运动锻炼上调M3R对MI大鼠心脏产生保护效应及其机制探讨
2015-02-15田振军
田振军,张 可,陈 婷,2
运动锻炼上调M3R对MI大鼠心脏产生保护效应及其机制探讨
田振军1,张 可1,陈 婷1,2
目的:探讨运动上调M3受体(M3R)对心肌梗死(MI)大鼠心脏产生保护效应及其机制。方法:雄性SD大鼠48只,随机分为正常对照组(C),心梗组(MI),心梗+中强度持续有氧运动组(ME1),心梗+高强度间歇运动组(ME2),每组12只。C组常规饲养,MI组采用左冠脉前降支结扎法(LAD)建立MI模型。ME1和ME2组心梗手术1周后进行跑台运动, 60 min/次,1次/d,5 d/1周×8周。ME1组以10 m/min×5 min,再以3 m/min速度递增至16 m/min。ME2组以10 m/min×10 min后,逐渐递增至25 m/min×7 min;之后以15 m/min×3 min间歇运动,依次交替进行。训练结束后次日,腹腔麻醉进行颈动脉插管测定LVSP、LVEDP、±dp/dtmax等指标,评定心功能变化。之后迅速开胸摘取心脏,分别进行组织学制片、Masson染色观察心肌胶原纤维变化,免疫荧光法观察分析心肌M3R表达,Western Blot法检测心肌M3R、MEK1/2、p-ERK1/2、ERK1/2、Bcl-2和Bax蛋白表达。结果:MI组胶原容积百分比(CVF%)和LVEDP较C组显著升高(P<0.01),LVSP和±dp/dtmax较C组均显著降低(P<0.01)。MI后可见心肌M3R阳性染色,且M3R、MEK1/2、p-ERK1/2/ERK1/2表达较C组均显著升高(P<0.01),Bcl-2/Bax比值较C组显著降低(P<0.01)。ME1和ME2组CVF%和LVEDP较MI组均显著降低(P<0.01),ME1组-dP/dt max较MI组显著升高(P<0.01),ME2组LVSP较MI组显著升高(P<0.01)。ME1和ME2组均可见心肌M3R阳性染色,M3R、MEK1/2、p-ERK1/2/ERK1/2表达较MI组显著增加 (P<0.01),Bcl-2/Bax比值较MI组显著升高(P<0.01,P<0.05),ME1组和ME2组间无显著差异。结论:持续有氧运动和高强度间歇运动均可上调心梗心肌M3R-MEK1/2- ERK1/2通路,抑制心肌细胞凋亡,改善心肌纤维化程度,保护心梗大鼠心功能。心梗心肌M3R-MEK1/2- ERK1/2-细胞凋亡途径与持续和间歇运动方式及运动强度关系不密切。
M3受体;MEK1/2-ERK1/2通路;心肌细胞凋亡;持续有氧运动;高强度间歇运动;心肌梗死
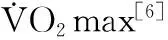
1 材料与方法
1.1 实验动物与分组
3月龄雄性Sprague Dawley大鼠48只,体重190~210 g,购于西安交通大学医学院实验动物中心(动物质量合格证号:陕医动证字08-004号),随机分为正常对照组(C),心梗组(MI),心梗+中等强度持续运动组(ME1),心梗+高强度间歇运动组(ME2)。每组12只。动物室温为18℃~23℃,湿度为50%~60%,标准啮齿类动物干燥饲料喂养,自由饮食。C组大鼠常规笼内安静饲养,MI组采用左冠脉前降支结扎法(LAD),建立MI模型。ME1和ME2组进行为期8周的小动物跑台运动。
1.2 主要仪器和试剂
主要仪器为PowerLab/8s生理信号采集处理系统,ALC-V8动物呼吸机,BM-Ⅱ型病理组织包埋机,生物组织摊烤片机,LEICA-RM 2126切片机,BX51奥林巴斯光学显微镜,Nikon Eclipse 55i荧光显微镜,低温高速离心机,酶标仪,Biorad电泳仪和转移槽,凝胶成像仪等。主要试剂为兔抗大鼠M3R多克隆抗体购于abcam公司,ERK1/2、p-ERK1/2、MEK1/2购于CST公司,Bcl-2和Bax购于博士德生物有限公司。
1.3 心梗模型制备与运动方案

1.4 血流动力学指标测定
运动8周结束后次日,腹腔麻醉PowerLab/8s记录心电图,右颈总动脉插管同步测试左室收缩压(LVSP)、左室舒张末压(LVEDP)、左室压力最大上升速率(+dp/dtmax)和最大下降速率(-dp/dtmax)等反映心功能的指标。数据采集完毕后,迅速开胸摘取心脏,进行后续实验。
1.5 取材、样品处理与组织学实验
待心功能指标测试完毕后,每组随机选取6只大鼠,迅速摘取心脏,置10%中性甲醛溶液固定24 h后,经流水冲洗、梯度乙醇脱水、二甲苯透明,石蜡包埋,连续切片(厚5 μm),常规制片,常规Masson染色,显微镜观察、摄片。另速摘取6只大鼠心脏铝箔纸包裹,液氮冷冻后移至-80℃低温冰箱保存,用于Western Blot实验。
免疫荧光实验的切片经脱蜡至水、PBS清洗和微波抗原修复后,用正常山羊血清在湿盒中37℃封闭30 min。滴加一抗(兔抗大鼠M3R多克隆抗体,1∶300),湿盒4℃过夜。室温复温45 min,PBS冲洗。滴加TRITC标记的二抗(1∶200)。37℃湿盒孵育1 h,PBS冲洗。滴加DAPI避光孵育2 min,PBS冲洗、封片。每次染色设置空白对照(PBS取代一抗和二抗)及阴性对照(PBS取代一抗)。采用荧光显微镜观察,低倍镜选位,400倍镜下拍照。各组选取6张石蜡切片,每张选10个视野,统计平均光密度(Mean Optical Density,MOD)。
1.6 Western Blot实验
采用RIPA试剂提取总蛋白,Bradford方法测定蛋白浓度。等量蛋白采用12% SDS聚丙烯酰胺凝胶垂直电泳分离,NC转膜,丽春红染膜,室温摇动封闭(3% BSA,TBST稀释)60 min后,分别加入兔抗M3R(1∶500)、MEK1/2(1∶1 000)、ERK1/2(1∶1 000)、p-ERK1/2(1∶1 000)、Bcl-2(1∶500)、Bax(1∶100)等多克隆抗体,4℃过夜,室温洗膜,加入HRP羊抗兔IgG抗体(Jackson,1∶10 000)孵育30 min,室温洗膜,化学发光底物ECL(Millipore)发光显迹。内参为GAPDH,计算目的蛋白与内参蛋白条带的积分光密度(Integral Optical Density,IOD)。
1.7 数据采集与统计学处理
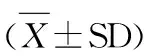
2 实验结果
2.1 心肌M3R表征结果
免疫荧光观察显示,M3R阳性染色为红色荧光颗粒。C组心肌M3R阳性荧光颗粒极少。MI后可见心肌M3R红色荧光颗粒呈点状分布,且较C组显著增加(P<0.01);ME1和ME2组M3R阳性颗粒较MI组均显著增加(P<0.01),两组间无显著差异(图1)。Western Blot结果显示,C组、MI组、ME1组和ME2组心肌M3R表征与免疫荧光观察统计结果一致(图1)。结果表明,心肌细胞上分布着M3R,心梗可引起心肌应激性M3R上调表达,有氧运动和高强度间歇运动均可显著上调M3R表达,且无运动强度差异。
2.2 心肌MEK1/2-ERK1/2通路和凋亡信号Bcl-2/Bax蛋白表征
Western Blot结果显示,MI组心肌MEK1/2、p-ERK1/2和ERK1/2蛋白表达较C组显著增加(P<0.01),ME1和ME2组较MI组显著增加(P<0.01),ME1和ME2组间无显著差异(图2)。MI组心肌Bcl-2/Bax比值较C组显著降低(P<0.01),ME1和ME2组较MI组显著增加(P<0.05),ME1和ME2组间无显著差异(图2)。结果表明,心梗心肌MEK1/2-ERK1/2通路发生应激性激活,而有氧运动和高强度间歇运动均可显著激活MEK1/2-ERK1/2通路,且无运动强度差异。
2.3 心肌纤维化观察分析和心功能测试结果
Masson染色结果显示,C组心肌细胞排列整齐,蓝色胶原纤维所占比例较少。与C组比较,MI组心肌组织结构紊乱,胶原纤维过度增生,胶原容积百分比(CVF%) 显著增加(P<0.01);与MI组比较,ME1和ME2组胶原减少,CVF%显著降低 (P<0.01),且两组间无显著差异(图3)。
心脏血流动力学参数测定结果显示,与C组比较,MI组心脏LVSP和±dp/dtmax显著降低(P<0.01),LVEDP显著升高(P<0.01)。与MI组比较,ME1和ME2组心脏LVSP和±dp/dtmax均显著升高(P<0.01),LVEDP显著降低(P<0.01)。ME1和ME2组间无显著差异,表明心梗后心功能显著降低,中等强度有氧运动和高强度间歇运动均可显著提升心功能,且无运动强度差异(图4)。

图1 本研究心肌M3R表征结果(×400)柱状图Figure 1. Characterization Results of Cardiac M3R(×400)
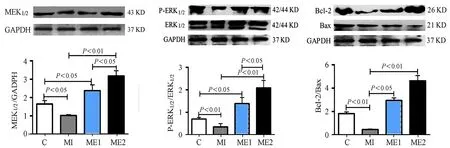
图2 本研究心肌MEK1/2、p-ERK1/2/ERK1/2和Bcl-2/Bax表达的Western Blot结果柱状图Figure 2. Western Blot Results of Cardiac MEK1/2、p-ERK1/2/ERK1/2 and Bcl-2/Bax
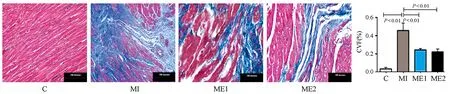
图3 本研究心肌组织Masson染色与分析(×400)切片图Figure 3. Masson Dyeing Results in Myocardium(×400)
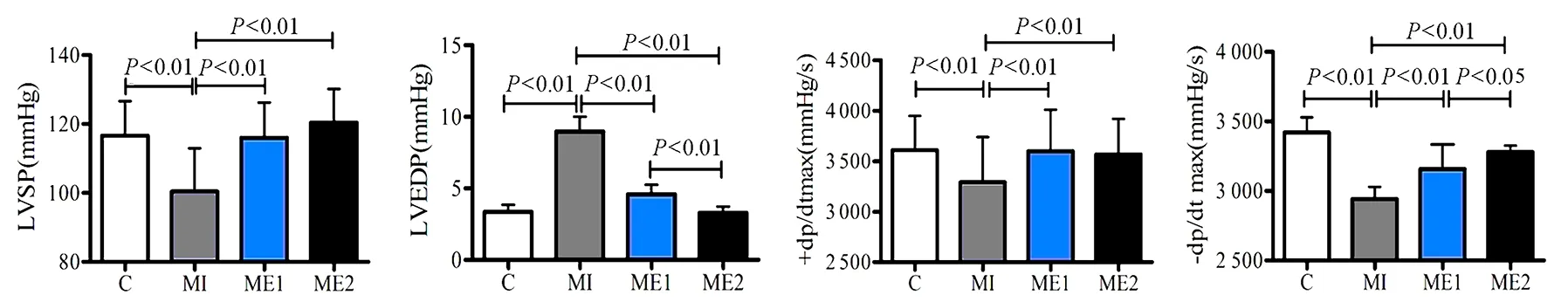
图4 本研究血流动力学参数变化柱状图Figure 4. The Index of Hemodynamic
3 分析与讨论
3.1 心梗后进行运动干预均可上调心肌M3R表达,激活其下游MEK1/2-ERK1/2通路
迷走神经通过刺激MR调节心脏起搏、房室传导和心肌收缩等功能[9,40]。过去认为M2R是心脏唯一的MR亚型[2],近年发现心肌细胞上存在着M3R。小鼠心脏窦房结、心房肌、心室肌均有M3R mRNA表达,且水平依次降低。M3R在窦房结表达密度是心室肌的2~3倍[17,20]。作为迷走神经递质作用的靶点之一,M3R的心肌保护作用日益受到重视[23],激活M3R可成为治疗心脏疾病新的靶点[36]。研究发现,激活窦房结M3R可导致窦性节律降低以及舒张期去极化减慢,增加动作电位上升速度[15]。激活心房和心室肌M3R可显著缩短动作电位持续时间,减慢心率,保护心功能。上调M3R可通过增加热休克蛋白-70和环加氧酶-2,调节蛋白激酶C-ε和β-链蛋白的活化与表达[24],同时抑制miRNA-1及miRNA-376b-5p的表达[29],发挥抗心律失常和改善缺血性心肌损伤的作用[24]。研究表明,心梗后的梗死区及梗死周边区均发生胆碱能失支配现象,且胆碱能神经纤维密度从6 h、12 h到48 h逐渐下降。而适宜运动可上调心血管疾病引起的心脏副交感神经活性降低,改善副交感神经重构[31,37],并增加心肌M2R的表达,从而保护心功能。但目前关于运动训练与心肌M3R表征研究报道少见。本研究发现,MI组、ME1组和ME2组均可见心肌M3R阳性表达。且与MI组比较,ME1和ME2组心肌M3R表达显著增加。表明,中强度持续有氧运动和高强度间歇运动两种干预方式,均可上调心梗心脏的M3R表达,并与运动对心功能的改善关系密切。
关于激活M3R保护心脏的机制研究日益增多[24]。上调心脏M3R抑制心肌缺血和肥厚及心律失常等有益作用,由抗细胞凋亡(增加抗凋亡因子Bcl-2和ERKs表达,降低凋亡因子Fas和P38表达)、缓解细胞内钙超载(调节胞内Ca2+通道,抑制胞内Ca2+超载)、降低氧化应激水平(增加SOD表达,减少MDA含量)、纠正离子通道失衡等多条信号通路实现[25,36]。而本研究重点探讨M3R及其下游MEK1/2-ERK1/2通路与心脏的保护作用。研究发现,在唾液腺细胞HSY和人类K652细胞中,活化MR可激活ERK1/2通路[4,22]。在原代新生大鼠心肌细胞中,胆碱不能活化ERK1/2,但在氧化应激状态下则可明显活化[40]。通过心肌M3R激活MEK1/2-ERK1/2,可促进细胞生存,提高细胞活力[12]。且有研究表明,M3R激活所发挥的抑制细胞凋亡效应与上调MEK1/2-ERK1/2通路关系密切[26,41]。本研究发现,心梗后运动可上调心脏M3R并激活其下游信号分子MEK1/2表达。表明有氧运动发挥心梗心脏保护效应与心肌M3R-MEK1/2-ERK1/2通路激活关系密切。
3.2 心梗后的不同强度运动干预均可抑制心肌细胞凋亡、减轻心肌纤维化程度、改善心功能
目前,临床上把运动作为治疗及预防心梗病人的重要干预措施[11]。心梗后进行中强度持续有氧运动或高强度间歇运动对提高患者心功能和再重构心室均具有积极意义[5,10,27]。运动作为心血管疾病干预手段,其有益作用备受学者关注,但相关机制研究仍显不足。运动可通过抑制心肌细胞凋亡[30],促进心肌细胞增殖和血管再生[44],改善神经体液调节[33],促进心脏发生生理学重塑[8],从而对心脏产生保护作用。目前研究认为,M3R无论在正常生理状态或心肌缺血及心律失常以及心衰等病理状态下,均可通过参与心率和收缩变力调节,心肌复极化过程、心房颤动的产生和恢复等[34],对缺血心脏发挥重要保护作用[14]。 激活M3R可上调心肌环氧化酶-2表达,抑制缺血诱导的Cx43的脱磷酸化,缩小心肌梗死面积,保护心功能[43]。本研究发现,M3R表征与持续和间歇运动方式、运动强度无关。MEK1/2-ERK1/2是细胞内重要的信号通路,对促进细胞生长、生存,抑制细胞凋亡等均发挥关键作用[23,26]。在病理生理条件导致的细胞凋亡中,G-蛋白偶联受体可激活心肌细胞MEK1/2-ERK1/2通路发挥抗凋亡作用[14,23,42]。本研究结果发现,两种运动方式干预均可增加心梗心肌Bcl-2/Bax比值,抑制胶原过度增生,改善心梗大鼠心功能;同时发现,运动可激活心梗心脏的M3R-MEK1/2-ERK1/2通路。上述结果与持续和间歇运动方式及运动强度无显著差异。中等强度有氧运动较高强度间歇运动对心梗心脏更安全。提示,采取中等强度有氧运动方式保护心梗心脏具有重要意义。
4 结论
持续有氧运动和高强度间歇运动均可上调心肌M3R-MEK1/2- ERK1/2通路,抑制心梗后心肌细胞凋亡,改善心肌纤维化程度,保护心梗大鼠心功能。心梗心脏的M3R-MEK1/2- ERK1/2-细胞凋亡途径与持续和间歇运动方式及运动强度关系不密切。
[1]陈婷,呼德尓朝鲁,张可,等.有氧运动对心梗大鼠左室β3-AR和一氧化氮合酶表达的影响[J].体育科学,2013,33(9):57-76.
[2]田振军,杜蕾.M2受体细胞表征及其运动与心脏、血管M2受体研究进展[J].体育科学,2009,29(11):65-71.
[3]田振军,贺志雄,刘智炜,等.持续和间歇有氧运动对心梗大鼠心肌Myostatin及其受体表达的影响[J].体育科学,2013,33(11):66-74.
[4]AYDIN B,KAN B,CABADAK H.The role of intracellular pathways in the proliferation of human K562 cells mediated by muscarinic receptors[J].Leuk Res,2013,37(9):1144-1149.
[5]BITO V,DE WAARD M C,BIESMANS L,etal.Early exercise training after myocardial infarction prevents contractile but not electrical remodelling or hypertrophy[J].Cardiovasc Res,2010,86(1):72-81.
[6]BOUTCHER S H,PARK Y,DUNN S L,etal.The relationship between cardiac autonomic function and maximal oxygen uptake response to high-intensity intermittent-exercise training[J].J Sports Sci,2013,31(9):1024- 1029.
[7]COCHRAN A J,PERCIVAL M E,TRICARICO S,etal.Intermittent and continuous high-intensity exercise induce similar acute but different chronic muscle training adaptations[J].Exp Physiol,2014,99(5):782-791.
[8]DUNCKER D J,VAN DEEL E D,DE WAARD M C,etal.Exercise training in adverse cardiac remodeling[J].Pflugers Arch,2014,466(6):1079-1091.
[9]EGLEN R M.Overview of muscarinic receptor subtypes[J].Handb Exp Pharmacol,2012,(208):3-28.
[10]GIALLAURIA F,ACAMPA W,RICCI F,etal.Exercise training early after acute myocardial infarction reduces stress-induced hypoperfusion and improves left ventricular function[J].Eur J Nucl Med Mol Imaging,2013,40(3):315-324.
[11]GIALLAURIA F,ACAMPA W,RICCI F,etal.Exercise training early after acute myocardial infarction reduces stress-induced hypoperfusion and improves left ventricular function[J].Eur J Nucl Med Mol Imaging,2013,40(3):315-324.
[12]GREENWOOD J M,DRAGUNOW M.M3muscarinic receptors promote cell survival through activation of the extracellular regulated kinase (ERK1/2) pathway[J].Eur J Pharmacol,2010,640(1-3):38-45.
[13]HANG P,ZHAO J,QI J,etal.Novel insights into the pervasive role of M(3) muscarinic receptor in cardiac diseases[J].Curr Drug Targets,2013,14(3):372-377.
[14]HANG P Z,ZHAO J,WANG Y P,etal.Reciprocal regulation between M3muscarinic acetylcholine receptor and protein kinase C-epsilon in ventricular myocytes during myocardial ischemia in rats[J].Naunyn Schmiedebergs Arch Pharmacol,2009,380(5):443-450.
[15]HARADA N,OCHI K,YAOSAKA N,etal.Immunohistochemical and functional studies for M muscarinic receptors and cyclo-oxygenase-2 expressed in the mouse atrium[J].Auton Autacoid Pharmacol,2012,32(3 Pt 4):41-52.
[16]HAYKOWSKY M J,TIMMONS M P,KRUGER C,etal.Meta-analysis of aerobic interval training on exercise capacity and systolic function in patients with heart failure and reduced ejection fractions[J].Am J Cardiol,2013,111(10):1466-1469.
[17]HELLGREN I,MUSTAFA A,RIAZI M,etal.Muscarinic M3receptor subtype gene expression in the human heart[J].Cell Mol Life Sci,2000,57(1):175-180.
[18]JIANG H K,MIAO Y,WANG Y H,etal.Aerobic interval training protects against myocardial infarction-induced oxidative injury by enhancing antioxidase system and mitochondrial biosynthesis[J].Clin Exp Pharmacol Physiol,2014,41(3):192-201.
[19]JIANG H K,WANG Y H,SUN L,etal.Aerobic interval training attenuates mitochondrial dysfunction in rats post-myocardial infarction:Roles of mitochondrial network dynamics[J].Int J Mol Sci,2014,15(4):5304-5322.
[20]KITAZAWA T,ASAKAWA K,NAKAMURA T,etal.M3muscarinic receptors mediate positive inotropic responses in mouse atria:A study with muscarinic receptor knockout mice[J].J Pharmacol Exp Ther,2009,330(2):487-493.
[21]KREJCIA,TUCEK S.Quantitation of mRNAs for M1 to M5 subtypes of muscarinic receptors in rat heart and brain cortex[J].Mol Pharmacol,2002,61(6):1267-1272.
[22]LIN A L,ZHU B,ZHANG W,etal.Distinct pathways of ERK activation by the muscarinic agonists pilocarpine and carbachol in a human salivary cell line[J].Am J Physiol Cell Physiol,2008,294(6):C1454-C1464.
[23]LIU Y,SUN H L,LI D L,etal.Choline produces antiarrhythmic actions in animal models by cardiac M3receptors:Improvement of intracellular Ca2+handling as a common mechanism[J].Can J Physiol Pharmacol,2008,86(12):860-865.
[24]LIU Y,SUN L,PAN Z,etal.Overexpression of M3muscarinic receptor is a novel strategy for preventing sudden cardiac death in transgenic mice[J].Mol Med,2011,17(11-12):1179-1187.
[25]LIU Y,WANG S,WANG C,etal.Upregulation of M3muscarinic receptor inhibits cardiac hypertrophy induced by angiotensin II[J].J Transl Med,2013,209:2-8.
[26]LORENZ K,SCHMIT T J P,VIDAL M,etal.Cardiac hypertrophy:Targeting Raf/MEK/ERK1/2-signaling[J].Int J Biochem Cell Biol,2009,41(12):2351-2355.
[27]MOREIRA J B,BECHARA L R,BOZI L H,etal.High-versus moderate-intensity aerobic exercise training effects on skeletal muscle of infarcted rats[J].J Appl Physiol,2013,114(8):1029-1041.
[28]NAVARRO-POLANCO R A,ARÉCHIGA-FIGUEROA I A,SALAZAR-FAJARDO P D,etal.Voltage sensitivity of M2 muscarinic receptors underlies the delayed rectifier-like activation of ACh-gated K(+) current by choline in feline atrial myocytes[J].J Physiol,2013,591(17):4273-4286.
[29]PAN Z,GUO Y,QI H,etal.M3subtype of muscarinic acetylcholine receptor promotes cardioprotection via the suppression of miR-376b-5p[J].PLoS One,2012,7(3):e32571 (1-8).
[30]QUINDRY J C,MILLER L,MCGINNIS G,etal.Ischemia reperfusion injury,KATP channels,and exercise- induced cardioprotection against apoptosis[J].J Appl Physiol(1985),2012,113(3):498-506.
[31]RANA O R,SAYGILI E,GEMEIN C,etal.Chronic neuronal stimulation increases cardiac parasympathetic tone by eliciting neurotrophic effects[J].Circ Res,2011,108(10):1209-1219.
[32]SAITO M,UESHIMA K,SAITO M,etal.Safety of exercise-based cardiac rehabilitation and exercise testing for cardiac patients in japan[J].Circ J,2014,78(7):1646-1653.
[33]SILVA JA Jr,SANTANA E T,MANCHINI M T,etal.Exercise training can prevent cardiac hypertrophy induced by sympathetic hyperactivity with modulation of kallikrein-kinin pathway and angiogenesis[J].PLoS One,2014,9(3):e91017.
[34]SONG W,YUAN M,ZHAO S.Variation of M3muscarinic receptor expression in different prostate tissues and its significance[J].Saudi Med J,2009,30(8):1010-1016.
[35]WANG S,HONG M,YA N,etal.Activation of cardiac M3muscarinic acetylcholine receptors has cardioprotective effects against ischaemia-induced arrhythmias[J].Clin Exp Pharmacol Physiol,2012,39(4):343-349.
[36]WANG H,LU Y,WANG Z.Function of cardiac M3receptors[J].Auton Autacoid Pharmacol,2007,27(1):1-11.
[37]WANG Y H,HU H,WANG S P,etal.Exercise benefits cardiovascular health in hyperlipidemia rats correlating with changes of the cardiac vagus nerve[J].Eur J Appl Physiol,2010,108(3):459-468.
[38]WISLØFF U,LOENNECHEN J P,CURRIE S,etal.Aerobic exercise reduces cardiomyocyte hypertrophy and increases contractility,Ca2+sensitivity and SERCA-2 in rat after myocardial infarction[J].Cardiovasc Res,2002,54(1):162-174.
[39]XU X,WAN W,JI L,etal.Exercise training combined with angiotensin II receptor blockade limits post-infarct ventricular remodelling in rats[J].Cardiovasc Res,2008,78(3):523-532.
[40]YANG B,LIN H,XU C,etal.Choline produces cytoprotective effects against ischemic myocardial injuries:Evidence for the role of cardiac M3subtype muscarinic acetylcholine receptors[J].Cell Physiol Biochem, 2005,16(4-6):163-174.
[41]ZHANG J,BIAN H J,LI X X,etal.ERK-MAPK signaling opposes rho-kinase to reduce cardiomyocyte apoptosis in heart ischemic preconditioning[J].Mol Med,2010,16(7-8):307-315.
[42]ZHANG Z,LI S,CUI M,etal.Rosuvastatin enhances the therapeutic efficacy of adipose-derived mesenchymal stem cells for myocardial infarction via PI3K/Akt and MEK/ERK pathways[J].Basic Res Cardiol,2013,108(2):333.
[43]ZHAO J,SU Y,ZHANG Y,etal.Activation of cardiac muscarinic M3receptors induces delayed cardioprotection by preserving phosphorylated connexin43 and up-regulating cyclooxygenase-2 expression[J].Br J Pharmacol,2010,159(6):1217-1225.
[44]ZHAO W,BAI J,ZHANG F,etal.Impact of completeness of revascularization by coronary intervention on exercise capacity early after acute ST-elevation myocardial infarction[J].J Cardiothorac Surg,2014,9:50(1-8).
Cardioprotective Effect and Its Mechanism of Exercise Training Up-Regulating the Expression of M3Receptor on Myocardial Infarction in Rats
TIAN Zhen-jun1,ZHANG Ke1,CHEN Ting1,2
objective:Cardioprotective effect and mechanism of exercise training up-regulating the expression of M3receptor on myocardial infarction.Methods:48 male Sprague-Dawley rats were randomly assigned to four groups (n=12,per group):control group (C),myocardial infarction group (MI),moderate-intensity aerobic exercise with myocardial infarction group (ME1) and high-intensity aerobic interval exercise with myocardial infarction group (ME2),Rats in C group are breed normally.MI was induced by ligation of the left anterior descending (LAD) coronary artery in MI group。Rats in ME1 and ME2 group take treadmill exercise for 8wk after 1wk post-operation.ME1 group running began at the speed of 10m/min for 5min,then accelerate from 3m/min to 16m/min.ME2 group running began at the speed of 10m/min for 10min,then the speed increases to 25 m/min,after 7min,slow down at the speed of 15m/min for 3min.Take the process alternatively.The total exercise time of ME1 and ME2 are both 60min,5d/1wk×8wk.LVSP,LVEDP,±dp/dtmaxand the cardiac function changes are measured after training.Myocardial collagen fibers were observed by histological section and Masson staining.The expression of myocardial M3R was observed and analyzed by immunoflourecence.The myocardial protein content of M3R,MEK1/2,P-ERK1/2,ERK1/2and apoptosis related Bcl-2 and Bax were assayed by Western Blot.Results:MI increased CVF and LVEDP (P<0.01),but decreased LVSP and -dp/dtmax(P<0.01).After MI myocardial M3positive staining,after MI M3protein expression significantly higher (P<0.01),MEK1/2,P-ERK1/2/ERK1/2protein expression were significantly increased (P<0.01,P<0.01),after the MI the Bcl-2/Bax expression significantly reduced (P<0.01).ME1 and ME2 group CVF%,LVEDP significantly reduced (P<0.01),ME1 -dp/dtmaxsignificantly increased (P<0.01),ME2 LVSP increased significantly (P<0.01).ME1 and ME2 groups were identified myocardial M3,ME1 and ME2 M3protein expression significantly increased (P<0.01,P<0.01).ME1 and ME2 group Bcl-2/Bax expression significantly reduced in the MI group (P<0.01,P<0.01).ME1 and ME2 index had no significant difference.Conclusions:Moderate-intensity aerobic exercise and high-intensity aerobic interval exercise can upregulate the M3R-MEK1/2-ERK1/2signaling pathway,thus inhibit the apoptosis of myocardial cells,reduce myocardial interstitial fibrosis and promote cardiac function after MI.
M3receptor(M3R);MEK1/2-ERK1/2signalingpathways;cardiomyocyteapoptosis;aerobicexercise;high-intensityintervalexercise;myocardialinfarction
1000-677X(2015)05-0055-07
10.16469/j.css.201505007
2014-07-08;
2015-03-16
国家自然科学基金资助项目(31171141),陕西师范大学“211工程”重点建设学科——运动生物学学科建设项目(2014-12)。
田振军(1965-),男,陕西绥德人,教授,博士研究生导师,主要研究方向为运动心血管生物学,E-mail:tianzj611@hotmail.com;张可(1989-),女,陕西西安人,在读硕士研究生,主要研究方向为运动心血管生物学;陈婷(1984-),女,陕西西安人,讲师,在读博士研究生,主要研究方向为运动心血管生物学。
1.陕西师范大学 体育学院 暨运动生物学研究所 运动与心血管研究室,陕西 西安 710062;2.西藏民族学院 体育学院,陕西 咸阳 712082 1.Shaanxi Normal University,Xi’an 710062,China;2.Tibet University for Nationalities,Xianyang 712082,China.
G 804.2
A
