中国东部南北样带典型针叶林土壤酶活性分布格局
2015-02-07王永生于贵瑞程淑兰方华军高文龙
王永生, 于贵瑞, 程淑兰, 方华军, 高文龙
1 中国科学院地理科学与资源研究所生态网络观测与模拟重点实验室CERN综合研究中心, 北京 100101 2 中国科学院大学, 北京 100049
中国东部南北样带典型针叶林土壤酶活性分布格局
王永生1,2, 于贵瑞1, 程淑兰2, 方华军1,*, 高文龙1,2
1 中国科学院地理科学与资源研究所生态网络观测与模拟重点实验室CERN综合研究中心, 北京 100101 2 中国科学院大学, 北京 100049
选取中国东部南北样带不同气候区(寒温带、温带和亚热带)的针叶林作为研究对象,分析了多酚氧化酶、过氧化物酶、几丁质酶以及β-葡萄糖苷酶活性的变化。结果表明,土壤溶解性有机碳(DOC)、微生物生物量碳(MBC)、微生物生物量氮(MBN)含量从北到南依次减小,寒温带显著高于亚热带;土壤pH值在3个气候区差异显著,温带最高,亚热带最低。分解木质素的多酚氧化酶和过氧化物酶活性不存在显著性差异;而与氮循环密切相关的几丁质酶活性表现为温带显著高于寒温带和亚热带;与碳循环密切的β-葡萄糖苷酶活性在温带最高,并显著高于亚热带地区。逐步回归分析表明,土壤MBN与pH值影响土壤几丁质酶活性,而土壤β-葡萄糖苷酶活性主要受pH的控制。研究认为,在中国东部南北样带针叶林中,土壤几丁质酶和β-葡萄糖苷酶活性存在显著性差异,而pH与MBN可能是影响土壤酶活性的主要因素。
气候区; 土壤酶活性; 纤维素; 木质素
土壤有机质碳库约为植物与动物碳库的3倍,是陆地上最大的碳库[1],土壤有机质的转化是全球碳循环中的关键过程。植物凋落物与根系是森林土壤有机质的主要来源[2- 3],碳贮存与矿化的平衡影响土壤有机质碳库的大小与周转,从而改变大气CO2浓度[4]。纤维素、木质素和几丁质是陆地生物区系中最主要的3类生物聚合物,是碳的主要存在与转化形式,也是碳循环过程中的主要基质和产物[5]。纤维素和木质素是凋落物的两类主要生物化学组分,能被不同的微生物胞外酶分解。木质素能被多酚氧化酶和过氧化物酶分解氧化,纤维素能被外切葡聚糖酶、内切葡聚糖酶以及β-葡萄糖苷酶水解[6]。几丁质并非来源于植物,而是主要来源于动物残体。几丁质的水解受内切几丁质酶、壳二糖酶和N-乙酰葡萄糖胺糖苷酶作用[5]。对活性和惰性有机质转化过程相关酶活性格局的探讨,有助于深入研究森林土壤有机质转化和固定的生物化学机制。
有机质的分解作用受凋落物数量和质量的影响[7- 8],同时也受水分和温度的影响[9- 10]。土壤酶活性除了受到底物有机质质量的影响外,还受到水热条件的影响[11- 13]。中国东部南北森林样带(NSTEC)是 (IGBP)的第15条样带,以热量为主要驱动因子,其次为降水。年均温、年均降水量与氮沉降通量呈现从南到北递减趋势,从北到南分布有寒温带针叶林、温带针阔混交林、暖温带落叶阔叶林、亚热带常绿阔叶林和热带季雨林,为探讨森林土壤活性分布格局和主控因子提供了很好的研究平台。有关NSTEC样带典型森林土壤碳储量动态、碳氮气体排放等关键过程的分布格局和主控因子已有大量的研究[14- 16],对不同森林群区典型森林土壤生物化学过程尤其是酶活性的分布格局和主控因子研究较少。本文主要研究目的是:(1)探讨不同气候区针叶林土壤活性碳氮和主要胞外酶活性的分布格局;(2)分析土壤活性碳氮含量与酶活性之间的内在联系。研究NSTEC针叶林土壤酶活性差异及其主控因子,对于加强该区域针叶林生态系统碳循环,尤其是研究从有机质到温室气体排放的“转换”过程提供科学依据。
1 材料与方法
1.1 研究区概况
沿着NSTEC由北向南选择寒温带大兴安岭站、温带东灵山站和亚热带鼎湖山站作为研究区域,分别代表寒温带、温带和亚热带气候特征。其中,大兴安岭站选择兴安落叶松成熟林作为研究对象;东灵山站选择油松林为研究对象;而鼎湖山站内选择马尾松作为研究对象。从北到南,3个站点的年均温,从寒温带的 -5.4 ℃到亚热带的21.4 ℃;多年平均降水量从500 mm到1600 mm[14]。0—20 cm的土壤理化性状以及森林基本情况见表1。
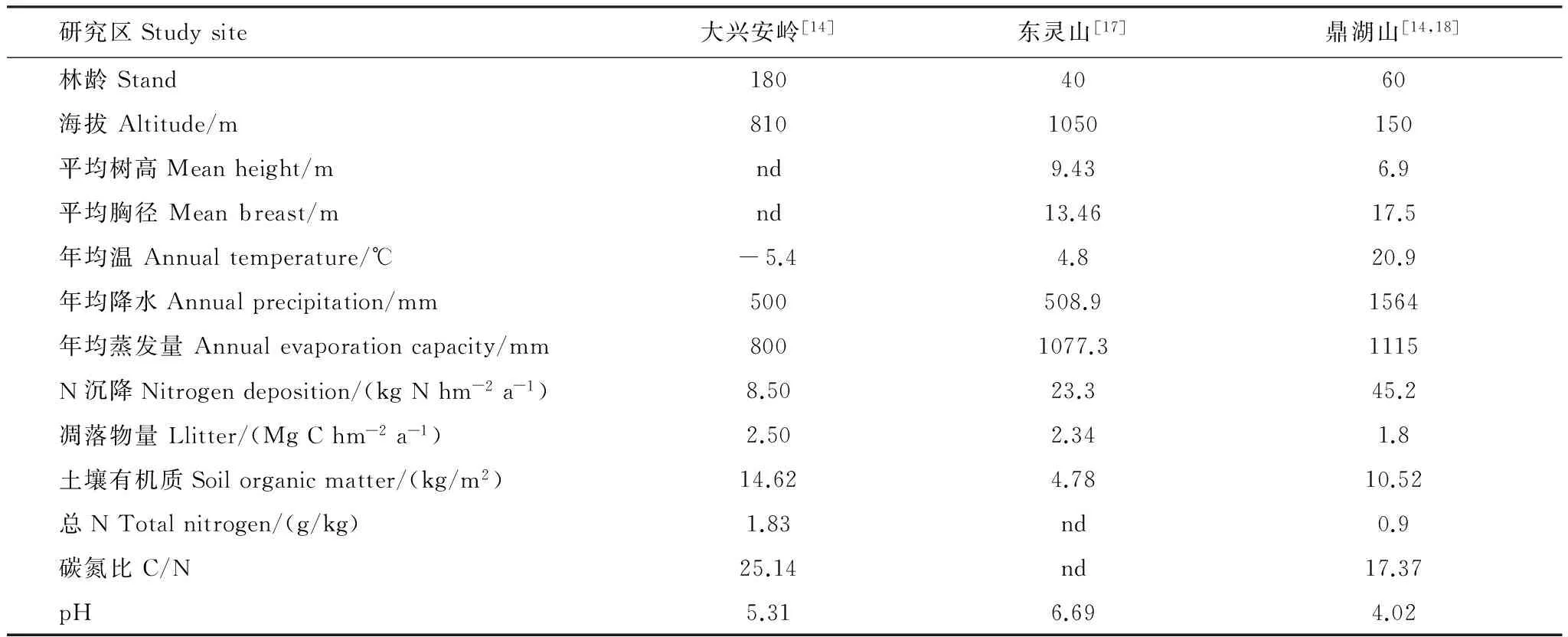
表1 NSTEC中3个森林站主要特征及0—20 cm土壤理化性状
1.2 样品采集与理化性状测定
在每个森林随机设置3个15 m×15 m的样方,在每个样方去除凋落物层后,采集0—10 cm的表层矿质土壤样品。大兴安岭土壤样品采自2011年8月15日,鼎湖山样品采集于2011年9月19日,东灵山样品采集于2011年9月21日。每个森林选择3个重复小区进行取样,每个小区内选择5点取样混合为一个样品,所有样品过2 mm筛,除根和砂石后,保存于4 ℃冰箱内。土壤可溶性有机碳(DOC)采用去离子水浸提,过0.45 μm滤膜后,利用TOC仪器测定分析;微生物碳(MBC)和微生物氮(MBN)采用氯仿熏蒸法测定[19];土壤pH 按2.5∶1的水土比,采用玻璃电极测定。
1.3 酶活性测定方法
多酚氧化酶和过氧化物酶的底物为5 mmol/L的 L- 3,4-dihydroxy phenyalanine (DOPA),采用改进的紫外分光光度计法测定[20- 21]。用50 mmol/L的pH值为5.0的醋酸钠缓冲液配制基质溶液,相当于1 g干土的样品中,加入2.5 mL 缓冲液和2.5 mL的DOPA底物溶液,在20 ℃下培养1 h,培养结束后,取滤液在分光光度计460 nm处测定吸光度值。其中,过氧化物酶的所有样品和对照均加0.3%的H2O2溶液。
β-葡萄糖苷酶和几丁质酶的底物为5 mmol/L的pNP-β-D-glucopyranoside与pNP N-acetyl-β-D-glucosaminide,采用改进的紫外分光光度计法测定[20,22]。用50 mmol/L的pH为5.0的醋酸钠缓冲液配制基质溶液,相当于1g干土的样品中,加入2.5 mL 缓冲液和2.5 mL的底物溶液,在25 ℃下培养4 h,培养结束后,加入0.2 mL的1.0 mol/L的NaOH溶液终止反应并显色。取滤液在紫外分光光度计410 nm处测定吸光度值。
以上实验,均设置不加基质的土样为空白。酶活性用每小时每克样品的基质(μmol)转化率表示(μmol h-1g-1土)。
1.4 数据分析
利用单因素方差分析分别比较3个气候区之间的土壤DOC、MBC、MBN和 pH值以及土壤酶活性的差异;利用逐步线性回归分析土壤理化性质和土壤酶活性之间的相关性。统计分析采用SPSS (16.0)进行,采用Sigma Plot 10.0 绘图。
2 结果与分析
2.1 土壤可溶性碳氮与pH分析
从图1中可以看出,土壤可溶性有机碳(DOC)与微生物碳(MBC)含量均呈现为由北到南不断减小的趋势,而且寒温带地区的兴安落叶松土壤DOC与MBC含量显著高于南亚热带的马尾松林土壤含量(P<0.05)。温带和寒温带土壤微生物氮(MBN)含量不存在显著性差异,但均显著高于亚热带地区(P<0.05)。而土壤pH则表现为,温带>寒温带>亚热带,而且均存在显著性差异(P<0.05)。
2.2 土壤酶活性研究
从图2中可以看出,3个不同气候区的针叶林土壤中,分解木质素的多酚氧化酶和过氧化物酶活性不存在显著性差异。温带东灵山地区的油松林土壤几丁质酶和β-葡萄糖苷酶活性最高;其中几丁质酶活性显著高于寒温带兴安落叶松和亚热带马尾松森林土壤酶活性,而β-葡萄糖苷酶活性仅显著高于亚热带马尾松森林土壤酶活性(P<0.05)。

图1 不同针叶林表层土壤可溶性碳氮与pH分析
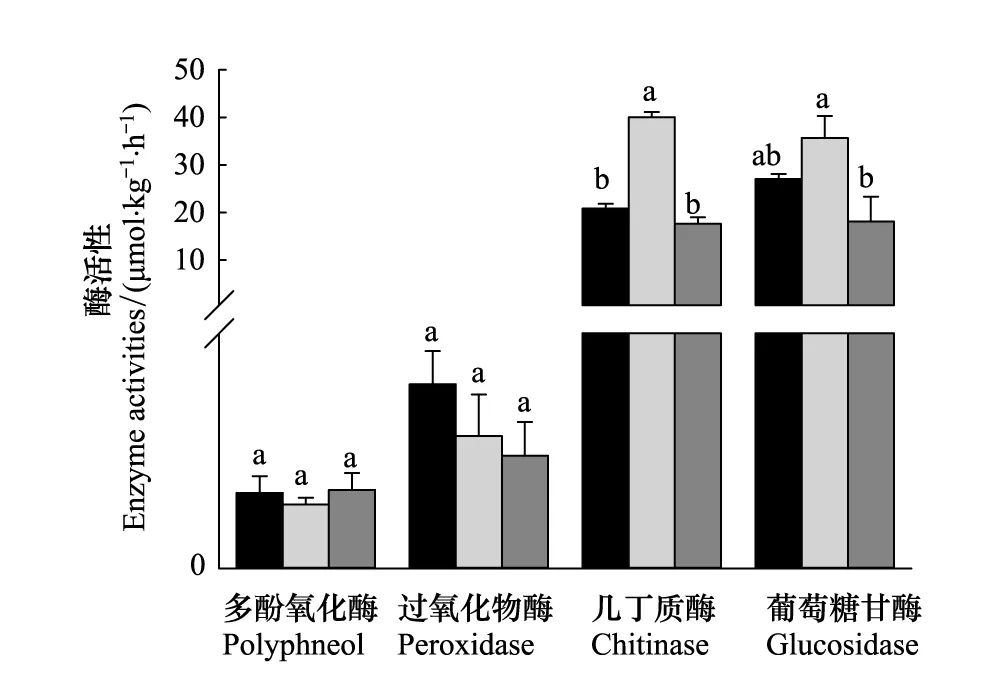
图2 不同针叶林表层土壤不同类型酶活性研究
2.3 土壤酶活性与理化性状相关性分析
利用线性回归分析了4种酶活性和土壤DOC、MBC、MBN以及pH值之间的相关性。结果见表2。多酚氧化酶和过氧化物酶与土壤碳氮以及pH值之间均不存在显著的相关性。几丁质酶与pH值存在显著相关性(R2=0.81,P<0.01),与MBN存在弱的相关性(R2=0.35,P=0.09);β-葡萄糖苷酶也与土壤MBN以及pH值存在显著的相关性,相关系数分别为0.46(P<0.05)和0.57(P<0.05)。
逐步线性回归的结果表明,几丁质酶主要受MBN与pH值的影响,两者可以解释几丁质酶变异的97.6%;而β-葡萄糖苷酶主要受土壤pH值的影响(R2=0.55,P=0.018)。

表2 土壤酶活性与理化性状的线性回归分析
3 讨论
土壤酶活性受土壤有机质质量和数量、养分有效性、底物(易氧化或难氧化)、湿度、温度以及pH的影响[23]。多酚氧化酶能利用O2作为最终的电子受体,催化腐殖质中难以利用的芳香族化合物转化分解为容易被利用的物质,该过程中释放的自由基和醌类是合成胡敏酸的重要组分[24]。过氧化物酶可以利用由于微生物活动和某些氧化酶的作用而在土壤中形成的H2O2和其他有机过氧化物中的氧,氧化土壤有机质,对腐殖质的形成具有重要作用。虽然本研究中3个针叶林所处的气候条件不同,而且土壤有机质、全氮以及碳氮比均存在一定差异(表1),但分解木质素的多酚氧化酶与过氧化物酶活性在亚热带、温带以及寒温带之间不存在显著性差异。因此我们推断,从土壤酶活性的角度来看,我国东部南北样带针叶林土壤碳氮的差异,可能主要是由分解几丁质和β-葡萄糖苷酶的酶活性差异造成的。相关性分析发现,多酚氧化酶与过氧化物酶活性与土壤DOC、MBC、MBN以及pH均不存在相关性。Sinsabaugh and Moorhead[9]也发现,土壤有机质含量与多酚氧化酶以及过氧化物酶活性不存在相关性,其他的研究也发现,多酚氧化酶和过氧化物酶活性与土壤肥力的相关性较差[25- 26]。
几丁质酶与土壤有机碳和氮的转化关系密切,能将几丁质转化为氨基糖,是土壤矿质氮的主要来源[27],本研究中,3个针叶林土壤几丁质酶活性在温带显著高于寒温带与亚热带地区。β-葡萄糖苷酶具有生物催化剂功能,能裂解二聚糖和多聚糖及β-葡萄糖苷中的β-葡萄糖苷糖苷键,在降解有机碳复合物的过程中发挥重要作用,其水解产物(糖类)是土壤微生物的主要能量来源[28],本研究中,温带针叶林土壤β-葡萄糖苷酶活性高于亚热带针叶林。温带地区几丁质酶与β-葡萄糖苷酶活性较高的原因在于:(1)温带东灵山地区的油松林凋落物量较大,但由于林龄较小(约40a),而且海拔较高,因此土壤有机质含量较低(表1);理论上处于碳氮解状态。因此会引起分解有机质合成酶的负反馈效应,因此几丁质酶和β-葡萄糖苷酶活性显著高于其他气候区。而大兴安岭地区的兴安落叶松土壤有机质丰富,DOC、MBC以及MBN含量较高(图1),分解有机质的酶产生负反馈效应,减少用于胞外酶合成的能量,使养分更高效的用于微生物生长,从而限制养分释放[29],因此酶活性反而不高。(2)干旱指数(降水量/蒸发量)也是影响酶活性的主要因素。本研究选取的3个气候区的降水量从北到南分别为500、508.9和1564 mm,然而蒸发量为800,1077.3与1115 mm,干旱指数分别为0.63,0.47与1.40,与几丁质酶以及β-葡萄糖苷酶活性的格局呈相反趋势。其他的研究也发现,水分(%)与几丁质酶以及β-葡萄糖苷酶活性呈现负相关关系[12],年均降水量、潜在蒸发蒸腾与实际蒸发蒸腾这些气候变量与凋落物分解具有高度相关[30]。
关于土壤DOC、MBC和MBN含量以及pH值与土壤酶活性的研究结论不一致,存在相关[31]与不相关[32]两种结果,这与土壤酶活性受复杂的生物以及非生物环境因素影响有关[33]。逐步线性回归分析结果显示,几丁质酶活性主要受MBN与pH控制,而β-葡萄糖苷酶活性主要受pH控制(表2)。可能的原因在于:(1)土壤pH不仅控制土壤微生物群落组成的多样性,而且直接影响土壤酶参与生化反应的速度。而本研究中3个气候区之间的土壤pH值也存在显著差异(图1),亚热带森林土壤pH值低于4,部分酶活性受到限制。因此,土壤pH可能是主要的控制因素之一;(2)北方温带和寒温带森林土壤多受N限制,其次本研究选取的亚热带马尾松林处于演替的初级阶段,土壤可以利用氮有限[34],而且MBN含量显著低于温带和寒温带针叶林土壤。因此3个针叶林土壤可利用氮的不足,成为限制土壤酶活性的主要因素之一。而MBN虽然占土壤氮的百分比较小,但生物有效性较高,也是影响土壤酶活性的一个重要的因素。由此可以看出,在中国东部南北样带针叶林中,pH与MBN可能是影响微生物活动,从而影响土壤酶活性的主要因素。
4 结论与展望
通过研究发现,分解木质素类物质的多酚氧化酶和过氧化物酶活性在3个气候区无显著性差异;而温带东灵山地区的油松林土壤几丁质酶和β-葡萄糖苷酶活性最高,亚热带马尾松土壤酶活性最低。因此推断,从土壤酶活性的角度来看,我国东部南北样带针叶林土壤碳氮的差异,可能主要是由分解几丁质和β-葡萄糖苷酶的酶活性差异造成的。森林土壤MBN含量与pH值控制土壤几丁质酶活性,而土壤β-葡萄糖苷酶活性主要受pH的控制。
下一步的研究应从土壤微生物的角度剖析,通过Biolog技术评价我国东部南北森林样带土壤微生物对不同碳源利用的能力,利用PLFA技术分析该条样带上土壤微生物群落结构的差异及其与土壤理化性状的分析,对于加强我国东部南北森林样带碳循环研究具有重要意义。
[1] Kogel-Knabner I, Ekschmitt K, Flessa H, Guggenberger G, Matzner E, von Lützow M, Marschner B. An integrative approach of organic matter stabilization in temperate soils: Linking chemistry, physics, and biology. Journal of Plant Nutrition and Soil Science, 2008, 171(1): 5- 13.
[2] Litton C M, Ryan M G, Knight D H, Stahl P D. Soil-surface carbon dioxide efflux and microbial biomass in relation to tree density 13 years after a stand replacing fire in a lodgepole pine ecosystem. Global Change Biology, 2003, 9(5): 680- 696.
[3] von Lützow M, Kögel-Knabner I, Ekschmitt K, Matzner E, Guggenberger G, Marschner B, Flessa H. Stabilization of organic matter in temperate soils: mechanisms and their relevance under different soil conditions - a review. European Journal of Soil Science, 2006, 57(4): 426- 445.
[4] Kögel-Knabner I, von Lutzow M, Guggenberger G, Flessa H, Marschner B, Matzner E, Ekschmitt K. Mechanisms and regulation of organic matter stabilisation in soils. Geoderma, 2005, 128(1/2): 1- 2.
[5] Baldrian P, Vorˇisˇková J, Dobiásˇová P, Merhautová V, Lisá L, Valásˇková V. Production of extracellular enzymes and degradation of biopolymers by saprotrophic microfungi from the upper layers of forest soil. Plant and Soil, 2011, 338(1/2): 111- 125.
[6] Waldrop M P, Balser T C, Firestone M K. Linking microbial community composition to function in a tropical soil. Soil Biology and Biochemistry, 2000, 32(13): 1837- 1846.
[7] Booth M S, Stark J M, Rastetter E. Controls on nitrogen cycling in terrestrial ecosystems: A synthetic analysis of literature data. Ecological Monographs, 2005, 75(2): 139- 157.
[8] Ruan H H, Li Y Q, Zou X M. Soil communities and plant litter decomposition as influenced by forest debris: Variation across tropical riparian and upland sites. Pedobiologia, 2005, 49(6): 529- 538.
[9] Sinsabaugh R L, Moorhead D L. Resource allocation to extracellular enzyme production: a model for nitrogen and phosphorus control of litter decomposition. Soil Biology and Biochemistry, 1994, 26(10): 1305- 1311.
[10] Jandl R, Lindner M, Vesterdal L, Bauwens, B, Baritz, R, Hagedorn F, Johnson D W, Minkkinen K, Byrne K A. How strongly can forest management influence soil carbon sequestration? Geoderma, 2007, 137(3/4): 253- 268.
[11] Baldrian P,najdr J, Merhautová V, Dobiásˇová P, Cajthaml T, Valásˇková V. Responses of the extracellular enzyme activities in hardwood forest to soil temperature and seasonality and the potential effects of climate change. Soil Biology and Biochemistry, 2013, 56: 60- 68.
[12] Brockett B F T, Prescott C E, Grayston S J. Soil moisture is the major factor influencing microbial community structure and enzyme activities across seven biogeoclimatic zones in western Canada. Soil Biology & Biochemistry, 2012, 44(1): 9- 20.
[13] McDaniel M D, Kaye J P, Kaye M W. Increased temperature and precipitation had limited effects on soil extracellular enzyme activities in a post-harvest forest. Soil Biology & Biochemistry, 2013, 56: 90- 98.
[14] Fang H J, Yu G R, Cheng S L, Zhu T H, Wang Y S, Yan J H, Wang M, Cao M, Zhou M. Effects of multiple environmental factors on CO2emission and CH4uptake from old-growth forest soils. Biogeosciences, 2010,7: 395- 407.
[15] Yu G R, Zheng Z M, Wang Q F, Fu Y L, Zhuang J, Sun X M, Wang Y S. Spatiotemporal pattern of soil respiration of terrestrial ecosystems in China: the development of a geostatistical model and its simulation. Environmental Science & Techinology, 2010, 44(16): 6074- 6080.
[16] Zheng Z M, Yu G R, Fu Y L, Wang Y S, Sun X M, Wang Y H. Temperature sensitivity of soil respiration is affected by prevailing climatic conditions and soil organic carbon content: A trans-China based case study. Soil Biology & Biochemistry, 2009, 41(7): 1531- 1540.
[17] Sheng W P, Ren S J, Yu G R, Fang H J, Jiang C M, Zhang M. Patterns and driving factors of WUE and NUE in natural forest ecosystems along the North-South Transect of Eastern China. Journal of Geographical Sciences, 2011, 21(4): 651- 665.
[18] Fang H J, Yu G R, Cheng S L, Zhu T H, Mo J M, Yan J H, Luo Y Q. Nitrogen- 15 signals of leaf-litter-soil continuum as a possible indicator of ecosystem nitrogen saturation by forest succession and N loads. Biogeochemistry, 2011, 102(1/3): 251- 263.
[19] Vance E D, Brookes P C, Jenkinson D S. An extraction method for measuring soil microbial biomass C. Soil Biology and Biochemistry, 1987, 19(6): 703- 707.
[20] Waldrop M P, Zak D R, Sinsabaugh R L, Gallo M, Lauber C. Nitrogen deposition modifies soil carbon storage through changes in microbial enzymatic activity. Ecological Applications, 2004, 14(4): 1172- 1177.
[21] Williams C J, Shingara E A, Yavitt J B. Phenol oxidase activity in peatlands in New York State: Response to summer drought and peat type. Wetlands, 2000, 20(2): 416- 421.
[22] 张鹏, 田兴军, 何兴兵, 宋富强, 任利利. 亚热带森林凋落物层土壤酶活性的季节动态.生态环境, 2007, 16(3): 1024- 1029.
[23] Decker K L M, Boerner R E J, Morris S J. Scale-dependent patterns of soil enzyme activity in a forested landscape. Canadian Journal of Forest Research-Revue Canadienne De Recherche Forestiere, 1999, 29(2): 232- 241.
[24] Dec J, Haider K, Bollag J M. Release of substituents from phenolic compounds during oxidative coupling reactions. Chemosphere, 2003, 52(3): 549- 556.
[25] 耿玉清, 白翠霞, 赵铁蕊,王树森,陈峻崎.北京八达岭地区土壤酶活性及其与土壤肥力的关系.北京林业大学学报, 2006, 28(5): 7- 11.
[26] 薛文悦.北京山地森林土壤酶特征及其与土壤理化性质的关系 [D]. 北京: 北京林业大学, 2009.
[27] Ekenler M, Tabatabai M. Beta-glucosaminidase activity of soils: effect of cropping systems and its relationship to nitrogen mineralization. Biology and Fertility of Soils, 2002, 36(5): 367- 376.
[28] Acosta-Martinez V, Tabatabai M A. Enzyme activities in a limed agricultural soil. Biology and Fertility of Soils, 2000, 31(1): 85- 91.
[29] Enrique A G, Bruno C, Christopher A, Virgile C, Stéven C. Effects of nitrogen availability on microbial activities, densities and functional diversities involved in the degradation of a Mediterranean evergreen oak litter (QuercusilexL.). Soil Biology & Biochemistry, 2008, 40(7): 1654- 1661.
[30] Prescott C E, Blevins L L, Staley C. Litter decomposition in British Columbia forests: controlling factors and influences of forestry activities. BC Journal of Ecosystem and Management, 2004, 5(2): 44- 57.
[31] Sinsabaugh R L, Lauber C L, Weintraub M N, Ahmed B, Allison S D, Crenshaw C, Contosta A R, Cusack D, Frey S, Gallo M E, Gartner T B, Hobbie S E, Holland K, Keeler B L, Powers J S, Stursova M, Takacs-Vesbach C, Waldrop M P, Wallenstein M D, Zak D R, Zeglin L H. Stoichiometry of soil enzyme activity at global scale. Ecology Letters, 2008, 11(11): 1252- 1264.
[32] Lagomarsino A, Moscatelli M C, Di Tizio A, Mancinelli R, Grego S, Marinari S. Soil biochemical indicators as a tool to assess the short-term impact of agricultural management on changes in organic C in a Mediterranean environment. Ecological Indicators, 2009, 9(3): 518- 527.
[33] Kang H J, Freeman C. Phosphatase and arylsulphatase activities in wetland soils: annual variation and controlling factors. Soil Biology & Biochemistry, 1999, 31(3): 449- 454.
[34] Fang Y T, Zhu W X, Mo J M, Zhou G Y, Gundersen P. Dynamics of soil inorganic nitrogen and their responses to nitrogen additions in three subtropical forests, south China. Journal of Environmental Sciences, 2006, 18(4): 752- 759.
Pattern of enzyme activities of typical coniferous forest soils along the North-South Transect of Eastern China
WANG Yongsheng1,2, YU Guirui1, CHENG Shulan2, FANG Huajun1,*, GAO Wenlong1,2
1SynthesisResearchCenterofCERN,KeyLaboratoryofEcosystemNetworkObservationandModeling,InstituteofGeographicSciencesandNaturalResourcesResearch,ChineseAcademyofSciences,Beijing100101,China2UniversityofChineseAcademyofSciences,Beijing100049,China
Soil organic carbon (SOC) is the largest terrestrial pool of carbon, and the amount of carbon in soils represents about two thirds of total ecosystem. Therefore, any change in the size and turnover rate of SOC pools will potentially alter the atmospheric CO2concentration and the subsequent global climate. Soil enzymes are involved in a series of catalyzing reactions and play pivotal roles in litter and soil organic matter decomposition as well as nutrient cycling in terrestrial ecosystems. Soil enzyme activity can be used as a proxy for plant and microbial substrate availability, the interference between soil ecosystem and external environment, and microbial community structure and metabolic capabilities. Therefore, the determination of soil enzyme activities is a powerful tool for understanding soil carbon and nitrogen biogeochemical processes and their responses to climate change. Soil enzyme activity has been a frontier of forest ecology research and has been widespread concern. Unfortunately, most of the studies to date have been limited to a single forest biome or a single forest site, and few studies concern the pattern and controlling factors of soil enzyme activity at a terrestrial transect scale. North-South Transect of Eastern China (NSTEC) is the fifteenth standard transect established by International Geosphere-Biosphere Program (IGBP) in 2005 and is mainly driven by heat, followed by precipitation. It covers almost all forest types from boreal forest to tropical rain forest, which provides an ideal tool to investigate the pattern and environmental control of soil enzyme activities in the boreal, temperate and tropical forest biomes. In the past decade, many studies were conducted to measure the main factors affecting the pattern of SOC storage and soil-atmosphere exchange of greenhouse gases in the typical forests along the NSTEC. However, little information is available on the pattern of soil enzyme activities involved in soil carbon and nitrogen cycles and they relate to soil microbial biomass at a large spatial scale. In this study, the typical coniferous forests distributed in the cold-temperate, temperate and subtropical climatic zones were selected along the NSTEC. The activities of polyphenol oxidase, peroxidase, chitinase and β-glucosaccharase, soil dissolved organic carbon (DOC), microbial biomass carbon (MBC) and microbial biomass nitrogen (MBN) as well as soil pH in the three coniferous forest soils were measured. The results showed that soil DOC, MBC, and MBN contents tended to decrease from north to south, and they were higher in the cold-temperate forest soils than in the subtropical forest soils. There was a significant difference in the soil pH values among the three coniferous forests, following the order cold-temperate > temperate > subtropical forests. Also, no significant differences were found in the soil polyphenol oxidase and peroxidase activities among the three typical coniferous forests. In contrast, the soil chitinase activity involved in soil nitrogen cycle was significantly higher in the temperate forest than in the cold-temperate and subtropical forests, and the soil β-glucosaccharase activity in the temperate forest was also significantly higher than that of the subtropical forest. Stepwise regression analysis showed that the soil chitinase activity was closely associated with soil MBN content and soil pH value, and the soil β-glucosaccharase activity was significantly and positively related to soil pH value. Our results suggest that soil cellulose- and chitin-degrading enzymes (i.e., chintinase and β-glucosaccharase) sensitively respond to climate zone. Soil MBN content and pH value are the main factors controlling soil enzyme activities of coniferous forests along the NSTEC.
climatic region; soil enzyme activities; cellulose; lignin
国家自然科学基金(31070435, 31290222, 31290221); 国家重点基础研究发展计划项目(2012CB417103, 2010CB833502); 中国科学院地理科学与资源研究所“秉维”优秀青年人才基金(2011RC202); 中国科学院战略性先导科技专项(XDA05050600)
2013- 09- 27;
2014- 08- 22
10.5846/stxb201309272377
*通讯作者Corresponding author.E-mail: fanghj@igsnrr.ac.cn
王永生, 于贵瑞, 程淑兰, 方华军, 高文龙.中国东部南北样带典型针叶林土壤酶活性分布格局.生态学报,2015,35(11):3636- 3642.
Wang Y S, Yu G R, Cheng S L, Fang H J, Gao W L.Pattern of enzyme activities of typical coniferous forest soils along the North-South Transect of Eastern China.Acta Ecologica Sinica,2015,35(11):3636- 3642.
