中东呼吸综合征冠状病毒及其疫苗和特异性药物的研发
2015-02-22瞿涤陆路姜世勃
瞿涤,陆路,姜世勃
复旦大学基础医学院病原生物学系,教育部/卫生部医学分子病毒学重点实验室,上海 200032
中东呼吸综合征冠状病毒及其疫苗和特异性药物的研发
瞿涤,陆路,姜世勃
复旦大学基础医学院病原生物学系,教育部/卫生部医学分子病毒学重点实验室,上海 200032
摘要:中东呼吸综合征冠状病毒(MERS-CoV)是引起MERS的病原。本文就MERS-CoV的分类、特性、决定宿主特异性的分子结构及其疫苗研究状况和特异性药物研究进展等方面进行综述。
关键词:中东呼吸综合征冠状病毒;中东呼吸综合征;疫苗;抗中东呼吸综合征冠状病毒中和抗体
2015年5月20日韩国报道了韩国首例中东呼吸综合征(Middle East respiratory syndrome,MERS)病例,至6月7日韩国已有64例确诊病例,其中5例死亡(http://edition.cnn.com/2015/06/05/asia/south-korea-mers/index.html)。MERS呈暴发趋势引起全球高度关注[1]。该病由一种新型冠状病毒感染所致,国际病毒命名委员会冠状病毒研究组(2013年5月)已将该新型冠状病毒命名为中东呼吸综合征冠状病毒(Middle East respiratory syndrome coronavirus,MERS-CoV)[2]。本文就MERS-CoV有关的主要研究综述如下。
1MERS的出现和病毒的来源
2012年3月约旦出现第1例由一种新型冠状病毒引起感染的病例[3],主要临床表现为发热、咳嗽、呼吸急促和呼吸困难等严重急性呼吸综合征(severe acute respiratory syndrome,SARS)样症状,并伴有肾衰竭等。2012~2013年约旦、卡塔尔、沙特阿拉伯、阿拉伯联合酋长国等中东国家,以及突尼斯、法国、德国、英国等相继报道此病,并发现这些病例均直接或间接与中东地区有关[4]。从沙特阿拉伯吉达肺炎伴肾衰竭患者的痰中分离到1株未知的新冠状病毒株,称为人乙型冠状病毒2c EMC(Erasmus Medical Center)(GenBank accession No. JX869059.2)[5];随后又分离到病毒株人乙型冠状病毒2C Jordan-N3 (GenBank accession No. KC776174.1)和乙型冠状病毒England 1(GenBank accession No. KC164505.2)。3株病毒全基因组测序分析结果显示,来自不同患者的病毒株序列同源性高达99%,30.1 kb的基因组中仅有100 个核苷酸变异[6]。为利于后续研究,国际病毒命名委员会冠状病毒研究组将该病毒命名为MERS-CoV。
截止2015年6月,已有20多个国家和地区,包括埃及、伊拉克、约旦、科威特、黎巴嫩、阿曼、塔尔、沙特阿拉伯、阿拉伯联合酋长国、也门、突尼斯、法国、德国、希腊、意大利、荷兰、英国、土耳其、马来西亚、菲律宾、韩国、中国、中国香港地区、美国、阿根廷、澳大利亚,报道了MERS病例。中国病例为输入性病例(来自韩国)。鉴于MERS-CoV给公共卫生造成了严重威胁,世界卫生组织(World Health Organization,WHO)建议有关国家和国际组织加强监测和疑似病例的检测和控制。WHO于2015年6月5日报道,实验室确诊病例1 185例,死亡443例,伴有基础疾病的老年患者病情严重[1,4]。流行病学分析结果显示,原发病例与排毒或血清学阳性的骆驼有接触史,而大多数二代病例为医疗护理相关感染(heath care-associated infection),MERS-CoV在人间传播限于飞沫和密切接触。韩国MERS的暴发提示MERS-CoV可能发生突变,人间传播力可能增强,但有待后续研究确证[1]。
鉴于原发病例均有骆驼接触史,Müller等检测了189份实验室保存的单峰骆驼的血清(1983~1984年,采自埃及、苏丹和索马里),发现81%为MERS-CoV中和抗体阳性,提示MERS-CoV在非洲东北区域的单峰骆驼中已流行数十年[7]。因此,WHO建议旅游者尽量减少与单峰骆驼的接触。
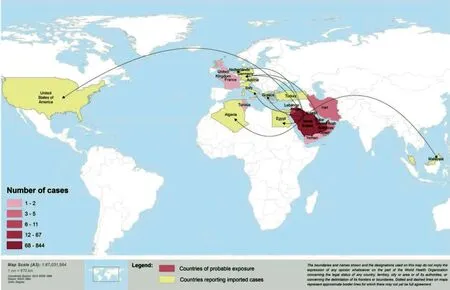
图1 截至2015年2月报道MERS-CoV感染病例的国家(WHO)Fig.1 Countries reporting MERS-CoV infection as of 5 February, 2015 (WHO)
2MERS-CoV的分类
在病毒分类上,MERS-CoV不同于普通的冠状病毒,也不同于SARS-CoV,虽然其在2013年常被称为SARS样病毒。MERS-CoV为单正链RNA病毒,属套式病毒目(Nidovirale)冠状病毒科(Coronaviridae)乙型冠状病毒属(Betacoronavirus)的C系群。序列进化分析显示,MERS-CoV与蝙蝠冠状病毒(Tylonycterisbat coronavirus HKU4和Pipistrellusbat coronavirus HKU5)近缘,同源性达81%,与猪血凝性脑脊髓炎病毒VW572的同源性达75%。根据国际病毒命名委员会的命名规则,病毒的保守复制酶功能域序列达90%同源者考虑为同种,因此该病毒是一种不同于以往报道的新型冠状病毒[2]。
3MERS-CoV的受体
MERS-CoV的形态和结构与冠状病毒相似。其包膜蛋白刺突蛋白(spike protein,S蛋白)决定该病毒的宿主范围。人和动物的冠状病毒均具有类似双层脂膜包膜结构,但不同的冠状病毒其结构蛋白和非结构蛋白的种类和数量有所差异。MERS-CoV的受体与SARS-CoV受体不同。SARS-CoV的受体是血管紧张素转换酶 2(angiotensin converting enzyme 2,ACE2)[8],而MERS-CoV的受体是二肽基肽酶4(dipeptidyl peptidase 4,DPP4;也称为CD26)[9,10]。CD26是继ACE2和氨基肽酶N(aminopeptidase N,APN;也称为CD13)之后[11]第3个被鉴定为功能性冠状病毒受体的肽酶,但其酶活性似乎与病毒入侵无明显关系。CD26参与MERS-CoV包膜S蛋白的相互作用,介导病毒吸附至宿主细胞并进一步融合,从而启动病毒感染。CD26在细胞中的表达水平和组织分布决定了MERS-CoV的细胞嗜性和致病性。人体多器官(肺、肾、小肠、肝、前列腺及免疫细胞)的上皮细胞、内皮细胞表达CD26,且CD26以可溶形式存在于血循环中[12]。此外,CD26在糖代谢、T细胞激活、免疫细胞趋化、细胞黏附及细胞凋亡等方面发挥重要作用。
与其他冠状病毒一样,MERS-CoV的S蛋白可被宿主细胞酶切割为S1和S2 亚单位(图2)。S1与受体相互作用,而S2参与膜融合[13]。蛋白晶体结构解析显示,MERS-CoV S1的C端367~606位氨基酸为受体结合功能域[9]。CD26与MERS-CoV的受体结合结构域(receptor binding domain,RBD)结合,解离常数(Kd)为16.7 nmol/L,但不与SARS-CoV的RBD结合。病毒-受体相互作用主要受亲水性氨基酸残基的影响,类似于腺苷脱氨酶(adenosine deaminase,ADA)与CD26的相互作用。ADA与CD26相互作用为诱导T细胞共刺激信号。与MERS-CoV S1相互作用的CD26 残基也参与与ADA的结合,提示ADA与MERS-CoV竞争结合CD26,MERS-CoV可能通过与ADA竞争识别位点而影响宿主的免疫系统。人和蝙蝠的CD26识别MERS-CoV RBD结合位点的氨基酸残基是保守的,仅有2个氨基酸变异(295T和R317Q),这就解释了为什么MERS-CoV可经蝙蝠感染人[14]。MERS-CoV RBD包括2个亚功能域(subdomain):RBD核心区和受体功能域(位于细胞外)。乙型冠状病毒属病毒具有与MERS-CoV S1的RBD核心区同源的结构区。MERS-CoV S1的RBD核心功能区与SARS-CoV S蛋白同源,但其外部受体结合基序域(external receptor binding motif region)参与受体识别,与特异的致病机制有关[10]。
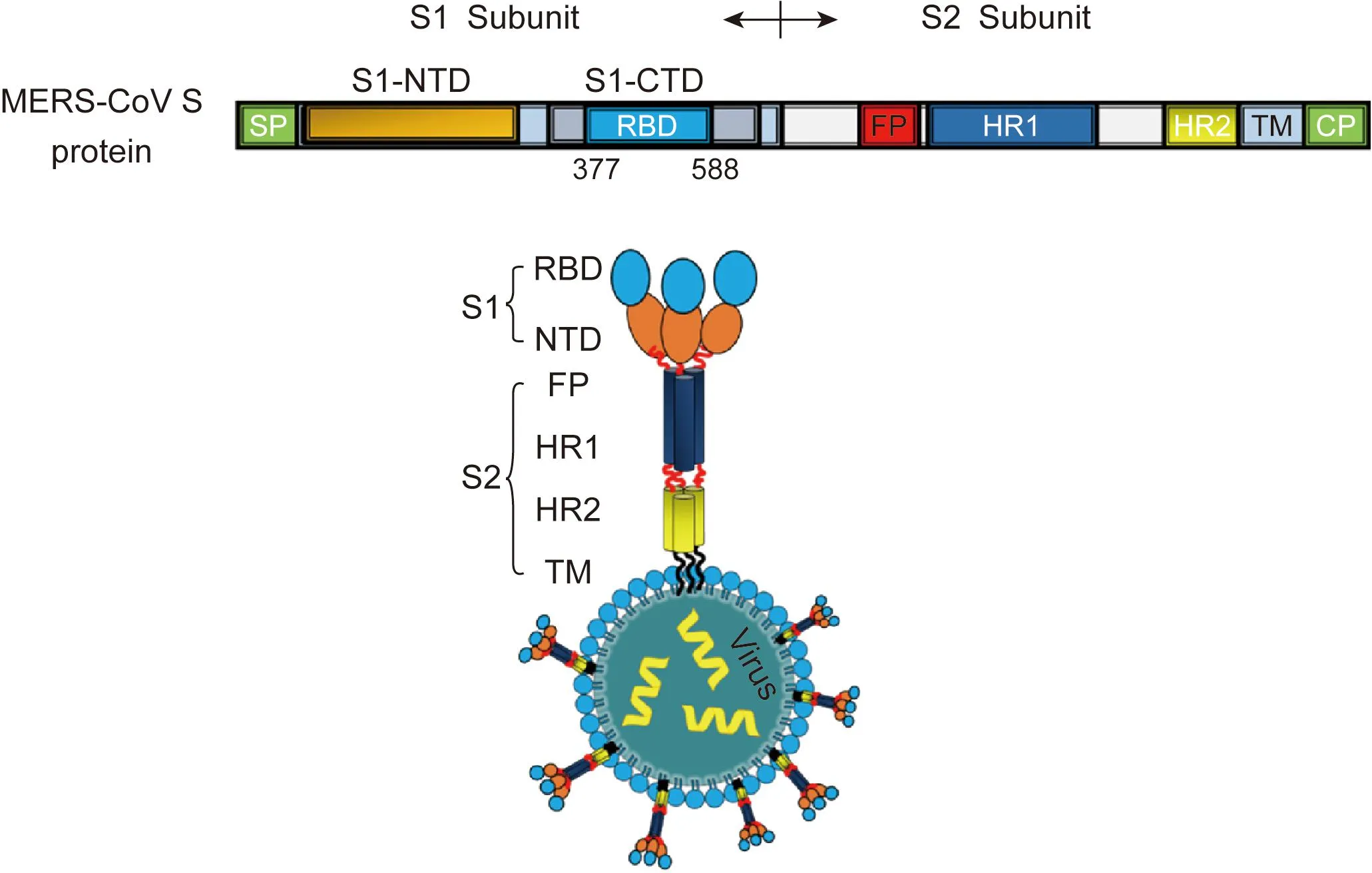
The schematic representation of MERS-CoV S protein consists of S1 subunit and S2 subunit. S1 subunit contains the N-terminal domain (S1-NTD) and the C-terminal domain (S1-CTD), and the later harbors the receptor binding domain (RBD). S2 subunit contains fusion peptide (FP), heptad repeat 1 domain (HR1), heptad repeat 2 domain (HR2), transmembrane domain (TM), and cytoplasmic domain (CP).图2 MERS-CoV的S蛋白结构Fig.2 Structure of MERS-CoV S protein
4MERS-CoV与细胞的融合及复制
MERS-CoV S2包含融合肽、七肽重复区1(heptad repeat 1,HR1)、HR2、跨膜区和胞质内区[15]。MERS-CoV S1与细胞RBD结合,启动S蛋白构象改变,融合肽插入靶细胞HR1与HR2之间,形成六螺旋的融合结构(six-helix bundle fusion core)(图3)。针对HR2的抗病毒融合肽可阻碍HR1与HR2的相互作用,从而抑制病毒融合[16]。
MERS-CoV的复制与其他冠状病毒相似,通过与受体结合、膜融合进入宿主细胞,病毒颗粒内的核衣壳和病毒RNA释放入细胞质,ORF1a/b翻译出病毒早期蛋白如病毒的复制酶、蛋白酶等非结构蛋白,以正链RNA病毒基因组为模板合成全长负链RNA,然后以后者为模板转录出正链全长病毒基因组及短链mRNA。病毒核蛋白与基因组RNA在胞质内形成螺旋形核衣壳,而病毒的S蛋白、包膜蛋白(envelope protein,E蛋白)和膜蛋白(membrane protein,M蛋白)插入内质网膜。病毒以出芽的方式,经内质网和高尔基复合体获得病毒包膜,通过细胞外排(exocytosis)释放到细胞外[17]。
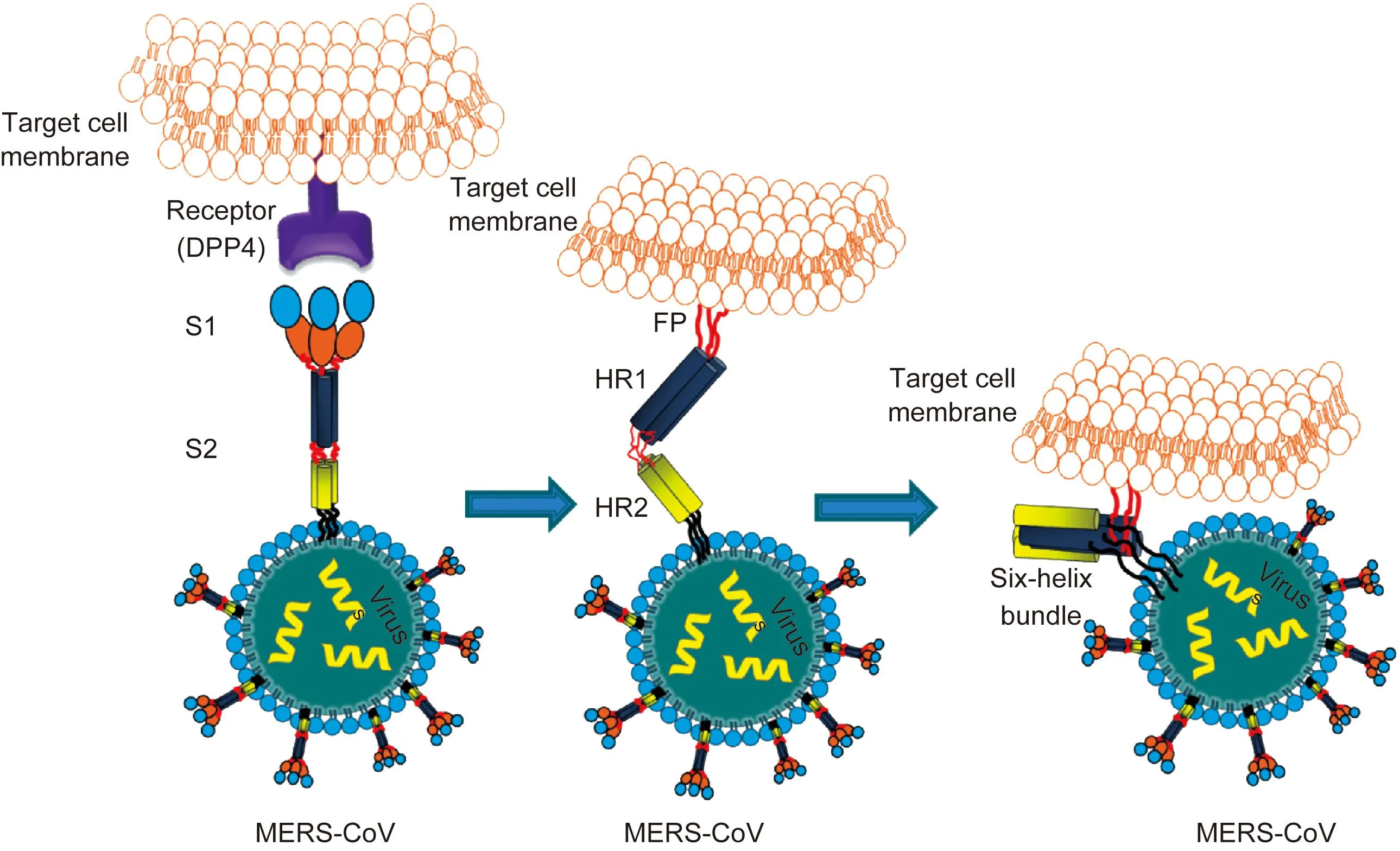
S1 subunit is responsible for binding with its receptor (DPP4), in which the receptor binding domain (RBD) is marked with blue. Then, it induces the change in the conformation of S2 subunit by inserting its FP into the target cell membrane. Its HR2 molecules then bind to the HR1 trimer to form a six-helix bundle (6-HB) core structure, which brings the viral and cellular membranes into close proximity to facilitate fusion.图3 MERS-CoV S蛋白与细胞受体的相互作用Fig.3 Interaction between MERS-CoV S1/S2 and cells
5抗MERS-CoV感染的免疫机制
免疫逃逸是冠状病毒在宿主细胞内复制、致病的重要策略。MERS-CoV编码的蛋白(ORF1a/b nsp3编码的木瓜蛋白酶样酶,ORF编码的M蛋白及ORF4a、ORF4b和ORF5编码的辅助蛋白)[18]可在干扰素(interferon,IFN)应答的不同水平拮抗IFN作用,如抑制IFN转录、延缓IFN诱导产生及降低IFN诱导基因的表达等。对IFN的抑制,可导致感染细胞的裂解及炎性细胞因子调控异常引起的免疫病理损伤。IFN表达下调可使主要组织相容性复合体(major histocompatibility complex,MHC)Ⅰ和Ⅱ类抗原呈递途径下调及CD4+Th1细胞激活的关键细胞因子﹝白细胞介素12(interleukin 12,IL-12)、IFN-γ)﹞表达水平下降。
在转导了人CD26-腺病毒的T细胞缺损小鼠(TCRα-/-)或T细胞和B细胞严重联合免疫缺损小鼠(RAG1-/-)中,MERS-CoV感染呈持续性,且不能被清除,提示T细胞在MERS-CoV急性感染中有清除病毒的作用[19]。针对MERS-CoV S蛋白优势免疫表位(immunodominant epitope)的CD8 T细胞在感染7~10 d后达高峰,与SARS-CoV T细胞免疫交叉的水平很低。在感染第12天的MERS患者血清中可检测到高水平的中和抗体,至少持续13个月以上;在患者症状出现的第16天可检测到针对S蛋白和核衣壳蛋白(nucleocapsid protein,N蛋白)的IgM和IgG抗体。关于MERS-CoV抗体对疾病进程的影响,还有待进一步研究。
6MERS-CoV的致病机制
体外研究发现,MERS-CoV在下呼吸道细胞系中复制的效率高于在上呼吸道上皮细胞系。因缺少产生细胞因子的树突细胞和巨噬细胞,人支气管上皮细胞不能及时产生适量的天然免疫应答,这部分解释了MERS的严重程度[20]。在体外培养系统中, MERS-CoV可感染肺泡中的多种细胞,包括无纤毛和有纤毛的上皮细胞、Ⅰ型和Ⅱ型肺细胞、肺血管内皮细胞和肺巨噬细胞,这与临床上重症MERS患者表现的全身病毒扩散一致[21]。此外,与其他冠状病毒不同的是MERS-CoV对肾细胞的嗜性,MERS-CoV感染原代肾上皮细胞时,可致明显细胞病变,且病毒滴度是原代支气管上皮细胞中的1 000倍[22]。人单核细胞来源的树突细胞和巨噬细胞可支持MERS-CoV复制(productive replication)[23],提示MERS患者出现的多器官损伤与CD26在多细胞中的分布并通过巨噬细胞扩散至全身多器官有关。
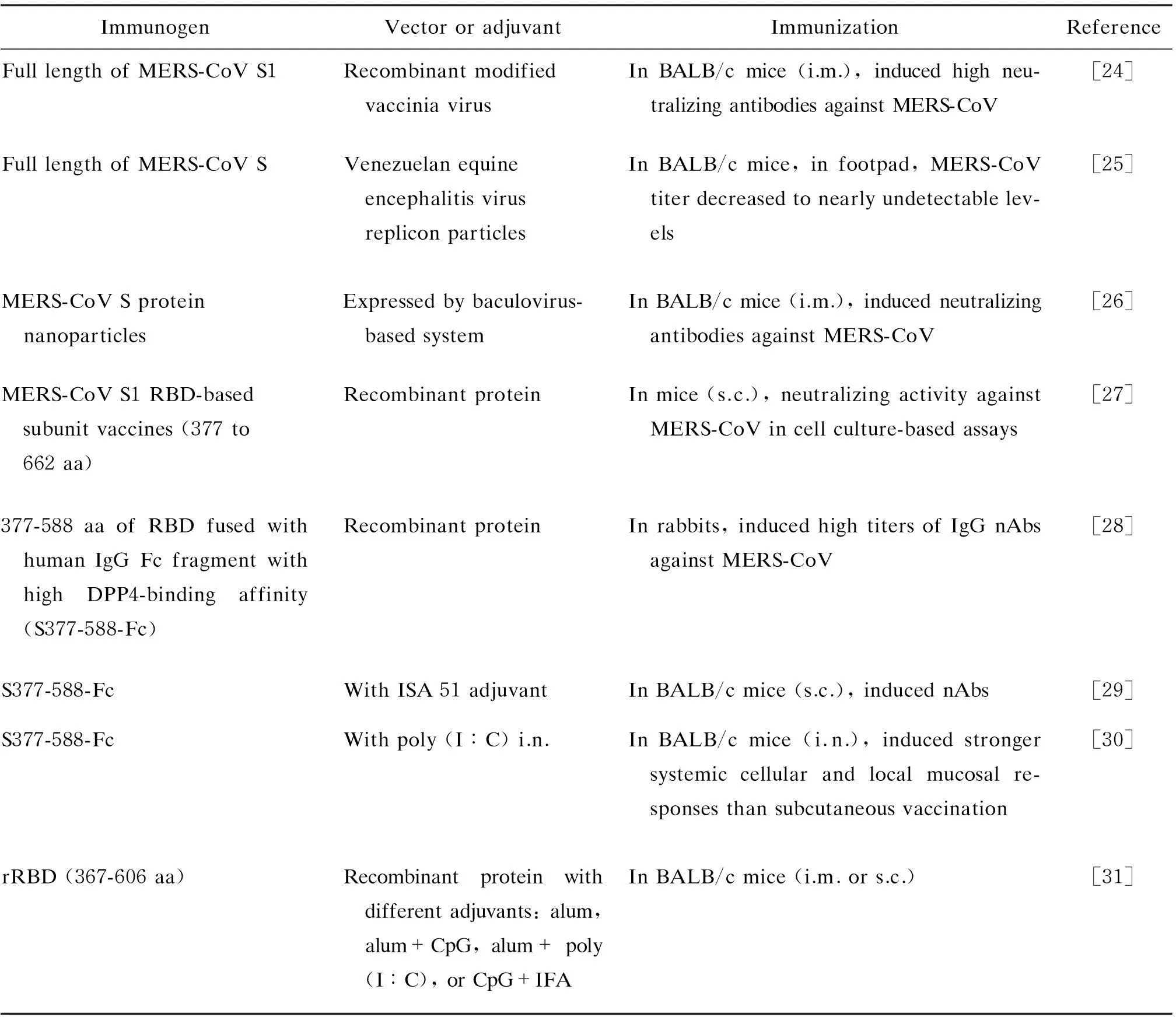
表1 研发中的MERS-CoV疫苗Tab.1 MERS-CoV vaccine understudying
7MERS-CoV疫苗的研制
目前尚无人用或动物(骆驼)用MERS-CoV疫苗。为控制MERS的流行,MERS-CoV疫苗的研发是首要任务之一(表1)。MERS-CoV S蛋白结构的研究显示,S蛋白可诱生中和抗体,S1蛋白的377~588位氨基酸是最稳定的中和区域,针对RBD的单克隆中和抗体可抑制病毒入侵宿主细胞和受体依赖的细胞融合(图3),且MERS-CoV的RBD在兔和小鼠体内可诱生高效价的中和抗体。基于上述基础,科学家对MERS-CoV疫苗进行了研究,动物体内免疫结果显示均可诱生中和MERS-CoV的抗体。在疫苗研究中,对MERS-CoV S蛋白的免疫主要区域、中和表位和非中和表位的鉴定将有利于增强疫苗的效果,同时降低抗体增强病毒感染的潜在危险。
8抗MERS-CoV药物研制的新进展
目前,临床上还没有治疗MERS-CoV感染的特异性药物,但在该领域已取得较大进展。例如,有些研究小组根据病毒自身S2六螺旋的晶体结构,设计了来自S2 HR2区的多肽类进入抑制剂,体外实验显示该类抑制剂可有效抑制MERS-CoV对靶细胞的感染[16,32]。近期,Jiang的团队在以前研究的基础上优化出更具优势的多肽抑制剂HR2P-M2,该多肽具有很好的抗MERS-CoV活性,且已顺利完成动物保护性实验:在MERS-CoV感染的小动物模型中,该多肽显示了较好的抗MERS-CoV的效果及安全性[33]。该多肽在发展为紧急防御和治疗的临床药物方面,具有很好的潜在应用前景。另外,在抗体药物方面也取得了很大进展,如抗体m336[34]、MERS-4[35]、3B11[36]和Mersmab1[37]均有很好的抗MERS的中和活性。其中,全人源化抗体m336的抗MERS效果最好,半数抑制浓度(half inhibitory concentration,IC50)可达0.47 nmol/L,且该抗体完全是一个胚系(germline)抗体,序列上不可能产生免疫原性的突变,因此预期具有更好的安全性。
参考文献
[1]WHO. Middle East respiratory syndrome coronavirus(MERS-CoV)—Republic of Korea.Disease outbreak news[EB/OL][2015-06-17]. http://www.who.int/csr/don/05-june-2015-mers-korea/en.
[2]de Groot RJ, Baker SC, Baric RS, Brown CS, Drosten C, Enjuanes L, Fouchier RA, Galiano M, Gorbalenya AE, Memish ZA, Perlman S, Poon LL, Snijder EJ, Stephens GM, Woo PC, Zaki AM, Zambon M, Ziebuhr J. Middle East respiratory syndrome coronavirus (MERS-CoV): announcement of the Coronavirus Study Group [J]. J Virol, 2013, 87(14): 7790-7792.
[3]Zaki AM, van Boheemen S, Bestebroer TM, Fouchier RA. Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia [J]. N Engl J Med, 2012, 367(19): 1814-1820.
[4]WHO. Middle East respiratory syndrome coronavirus(MERS-CoV):summary of current situation,literature update and risk assessment—as of 5 February 2015 [EB/OL][2015-06-17]. http://www.who.int/csr/disease/coronavirus_ infections/MERS_CoV_Update_09_May_2014.pdf?ua=1.
[5]van Boheemen S, de Graaf M, Lauber C, Bestebroer TM, Raj VS, Zaki AM, Osterhaus AD, Haagmans BL, Gorbalenya AE, Snijder EJ, Fouchier RA. Genomic characterization of a newly discovered coronavirus associated with acute respiratory distress syndrome in humans [J/OL]. MBio, 2012. http://mbio.asm.org/content/3/6/e00473-12.
[6]Cotten M, Lam TT, Watson SJ, Palser AL, Petrova V, Grant P, Pybus OG, Rambaut A, Guan Y, Pillay D, Kellam P, Nastouli E. Full-genome deep sequencing and phylogenetic analysis of novel human betacoronavirus [J]. Emerg Infect Dis, 2013, 19(5): 736-742.
[7]Müller MA, Corman VM, Jores J, Meyer B, Younan M, Liljander A, Bosch BJ, Lattwein E, Hilali M, Musa BE, Bornstein S, Drosten C. MERS coronavirus neutralizing antibodies in camels, Eastern Africa, 1983-1997 [J]. Emerg Infect Dis, 2014, 20(12): 2093-2095.
[8]Kuba K, Imai Y, Rao S, Gao H, Guo F, Guan B, Huan Y, Yang P, Zhang Y, Deng W, Bao L, Zhang B, Liu G, Wang Z, Chappell M, Liu Y, Zheng D, Leibbrandt A, Wada T, Slutsky AS, Liu D, Qin C, Jiang C, Penninger JM. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus-induced lung injury [J]. Nat Med, 2005, 11(8): 875-879.
[9]Lu G, Hu Y, Wang Q, Qi J, Gao F, Li Y, Zhang Y, Zhang W, Yuan Y, Bao J, Zhang B, Shi Y, Yan J, Gao GF. Molecular basis of binding between novel human coronavirus MERS-CoV and its receptor CD26 [J]. Nature, 2013, 500(7461): 227-231.
[10]Wang N, Shi X, Jiang L, Zhang S, Wang D, Tong P, Guo D, Fu L, Cui Y, Liu X, Arledge KC, Chen YH, Zhang L, Wang X. Structure of MERS-CoV spike receptor-binding domain complexed with human receptor DPP4 [J]. Cell Res, 2013, 23(8): 986-993.
[11]Yeager CL, Ashmun RA, Williams RK, Cardellichio CB, Shapiro LH, Look AT, Holmes KV. Human aminopeptidase N is a receptor for human coronavirus 229E [J]. Nature, 1992, 357(6377): 420-422.
[12]Gorrell MD, Gysbers V, McCaughan GW. CD26: a multifunctional integral membrane and secreted protein of activated lymphocytes [J]. Scand J Immunol, 2001, 54(3): 249-264.
[13]Song F, Fux R, Provacia LB, Volz A, Eickmann M, Becker S, Osterhaus AD, Haagmans BL, Sutter G. Middle East respiratory syndrome coronavirus spike protein delivered by modified vaccinia virus Ankara efficiently induces virus-neutralizing antibodies [J]. J Virol, 2013, 87(21): 11950-11954.
[14]Wang Q, Qi J, Yuan Y, Xuan Y, Han P, Wan Y, Ji W, Li Y, Wu Y, Wang J, Iwamoto A, Woo PC, Yuen KY, Yan J, Lu G, Gao GF. Bat origins of MERS-CoV supported by bat coronavirus HKU4 usage of human receptor CD26 [J]. Cell Host Microbe, 2014, 16(3): 328-337.
[15]Millet JK, Whittaker GR. Host cell entry of Middle East respiratory syndrome coronavirus after two-step, furin-mediated activation of the spike protein [J]. Proc Natl Acad Sci USA, 2014, 111(42): 15214-15219.
[16]Lu L, Liu Q, Zhu Y, Chan KH, Qin L, Li Y, Wang Q, Chan JF, Du L, Yu F, Ma C, Ye S, Yuen KY, Zhang R, Jiang S. Structure-based discovery of Middle East respiratory syndrome coronavirus fusion inhibitor [J]. Nat Commun, 2014, 5: 3067.
[17]Chan JF, Lau SK, To KK, Cheng VC, Woo PC, Yuen KY. Middle East respiratory syndrome coronavirus: another zoonotic betacoronavirus causing SARS-like disease [J]. Clin Microbiol Rev, 2015, 28(2): 465-522.
[18]Yang Y, Zhang L, Geng H, Deng Y, Huang B, Guo Y, Zhao Z, Tan W. The structural and accessory proteins M, ORF 4a, ORF 4b, and ORF 5 of Middle East respiratory syndrome coronavirus (MERS-CoV) are potent interferon antagonists [J]. Protein Cell, 2013, 4(12): 951-961.
[19]Zhao J, Li K, Wohlford-Lenane C, Agnihothram SS, Fett C, Zhao J, Gale MJ, Baric RS, Enjuanes L, Gallagher T, McCray PB, Perlman S. Rapid generation of a mouse model for Middle East respiratory syndrome [J]. Proc Natl Acad Sci USA, 2014, 111(13): 4970-4975.
[20]Chan JF, Chan KH, Choi GK, To KK, Tse H, Cai JP, Yeung ML, Cheng VC, Chen H, Che XY, Lau SK, Woo PC, Yuen KY. Differential cell line susceptibility to the emerging novel human betacoronavirus 2c EMC/2012: implications for disease pathogenesis and clinical manifestation [J]. J Infect Dis, 2013, 207(11): 1743-1752.
[21]Hocke AC, Becher A, Knepper J, Peter A, Holland G, Tönnies M, Bauer TT, Schneider P, Neudecker J, Muth D, Wendtner CM, Rückert JC, Drosten C, Gruber AD, Laue M, Suttorp N, Hippenstiel S, Wolff T. Emerging human Middle East respiratory syndrome coronavirus causes widespread infection and alveolar damage in human lungs[J]. Am J Respir Crit Care Med, 2013, 188(7): 882-886.
[22]Eckerle I, Müller MA, Kallies S, Gotthardt DN, Drosten C. In-vitro renal epithelial cell infection reveals a viral kidney tropism as a potential mechanism for acute renal failure during Middle East respiratory syndrome (MERS) coronavirus infection [J]. Virol J, 2013, 10: 359.
[23]Zhou J, Chu H, Li C, Wong BH, Cheng ZS, Poon VK, Sun T, Lau CC, Wong KK, Chan JY, Chan JF, To KK, Chan KH, Zheng BJ, Yuen KY. Active replication of Middle East respiratory syndrome coronavirus and aberrant induction of inflammatory cytokines and chemokines in human macrophages: implications for pathogenesis [J]. J Infect Dis, 2014, 209(9): 1331-1342.
[24]Volz A, Kupke A, Song F, Jany S, Fux R, Shams-Eldin H, Schmidt J, Becker C, Eickmann M, Becker S, Sutter G. Protective efficacy of recombinant Modified Vaccinia virus Ankara (MVA) delivering Middle East Respiratory Syndrome coronavirus spike glycoprotein [J/OL]. J Virol, 2015. doi: 10.1128/JVI.00614-15. http://jvi.asm.org/content/early/2015/05/22/JVI.00614-15.long.
[25]Agnihothram S, Gopal R, Yount BL Jr, Donaldson EF, Menachery VD, Graham RL, Scobey TD, Gralinski LE, Denison MR, Zambon M, Baric RS. Evaluation of serologic and antigenic relationships between Middle Eastern respiratory syndrome coronavirus and other coronaviruses to develop vaccine platforms for the rapid response to emerging coronaviruses [J]. J Infect Dis, 2014, 209(7): 995-1006.
[26]Coleman CM, Liu YV, Mu H, Taylor JK, Massare M, Flyer DC, Glenn GM, Smith GE, Frieman MB. Purified coronavirus spike protein nanoparticles induce coronavirus neutralizing antibodies in mice [J]. Vaccine, 2014, 32(26): 3169-3174.
[27]Du L, Zhao G, Kou Z, Ma C, Sun S, Poon VK, Lu L, Wang L, Debnath AK, Zheng BJ, Zhou Y, Jiang S. Identification of a receptor-binding domain in the S protein of the novel human coronavirus Middle East respiratory syndrome coronavirus as an essential target for vaccine development [J]. J Virol, 2013, 87(17): 9939-9942.
[28]Mou HH, Raj VS, van Kuppeveld FJ, Haagmans BL, Bosch BJ. The receptor binding domain of the new Middle East respiratory syndrome coronavirus maps to a 231-residue region in the spike protein that efficiently elicits neutralizing antibodies [J]. J Virol, 2013, 87(16): 9379-9383.
[29]Du L, Kou Z, Ma C, Tao X, Wang L, Zhao G, Chen Y, Yu F, Tseng CT, Zhou Y, Jiang S. A truncated receptor-binding domain of MERS-CoV spike protein potently inhibits MERS-CoV infection and induces strong neutralizing antibody responses: implication for developing therapeutics and vaccines [J]. PLoS One, 2013, 8(12): e81587.
[30]Ma C, Li Y, Wang L, Zhao G, Tao X, Tseng CT, Zhou Y, Du L, Jiang S. Intranasal vaccination with recombinant receptor-binding domain of MERS-CoV spike protein induces much stronger local mucosal immune responses than subcutaneous immunization: Implication for designing novel mucosal MERS vaccines [J]. Vaccine, 2014, 32(18): 2100-2108.
[31]Lan J, Deng Y, Chen H, Lu G, Wang W, Guo X, Lu Z, Gao GF, Tan W. Tailoring subunit vaccine immunity with adjuvant combinations and delivery routes using the Middle East respiratory coronavirus (MERS-CoV) receptor-binding domain as an antigen [J]. PLoS One, 2014, 9(11): e112602.
[32]Gao J, Lu G, Qi J, Li Y, Wu Y, Deng Y, Geng H, Li H, Wang Q, Xiao H, Tan W, Yan J, Gao GF. Structure of the fusion core and inhibition of fusion by a heptad repeat peptide derived from the S protein of Middle East respiratory syndrome coronavirus[J]. J Virol, 2013, 87(24): 13134-13140.
[33]Channappanavar R, Lu L, Xia S, Du L, Meyerholz DK, Perlman S, Jiang S. Protective effect of intranasal regimens containing peptidic Middle East respiratory syndrome coronavirus fusion inhibitor against MERS-CoV infection [J/OL]. J Infect Dis, 2015. http://jid.oxfordjournals.org/content/early/2015/07/11/infdis.jiv325.full.pdf+html.
[34]Ying TL, Du LY, Ju TW, Prabakaran PA, Lu L, Liu Q, Wang LL, Feng Y, Wang YP, Zheng BJ, Yuen KY, Jiang SB, Dimitrov DS. Exceptionally potent neutralization of Middle East respiratory syndrome coronavirus by human monoclonal antibodies[J]. J Virol, 2014, 88(14): 7796-7805.
[35]Jiang L, Wang N, Zuo T, Shi X, Poon KM, Wu Y, Gao F, Li D, Wang R, Guo J, Fu L, Yuen KY, Zheng BJ, Wang X, Zhang L. Potent neutralization of MERS-CoV by human neutralizing monoclonal antibodies to the viral spike glycoprotein [J]. Sci Transl Med, 2014, 6(234): 234ra59.
[36]Tang XC, Agnihothram SS, Jiao YA, Graham RL, Peterson EC, Avnir Y, Tallarico AS, Sheehan J, Zhu Q, Baric RS, Marasco WA. Identification of human neutralizing antibodies against MERS-CoV and their role in virus adaptive evolution [J]. Proc Natl Acad Sci USA, 2014, 111(19): E2018-E2026.
[37]Du L, Zhao G, Yang Y, Qiu H, Wang L, Kou Z, Tao X, Yu H, Sun S, Tseng CT, Jiang S, Li F, Zhou Y. A conformation-dependent neutralizing monoclonal antibody specifically targeting receptor-binding domain in Middle East respiratory syndrome coronavirus spike protein [J]. J Virol, 2014, 88(12): 7045-7053.
·特约专稿·
Corresponding authors. JIANG Shi-Bo, E-mail: shibojiang@fudan.edu.cn; QU Di, E-mail: dqu@shmu.edu.cn
Middle East respiratory syndrome coronavirus and development of vaccines and specific drugs for its control
QU Di, LU Lu, JIANG Shi-Bo
Department of Medical Microbiology and Parasitology, Key Laboratory of Medical Molecular Virology of Ministries of Education and Health, School of Basic Medical Sciences, Fudan University, Shanghai 200032, China
Abstract:Middle East respiratory syndrome (MERS) is a viral infection caused by a novel coronavirus (MERS-CoV). In this review, we focus on the classification, molecular structure and the proteins related to tissue tropisms and host range of MERS-CoV. The efforts on development of drugs and vaccines for the control of MERS-CoV are also discussed.
Key words:Middle East respiratory syndrome coronavirus;Middle East respiratory syndrome; Vaccine; Anti-Middle East respiratory syndrome coronavirus neutralization antibody
收稿日期:(2015-06-12)
通信作者:姜世勃,瞿涤
基金项目:国家自然科学基金(81173098)
