4B)和ZO-1(图5B)的表达均明显减少(P<0.01);与模型组相比,模型+地塞米松组小鼠肺内Cx43(图3C)、闭锁蛋白(图4C)、ZO-1(图5C)的表达明显增多(P<0.01),模型+LHQW 4和8 g·kg-1组Cx43、闭锁蛋白和ZO-1表达亦明显增加(P<0.05,P<0.01);模型+LHQW 2 g·kg-1组3个蛋白的表达均无明显变化。
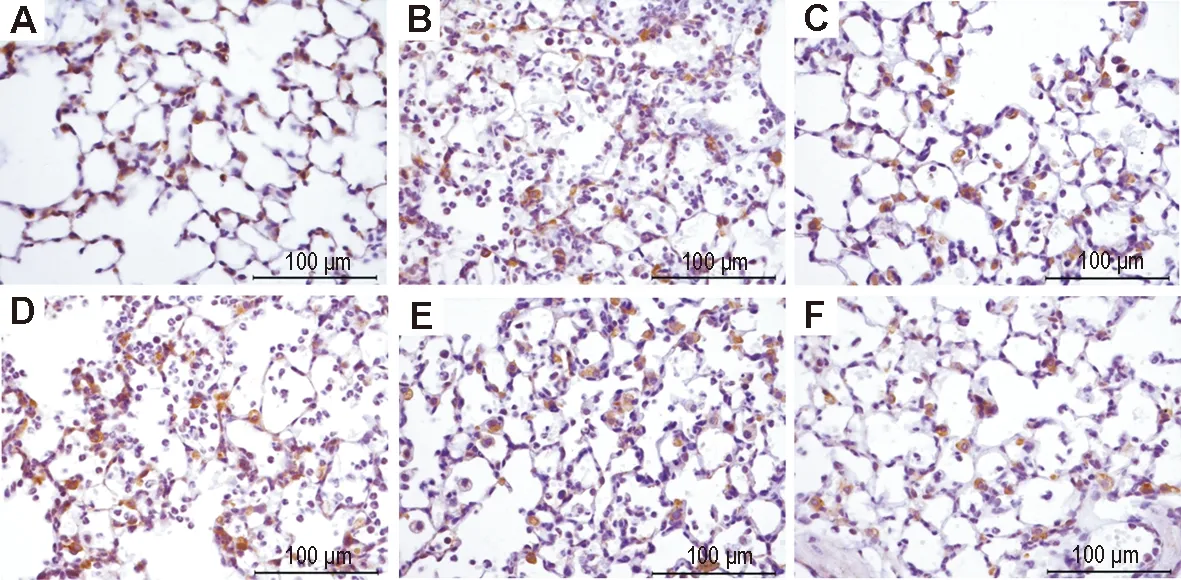
Fig.3 Effect of LHQW on expression of connexin 43 (Cx43) in lung tissue of ALI mice induced by LPS (immunohistochemistry). See Fig.1 for the mouse treatment. A:normal control group; B: model group; C:model+dexamethasone 5 mg·kg-1group; D, E and F: model+LHQW 2, 4 and 8 g·kg-1, respectively.
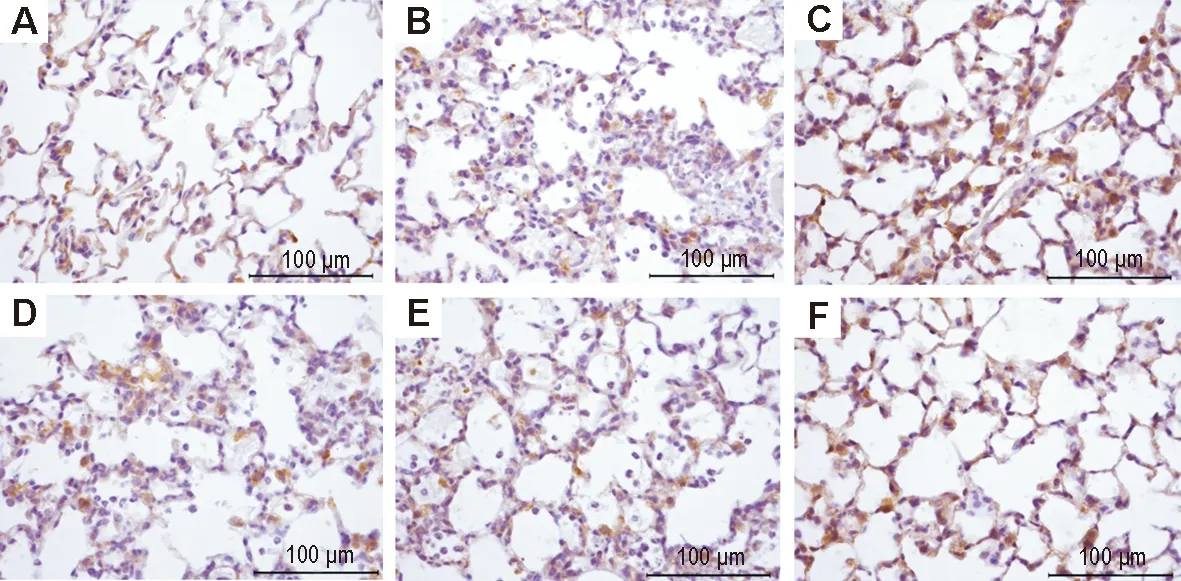
Fig.4 Effect of LHQW on expression of occludin in lung tissue of ALI mice induced by LPS (immunohistochemistry). See Fig.1 for the mouse treatment. A: normal control group; B: model group; C:model+dexamethasone 5 mg·kg-1group; D, E and F: model+LHQW 2, 4 and 8 g·kg-1, respectively.
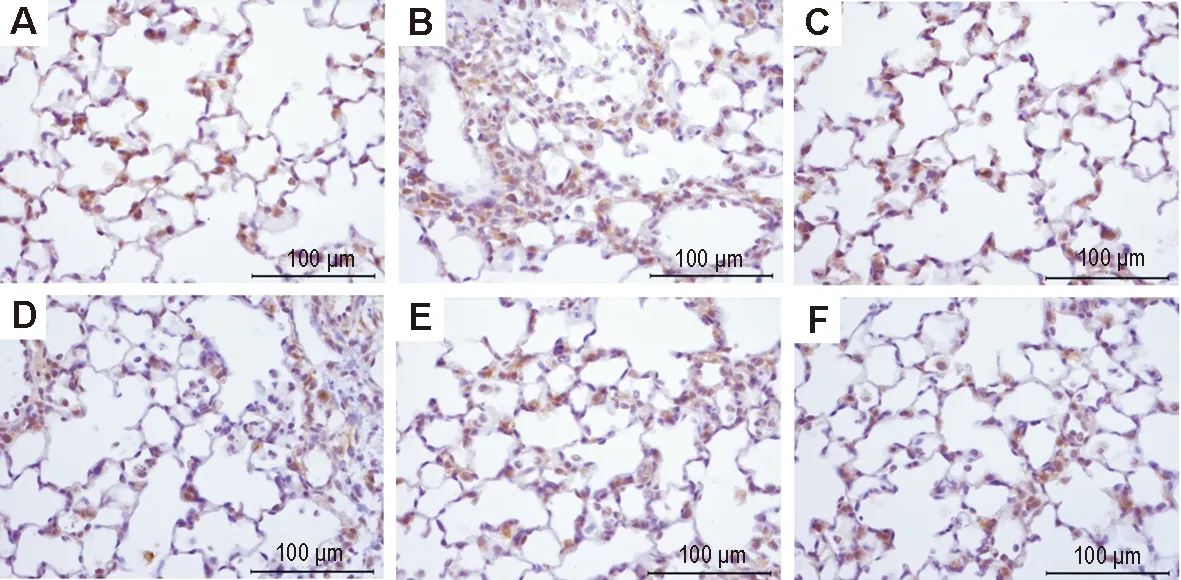
Fig.5 Effect of LHQW on expression of zonula occludens protein-1 (ZO-1) in lung tissue of ALI mice induced by LPS (immunohistochemistry). See Fig.1 for the mouse treatment. A: normal control group; B: model group; C:model+dexamethasone 5 mg·kg-1group; D, E and F: model+LHQW 2, 4 and 8 g·kg-1, respectively.
Tab.2 Effect of LHQW on Cx43, occludin and ZO-1 expression in lung tissue of ALI mice induced by LPS

GroupDose/g·kg-1Proteinexpression(Integratedabsorbance)Cx43OccludinZO⁃1Normalcontrol0.37±0.060.27±0.070.21±0.06Model0.23±0.06∗∗0.18±0.04∗∗0.12±0.03∗∗Model+dexamethasone0.35±0.07##0.25±0.05##0.21±0.06##Model+LHQW20.28±0.070.20±0.040.15±0.0540.31±0.08#0.23±0.03#0.18±0.06#80.35±0.10##0.25±0.05##0.19±0.09##
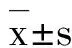
3 讨论
本研究观察肺组织病理改变和超微结构发现,气管内滴注LPS能够引起肺泡上皮和内皮的损伤,使细胞间隙增宽,通透性增加,进而致大量的炎症细胞浸润在肺泡腔内,引发肺水肿。这与Zemans等[1]和杨茂宪等[10]的研究结果一致。ALI小鼠外周血中TNF-α阳性表达细胞百分率的增加,进一步说明LPS进入肺内损伤肺泡上皮细胞,激活炎症细胞释放炎症因子,由于血管通透性的改变和肺毛细血管内皮的损伤,大量的炎症因子进入血管内,导致血液内炎症因子的含量增加。Matute-Bello等[11]也认为,制备动物ALI模型时,应该复制出肺泡上皮细胞和肺血管内皮细胞的病理损伤变化和炎症反应,才更符合临床ALI的病理特征。紧密连接和缝隙连接是肺泡上皮细胞和肺毛细血管内皮细胞间的2种重要连接方式。它们的存在对调节细胞通透性、维持细胞膜极性起到很大的作用,充分保持肺组织血气屏障的完整性[6,12]。紧密连接作为细胞之间的“屏障”,主要存在于上皮细胞、内皮细胞间的连接复合体中,保证机体内环境的相对稳定[13]。紧密连接蛋白主要是由3种完整的跨膜蛋白闭锁蛋白、封闭蛋白、连接黏附分子和3种闭锁小带蛋白ZO-1,ZO-2和ZO-3组成的。其中,闭锁蛋白和ZO-1是构成紧密连接屏障功能的调节蛋白,闭锁蛋白是跨膜整合蛋白,形成了连接在细胞间的紧密结合点;ZO-1属于细胞内蛋白,与闭锁蛋白的胞质结合,将闭锁蛋白定位于细胞骨架,充分保证了紧密连接的完整性[14]。因而,这两种紧密连接蛋白在肺泡上皮细胞和肺血管内皮细胞中的表达,对维持肺泡上皮屏障结构和血管通透性的稳定起到了重要作用。缝隙连接是由缝隙连接蛋白组合而成的,参与细胞间的物质交换和信号传导。Haefliger等[15]研究发现,Cx43是一种重要的细胞缝隙连接蛋白,它在肺泡上皮细胞和血管内皮细胞均有表达,也是肺组织细胞表达最主要的缝隙连接蛋白,能够调控对ALI至关重要的 Ca2+信号通路,与肺泡-毛细血管通透性也息息相关,是肺部炎症反应的基础[16]。LHQW是具有清瘟解毒、宣肺泄热作用的广谱抑菌、抗炎的中药复方制剂。研究发现,它对肺炎链球菌、金黄色葡萄球菌、甲型和乙型溶血性链球菌等具有明显的抑制作用,能降低流感病毒感染肺组织炎性因子TNF-α, 白细胞介素1b和白细胞介素6含量,对急性放射性肺损伤大鼠的炎症反应具有缓解作用[17-19]。综合本研究结果,即LPS组小鼠肺组织病理变化、肺部超微结构变化和全血中TNF-α阳性表达细胞百分率升高,表明已成功复制ALI模型。LHQW能不同程度地提高闭锁蛋白、ZO-1和Cx43的表达,表明LPS进入肺部后可能破坏了肺泡上皮细胞和血管内皮细胞间紧密连接蛋白构成的保护结构,增加血管通透性,影响了细胞间的信号传递和物质交换,从而致使炎症反应蔓延导致ALI;LHQW可以改善上述变化,抑制炎症反应的发展,缓解LPS造成的ALI。综上所述,LHQW对LPS导致的ALI肺组织紧密连接蛋白和缝隙连接蛋白的表达均有影响,提示LHQW可通过增加这两类连接蛋白在肺组织的表达、改善肺组织血气屏障和细胞通透性,但对肺泡上皮细胞和肺毛细血管内皮细胞的具体保护作用机制尚不清楚,需进一步研究。
[1] Zemans RL, Colgan SP, Downey GP. Transepithelial migration of neutrophils: mechanisms and implications for acute lung injury[J].AmJRespirCellMolBiol, 2009, 40(5):519-535.
[2] Ware LB, Matthay MA. The acute respiratory distress syndrome[J].NEnglJMed, 2000, 342(18):1334-1349.
[3] Dong HY. Phsp70/IκBαm prevents acute lung injury controllablly and effectively(Phsp70/IκBαm抑制NF-κB防治小鼠急性肺损伤的自控性和有效性研究)[D]. Xi′an: the Fourth Military Medical University, 2012.
[4] Gerber A, Heimburg A, Reisenauer A, Wille A, Welte T, Bühling F. Proteasome inhibitors modulate chemokine production in lung epithelial and monocytic cells[J].EurRespirJ, 2004, 24(1):40-48.
[5] Anderson JM, Van Itallie CM. Tight junctions and the molecular basis for regulation of paracellular permeability[J].AmJPhysiol, 1995, 269(4 Pt 1):G467-G475.
[6] Bazzoni G, Dejana E. Endothelial cell-to-cell junctions: molecular organization and role in vascular homeostasis[J].PhysiolRev, 2004, 84(3):869-901.
[7] Jiao HC, Liu CY, Guo L. Research progress of connexin 43 and arrhythmia relationship[J].PractJCardCerebrPneumalVascDis(实用心脑肺血管病杂志), 2013, 21(4):36-37.
[8] Scheckenbach KE, Crespin S, Kwak BR, Chanson M. Connexin channel-dependent signaling pathways in inflammation[J].JVascRes, 2011, 48(2):91-103.
[9] Lei Z, Lu HD, Dong KC, Lu C, Chen WQ, Yuan JP,etal.Lianhuaqingwencapsules inhibited the expression and effect of MCP-1 in rats with radiation-induced acute lung injury[J].HerMed(医药导报), 2014, 33(7):845-849.
[10] Yang MX, Zhao WJ. To compare two acute lung injury rat models induced by lipopolysaccharide in different pathways[J].ChinJCritCareMed(中国急救医学), 2012, 32(12):1102-1105.
[11] Matute-Bello G, Downey G, Moore BB, Groshong SD, Matthay MA, Slutsky AS,etal. An official American Thoracic Society workshop report: features and measurements of experimental acute lung injury in animals[J].AmJRespirCellMolBiol, 2011, 44(5):725-738.
[12] Xie WL. Resolvin D1 reduces deterioration of tight junction proteins by up-regulating HO-1 in LPS-induced mice(消退素D1对脂多糖诱导的小鼠肺紧密连接蛋白破坏的作用及机制)[D]. Wuhan: Huazhong University of Science and Technology, 2013.
[13] Li CF, Jiao GY, Liu CL. The expression of tight junction in rat lung tissue after LPS-induced acute lung injury[J].ChinaModDoct(中国现代医生), 2011, 49(15):16-17.
[14] Zhao XY, Li B, Cao LY, Fang WG, Zhu L, Chen YH. Opening of tight junction of human brain microvascular endothelial cells induced by small cell lung cancer cells[J].ProgAnatSci(解剖科学进展), 2009, 15(2):183-187,191.
[15] Haefliger JA, Nicod P, Meda P. Contribution of connexins to the function of the vascular wall[J].CardiovascRes, 2004, 62(2):345-356.
[16] Scheckenbach KE, Crespin S, Kwak BR, Chanson M. Connexin channel-dependent signaling pathways in inflammation[J].JVascRes, 2011, 48(2):91-103.
[17] Mo HY, Yang ZF, Zheng JP, Li YM, Xiao ZL. Experimental study of Lianhuaqingwen capsules for prevention and treatment of influenza virus FM1 infected mice[J].JChinMedMater(中药材), 2008, 32(8):1230-1233.
[18] Lei HT, Liu MY, Ouyang JF, Wang HT, Ma SH, Wang YZ,etal. Study on Lianhua Qingwen capsule resistingStaphylococcusaureusbiofilm[J].ChinJExpTraditMedForm(中国实验方剂学杂志), 2013, 19(22):161-164.
[19] Hu YF. Analysis of pharmacological and clinical curative effects of Lianhuaqingwen capsules[J].ModDiagnTreat(现代诊断与治疗), 2013, 24(9):2012.
(本文编辑: 齐春会)
Effect of Lianhuaqingwen capsules on inflammatory cytokinesand junction protein expression in mice with acutelung injury induced by lipopolysaccharides
CUI Wen-wen1, JIN Xin1, ZHANG Yan-Fen2,3, WANG Hong-tao1,2, MI Yao2,4, HE Qi-long2,4
〔1.CollegeofPharmacy,HeilongjiangUniversityofChineseMedicine,Harbin150040,China;2.YilingMedicalResearchInstituteofHebeiProvince,Shijiazhuang050035,China; 3.KeyLaboratoryofNetworkDiseaseofHebeiProvince,Shijiazhuang050035,China; 4.KeyLaboratoryofStateAdministrationofTraditionalChineseMedicine, (Cardio-CerebralVascularNetworkDisease),Shijiazhuang050035,China〕
OBJECTIVE To explore the effect ofLianhuaqingwencapsules (LHQW) on junction protein expression in mouse lung tissue of lipopolysaccharide (LPS)-induced acute lung injury (ALI). METHODS 120 male mice were randomly divided into six groups: normal control, model, model+dexamethasone 5 mg·kg-1, model+LHQW 2, 4 and 8 g·kg-1groups. Dexamethasone and LHQW were administered orally, once daily, for 7 d. 24 h after the last administration, LPS solution was instilled into the tracheas of mice except the normal control group to prepare the mouse model of ALI. 24 h after the establishment of the ALI model, the mice were sacrificed and the pathological changes in the mouse lung tissue were observed by optical microscopy and ultrastructure of alveolar epithelium was observed by transmission electron microscopy. The cell percentage of positive expression of tumor necrosis factor-α (TNF-α) in the peripheral blood T lymphocytes was detected by flow cytometry. The expressions of connexin 43 (Cx43), occludin and zonula occludens protein-1 (ZO-1) in lung tissues were detected by immunohistochemistry. RESULTS Under the light microscope, the mouse lung of model group showed a large amount of inflammatory cell infiltration and alveolar wall thickening. Compared with model group, inflammatory cell infiltration was reduced in model+dexamethasone, model+LHQW 2,4 and 8 mg·kg-1groups. Under the electron microscope, the mouse alveolar epithelial cells of model group showed injury. Compared with model group, the damage was reduced in model+dexamethasone, and model+LHQW 2, 4 and 8 mg·kg-1groups. The cell percentage of TNF-α positive expression in peripheral blood T lymphocytes in normal control, model, model+dexamethasone, model+LHQW 2,4 and 8 mg·kg-1groups was (3.6±0.9)%, (6.4±0.8)%, (2.8±0.7)%, (4.7±1.6)%, (4.0±1.5)% and (3.6±1.2)%, respectively. The percentage in model group was obviously higher than that in normal control group(P<0.01), but was lower in the four drug treatment groups than in model group(P<0.05,P<0.01). The expression of Cx43, occludin and ZO-1 in lung tissue of model group was lower than that of normal control group(P<0.01), but higher in model+dexamethasone, model + LHQW 4 and 8 mg·kg-1groups than in model group(P<0.05). CONCLUSION LHQW may alleviate ALI induced by LPS and play a protective role by inhibiting inflammatory cell infiltration and improving protein connection expression in alveolar epithelial cells and pulmonary vascular endothelial cells.Key words:Lianhuaqingwencapsules; lipopolysaccharides; acute lung injury; junction protein
WANG Hong-tao,E-mail: wanghongtao@yiling.cn, Tel: (0311)85901553
国家科技重大专项(2011ZX09201-201-27); 河北省中医药管理局科研计划(2014227)
崔雯雯(1991-),女,中药学硕士,主要从事中药药理学与呼吸药理学研究,Tel: (0311)85901553, E-mail: reaishenghuo0506@163.com
王宏涛,E-mail: wanghongtao@yiling.cn, Tel: (0311)85901553
Foundation item: The project supported by National Science and Technology Major Project of Original New Drug Research of China(2011ZX09201-201-27);and Scientific Research Program of Hebei Province Administration of Traditional Chinese Medicine(2014227)
2014-09-23 接受日期: 2015-03-11)
R285.5,R974
A
1000-3002(2015)02-0213-07
10.3867/j.issn.1000-3002.2015.02.005
--------------------
