Neuroprotective ef ects of ultrasound-guided nerve growth factor injections after sciatic nerve injury
2015-02-07HongfeiLiYiruWangHuipingHuoYuexiangWangJieTang
Hong-fei Li, Yi-ru Wang, Hui-ping Huo, Yue-xiang Wang, Jie Tang
Department of Ultrasound, Chinese PLA General Hospital, Beijing, China
Neuroprotective ef ects of ultrasound-guided nerve growth factor injections after sciatic nerve injury
Hong-fei Li, Yi-ru Wang, Hui-ping Huo, Yue-xiang Wang*, Jie Tang*
Department of Ultrasound, Chinese PLA General Hospital, Beijing, China
Nerve growth factor (NGF) plays an important role in promoting neuroregeneration after peripheral nerve injury. However, its ef ects are limited by its short half-life; it is therefore important to identify an ef ective mode of administration. High-frequency ultrasound (HFU) is increasingly used in the clinic for high-resolution visualization of tissues, and has been proposed as a method for identifying and evaluating peripheral nerve damage after injury. In addition, HFU is widely used for guiding needle placement when administering drugs to a specif c site. We hypothesized that HFU guiding would optimize the neuroprotective ef ects of NGF on sciatic nerve injury in the rabbit. We performed behavioral, ultrasound, electrophysiological, histological, and immunohistochemical evaluation of HFU-guided NGF injections administered immediately after injury, or 14 days later, and compared this mode of administration with intramuscular NGF injections. Across all assessments, HFU-guided NGF injections gave consistently better outcomes than intramuscular NGF injections administered immediately or 14 days after injury, with immediate treatment also yielding better structural and functional results than when the treatment was delayed by 14 days. Our f ndings indicate that NGF should be administered as early as possible after peripheral nerve injury, and highlight the striking neuroprotective ef ects of HFU-guided NGF injections on peripheral nerve injury compared with intramuscular administration.
nerve regeneration; high frequency ultrasound; peripheral nerve injury; nerve growth factor; sciatic nerve; neurotrophic factor; intramuscular injection; mediation time; noural regeneration
Funding: This study was supported by the National Natural Science Foundation of China, No. 81100922.
Li HF, Wang YR, Huo HP, Wang YX, Tang J (2015) Neuroprotective ef ects of ultrasound-guided nerve growth factor injections after sciatic nerve injury. Neural Regen Res 10(11):1846-1855.
Introduction
Peripheral nerve injury is common, accounting for one million injuries per year worldwide (Dahlin, 2008; Siemionow and Brzezicki, 2009). Despite advances in surgical techniques such as microsurgery, restoration of nerve function after injury remains a major challenge in medicine (Zochodne, 2012). The degree of recovery of nerve function after injury depends on axonal regeneration (Siemionow and Brzezicki, 2009), and peripheral nerve injury often involves damage to the axon itself. Therefore, enhancing the rate of axonal regeneration to accelerate repair after peripheral nerve damage has been the focus of many studies to date.
The structural recovery of peripheral nerves is inf uenced by the microenvironment of the injured nerve (Zochodne, 2012). An important constituent of this microenvironment is nerve growth factor (NGF), which has been studied extensively (Ebendal, 1992; Moges et al., 2011; Pettersson et al., 2011). NGF plays an important role in the nutrition and growth-promotion of axons, and contributes to the regulation of the development, proliferation, dif erentiation, growth, regeneration and overall function of central and peripheral neurons (Shen et al., 2010a; Barmpitsioti et al., 2011; Kaiser and Haninec, 2012; Shakhbazau et al., 2012; Euler de Souza Lucena et al., 2014; Martinez et al., 2014). The signif cant and wide-ranging functions of NGF have led to its use in many preclinical and clinical trials for the treatment of nervous system disorders, such as Alzheimer’s and Parkinson’s diseases, and peripheral neuropathies (Olson et al., 1991; Apfel, 2002; Mandel, 2010; Wahlberg et al., 2012). The ef ect of treatment depends largely on two factors: the route and timing of administration, which can be via local, intramuscular (i.m.), or systemic (intravenous or oral) routes, or by gene therapy. However, all these methods have disadvantages that limit the clinical success of a treatment. For example, systemic administration cannot ensure an optimal dose at the site of action, and gene therapy is costly and presents a number of risks. Local injections of NGF are used widely in the treatment of nerve injury (Fortun et al., 2009), but this route is not suitable in all cases, such as those in which surgery is not required (Walshe et al., 2001). Moreover, the duration and concentration of NGF cannot be guaranteed (Kubo et al., 2000). Regarding timing, early administration of NGF consistently results in better outcomes. However, its short half-life means that the administration schedule forNGF still needs further investigation to optimize the treatment ef ect after peripheral nerve injury.
High frequency ultrasound (HFU) is an ultrasonic diagnostic technique which allows the identif cation of the f ne structure of peripheral nerves and of the exact position of the damaged nerve (Toros et al., 2009; Liu et al., 2012). Used soon after injury, HFU can help to determine the nature and extent of the damage. Furthermore, HFU can be used to guide needle placement when administering drugs directly to the site of action. HFU is therefore increasingly widely used as a tool for the diagnosis and treatment of nerve injury.
The aim of the present study was to determine the ef cacy of immediate and delayed (14 days after injury) administration of NGF directly into the damaged area of the sciatic nerve via HFU-guided injection, and compare it with immediate and delayed i.m. NGF administration, in a rabbit model of sciatic nerve crush injury.
Materials and Methods
Animals and surgery
Sixty male New Zealand white rabbits, aged 4–6 months and weighing 2.5–3.0 kg, were provided by the Laboratory Animal Center, PLA General Hospital, Beijing, China (license No. SCXK (Jing) 2010-0001). Rabbits were anesthetized with Sumianxin (ShengDa Animal Drugs Ltd., Donghua, Jilin Province, China; 0.2 mL/kg, i.m.) and f xed on a surgical table. Following routine sterilization, an incision, 3 cm long, was made longitudinally on the posterior thigh of the left hindlimb. The biceps f exor cruris muscle was separated bluntly to expose the sciatic nerve. The hindlimb contracted upon mild stimulation of the sciatic nerve. Intestinal forceps (25 cm; Shanghai Medical Instrument Co., Ltd., Shanghai, China) were used to clamp the nerve vertically at three points, 1.5 cm below the ossa sedentarium (Schmitz and Beer, 2001; Wang et al., 2010). Clamping lasted approximately 5 minutes and was repeated once after a 10 second release. The damaged area was 10 mm wide and the clamped parts were translucent but not broken, and was marked by placing a sterile piece of catheter tubing in the epineurium. The wounds were closed and the rabbits were allowed to recover. Three days after modeling, one rabbit was selected at random and killed using an overdose of Sumianxin. A 5 mm length of spinal cord, with the injury site at the center, was harvested, dehydrated, embedded in paraf n, cut longitudinally into 5 μm sections, and dyed with toluidine blue to conf rm that the model had been established successfully. The experiments were approved by the Animal Ethics Committee of PLA General Hospital, Beijing, China, and were conducted in accordance with the NIH Guide for the Care and Use of Laboratory Animals. All efforts were made to minimize animal discomfort and reduce the number of animals used.
HFU-guided NGF administration
All 60 rabbits with sciatic nerve injury were randomly divided into three groups: model (sciatic nerve injury), HFU (HFU-guided NGF injection), and IM (i.m. NGF injection). The normal nerve tissue of the right hindlimb was used as control tissue. The two NGF treatment groups were then divided into two subgroups (n = 10) according to the time of treatment: immediately after injury (I-HFU, I-IM) or 14 days after injury (14-HFU, 14-IM). Rabbits in the model group (n = 20) received no treatment after injury. An HFU array probe was used to perform bilateral horizontal scans of the sciatic nerves to identify the damaged area, which was then marked by placing a sterile infusion tube in the epineurium 5 mm above the wound. Rabbits in the I-HFU group received injections of NGF (Staidson Biopharmaceuticals Co.,Ltd., Beijing, China) into the area surrounding the injured nerve via a 22-gauge needle guided by HFU, and rabbits in the I-IM group received i.m. NGF (2 μg/kg), daily from 1 to 14 days after injury. Rabbits in the 14-NGF and 14-IM groups received NGF under the same protocol but from 14 to 28 days after injury.
General observation
Limb movement, wound healing, degree of crus triceps paralysis, foot and ankle anabrosis, and signs of distress were monitored daily after surgery.
Neurological function assessment
Neurological function was assessed at 2, 4, 6, and 8 weeks after injury. Toe spreading (Shen et al., 2010b) was graded from 1 to 4, and modif ed Tarlov scoring (Matsuyama et al., 2000) from 0 to 4 was also conducted, with higher scores representing better function. The two scores were added to obtain the total neurological function score, and the mean was calculated for the group.
Ultrasound testing
At 2, 4, 6, 8 weeks after injury, the sciatic nerves of all rabbits were examined using HFU diagnostic apparatus (GE Healthcare, Bethesda, MD, USA). The rabbits were anesthetized with an injection of Sumianxin (0.2 mL/kg, i.m.) into the neck, and f xed in the prone position. Both hindlimbs were straightened and separated. The HFU array probe was used to conduct bilateral horizontal scans of the sciatic nerves, and the damaged area on the left nerve was identif ed by the catheter tubing in the epineurium. The inner diameter, continuity, and integrity of the myelinated nerve f bers were observed.
Electrophysiological measurements
At 8 weeks after surgery, we performed electrophysiological measurements to evaluate the recovery condition of triceps surae with an electromyography instrument (Medtronic Keypoint, Skovlunde, Denmark). Two rabbits from each group were chosen and anesthetized with an injection of Sumianxin (0.2 mL/kg, i.m.) into the neck, and sciatic nerves were exposed bilaterally. The stimulating electrode was placed on the distal and proximal ends of the injured segment (or equivalent position on the control nerve), and the recording electrode was placed in the triceps surae. Recovery of the triceps surae was evaluated by measuring the peak amplitude and latency of the compound muscle actionpotential (CMAP). The rabbits were then sacrificed with an overdose of Sumianxin for histological examination, described below.
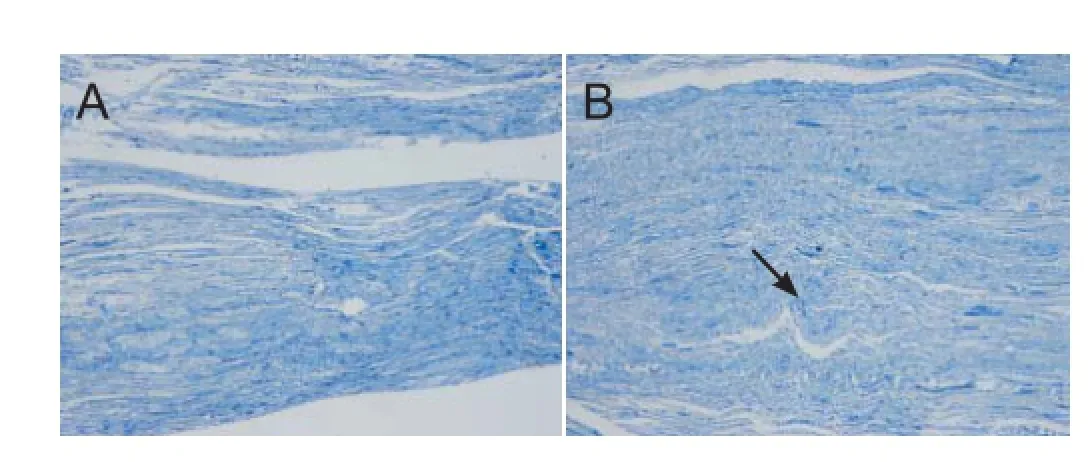
Figure 1 Longitudinal sciatic nerve sections (toluidine blue staining, × 200 magnif cation).
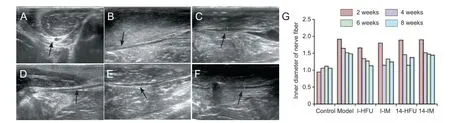
Figure 3 Ultrasonic testing of sciatic nerve injuries.
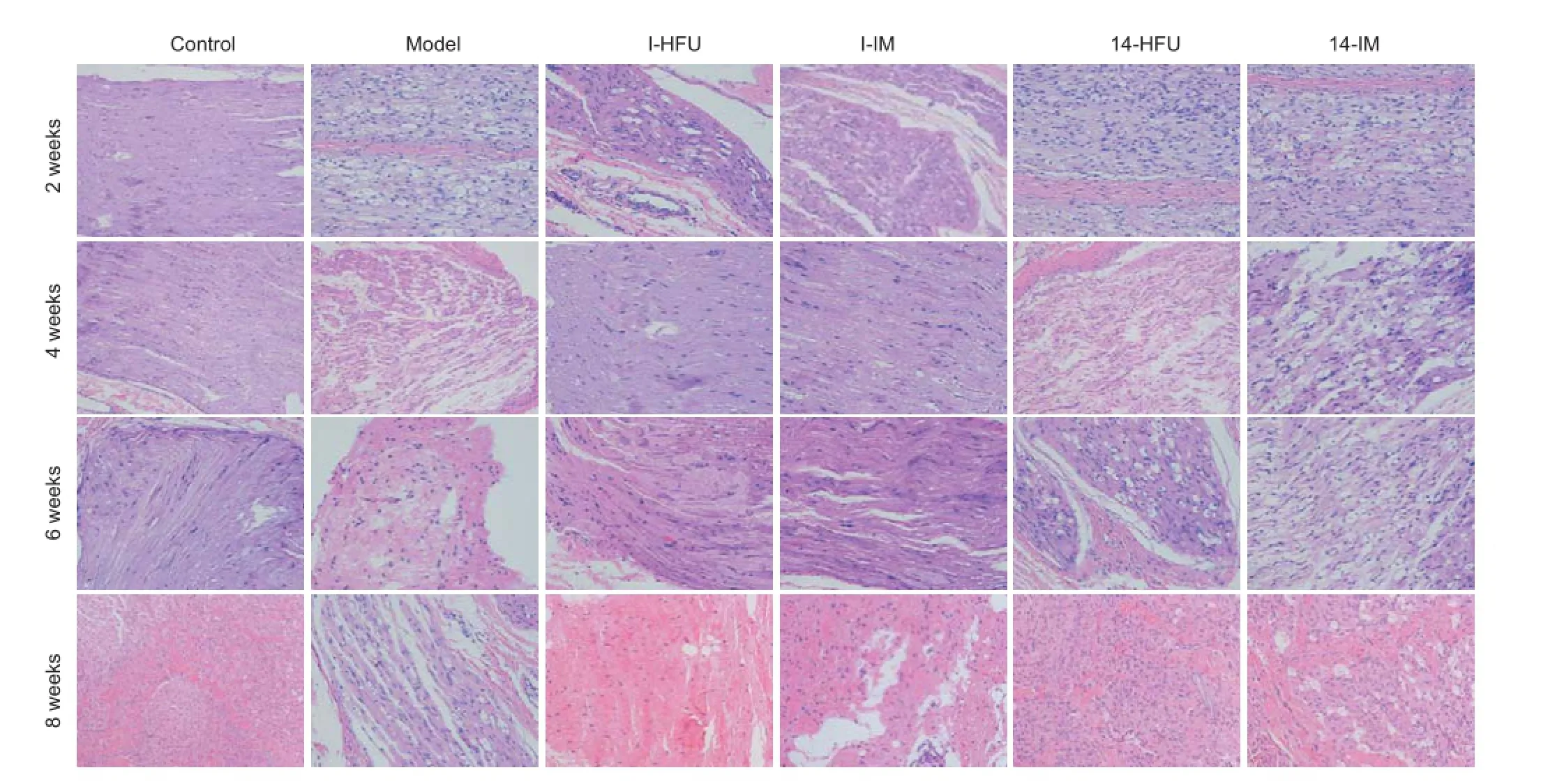
Figure 6 Pathology of rabbit sciatic nerve after injury and treatment with high-frequency ultrasound (HFU)-guided nerve growth factor (NGF) injection (hematoxylin-eosin staining, × 200).
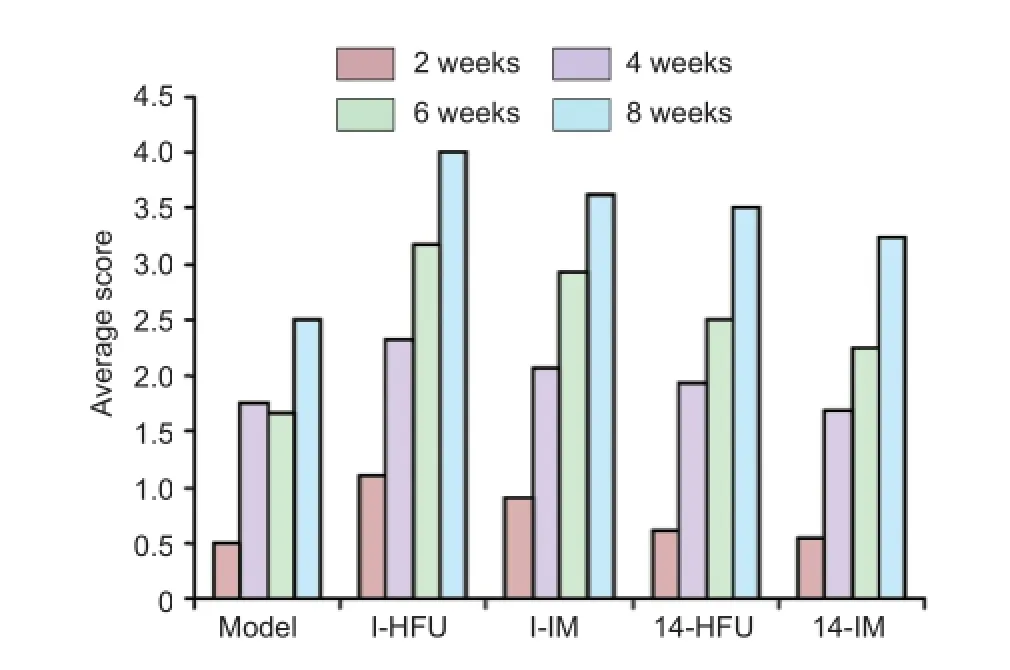
Figure 2 Neurological function in rabbits with sciatic nerve injury after high-frequency ultrasound (HFU)-guided nerve growth factor (NGF) injection.
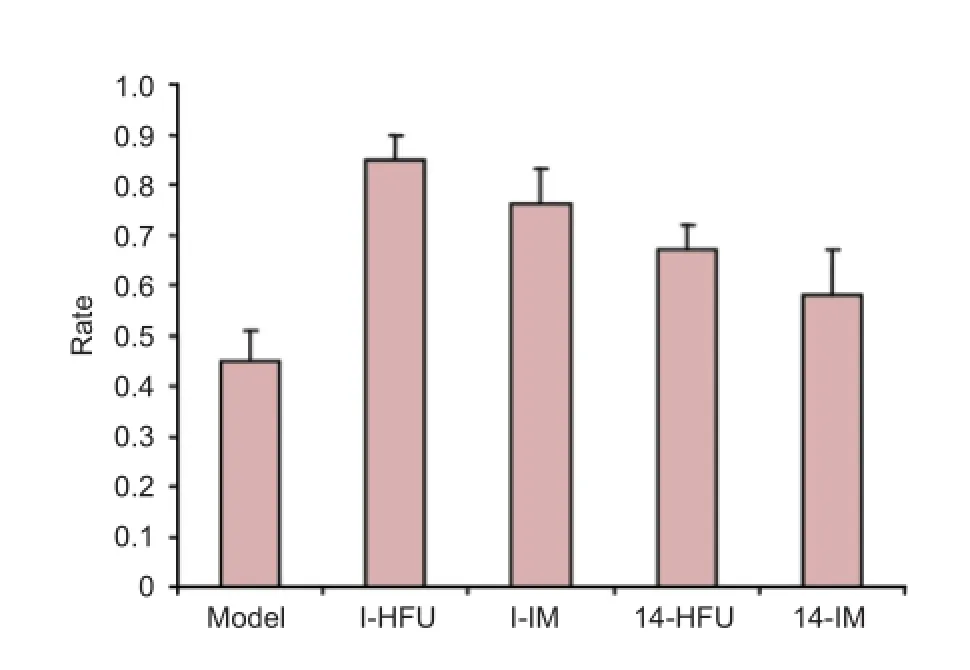
Figure 5 Wet weight recovery rate of the triceps surae after sciatic nerve injury treated by high-frequency ultrasound (HFU)-guided nerve growth factor (NGF) injection.
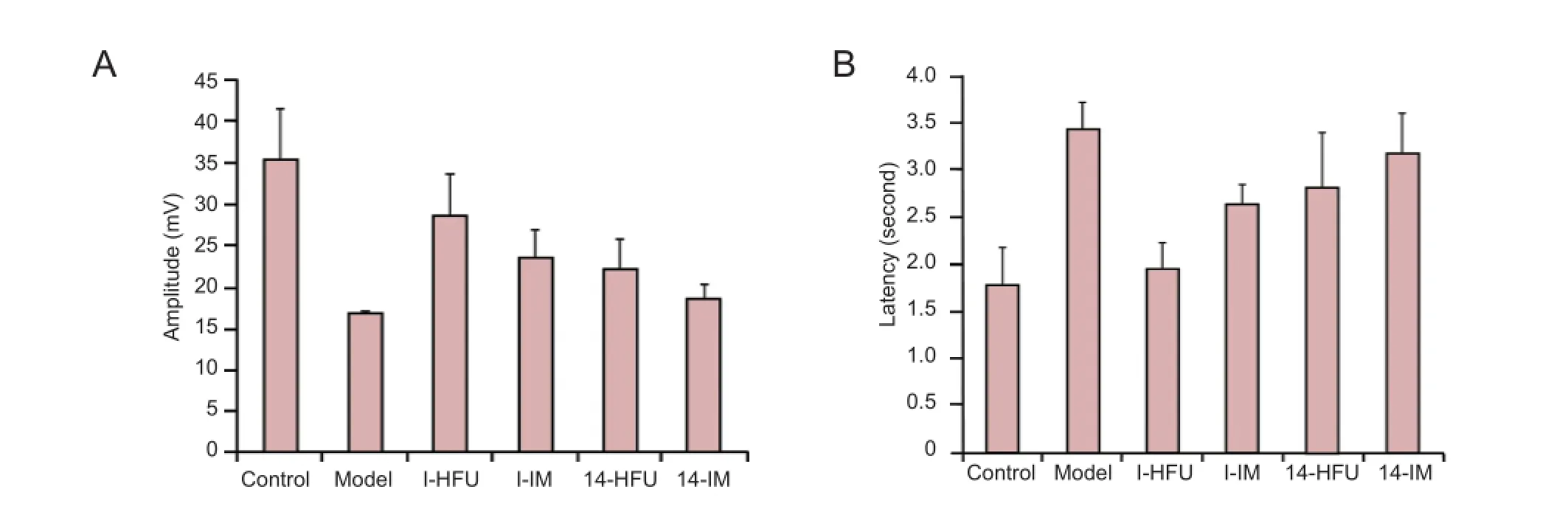
Figure 4 Compound muscle action potential of the triceps surae after sciatic nerve injury treated with high-frequency ultrasound (HFU)-guided injection of nerve growth factor (NGF).
Measurement of recovery rate of wet weight of the triceps surae
The triceps surae was dissected out from the two animals per group used for electrophysiological measurements, 8 weeks after injury. Excessive f brous connective tissues were removed, the tissue was rinsed with physiological saline and blotted with f lter paper, and the wet weight was measured. Recovery rate was calculated as the ratio of wet weight of the tissue from the injured limb to that of the healthy limb in each group. All measurements were carried out under temperature- and humidity-controlled conditions and by the same technician.
Masson trichrome staining
After weighing, the triceps surae tissue was f xed in paraformaldehyde (10%) for 48 hours, dehydrated, and embeddedin paraf n. The paraf n blocks were cut into 10-μm-thick sections and stained with Masson trichrome. The slices were observed under an optical microscope at 200× magnif cation, and 10 independent muscle f bers were chosen for analysis. The area of muscular f bers was calculated using Image Pro Plus 6.0.
Semi-thin sectioning
The sciatic nerve was dissected out from the two animals per group used for electrophysiological measurements, 8 weeks after injury. A 5 mm length of nerve was removed from the distal stump of the damaged sciatic nerve, f xed in glutaraldehyde (2.5%) for 24 hours, dehydrated, and embedded in epoxy resin. The tissue was cut into sections 1.5 μm thick, stained with toluidine blue, mounted, and observed under an optical microscope (Olympus). Three visual f elds (200× magnif cation) were selected from each slide, and 10 nerve f bers were chosen for analysis. Image Pro Plus 6.0 was used to calculate the density of nerve f bers, diameter of myelinated nerve f bers, and thickness of the myelin sheath.
Transmission electron microscopy
At 8 weeks after injury, one of the rabbits which were tested for electrophysiological measurements was chosen and killed. A 5 mm length of sciatic nerve tissue was removed from the distal stump of the damaged nerve and cut into several sections approximately 1 mm3. The nerve sections were f xed with glutaraldehyde (2.5%), washed by PBS three washes for 15 minutes each, f xed by osmic acid (1%) including 1 × PBS (pH 7.4) at 20°C for 2 hours, washed by PBS (pH 7.4) three times for 15 minutes each, dehydrated in ethanol gradients (50%, 60%, 70%, 80%, 90%, 100%) in turn for 15 minutes each, treated by acetone plus 812 embedding agent (1:1) overnight, emdedded at 60°C for 48 hours, sliced into 60–80 nm thick sections with a HM 360 microtome (Microm, Walldorf, Germany), dyed with saturated aqueous uranyl acetate (2%) for 15 minutes, counterstained with citrate for 15 minutes, and f nally dried at room temperature overnight. Microscope sections were prepared, observed and analyzed under the electron microscope (FEI Tecnai G2 20 TWIN, USA).
Hematoxylin-eosin (HE) staining
At 2, 4, 6 and 8 weeks after injury, the rabbits were killed with an overdose of Sumianxin. The skin and subcutaneous fascia of the left hindlimb were cut along the original incision, the muscles were separated, and the damaged nerve was exposed. A 5 mm length of nerve tissue was taken from the injury site, f xed, and embedded in paraf n. Sections were cut and stained with HE for observation under an optical microscope (Olympus, Japan). Images were taken and analyzed using Image Pro Plus 6.0 (Media Cybernetics, Silver Spring, MD, USA).
Immunohistochemistry
At 8 weeks after injury, the remaining animals were killed by an overdose of anesthetic. The damaged nerve was removed, embedded in paraf n, and dewaxed. Antigen retrieval was carried out in a microwave oven (Galanz, China) for 10 minutes on the low setting, using citric acid buf er (pH 6.0), and the samples were washed three times, for 5 minutes each, in PBS (pH 7.4) on a decoloring shaker (Beijing LiuYi Instrument, Beijing, China). Endogenous peroxidase was blocked with 3% H2O2, incubated for 25 minutes in the dark, and then washed in PBS as before. The sections were blocked with 3% bovine serum albumin for 30 minutes, incubated in rabbit anti-mouse S100 β antibody (Abcam, Cambridge, UK) at 1:1,000 dilution overnight at 4°C, followed by goat anti-rabbit IgG H&L (HRP) (Abcam) at 1:500 dilution for 2 hours at room temperature. Proteins were visualized using a 3,3′-diaminobenzidine kit (Dako, Glostrup, Denmark), according to the manufacturer’s instructions, and the sections were mounted using neutral gum for viewing under an optical microscope (Olympus). The gray value of each image was calculated using Image Plus Pro 6.0, representing the relative expression of S100 β, and the mean taken for each group.
Statistical analysis
All data were analyzed using SPSS 13.0 software (SPSS, Chicago, IL, USA) and are expressed as the mean ± SD. Analysis of variance and Student’s t-test were used to compare means. P < 0.05 was considered statistically signif cant.
Results
Conf rmation of successful rabbit model of sciatic nerve injury
Toluidine blue staining conf rmed that the model of sciatic nerve injury was established successfully, resulting in a Sunderland grade III lesion (Sunderland, 1951). Damaged sciatic tissue showed weaker staining than control tissue, and more tortuous f ber tracts. The axon, myelin sheath and endoneurium were broken, but the perineurium and perilemma remained intact. Nerve f ber growth was maintained (Figure 1).
General observations
All rabbits were subdued after the surgery. Animals dragged the injured leg or avoided putting weight on it. The acrotarsium was weak and the toes were notably downturned; these signs were more pronounced when jumping. After 10 days, the model group showed ulceration at the bottom of the left hind foot, blackened toe skin, and self-mutilation of the injured limb; these ef ects were less severe in the four treatment groups. By 6 weeks after injury, animals in the treatment groups were able to bear more weight on the injured leg than previously, and recovered normal gait by 8 weeks.
Neurofunctional assessments
The mean neurological score was highest in the I-HFU group. Scores in all treatment groups increased with time after injury (P < 0.05; Figure 2).
Ultrasonic evaluation of the sciatic nerve
In the control group, the echo structure in the cross-sectionswas round or oval, and contained scattered hyperechoic spots (Figure 3A). By contrast, linear parallel structures were observed in the echo structure of the longitudinal sections (Figure 3B). In all groups, the damaged area changed with time after injury. At 2 weeks, the continuity of the linear echo structure was poor, with a prominent, hypoechoic interruption. The nerve tract was tortuous, the inner diameter was notably thicker than in control tissue, and the dividing line between the nerve perilemma and the surrounding tissues was obscure (Figure 3C). At 4 weeks, the extent of the variance and the inner diameter was smaller than at 2 weeks, the echogenicity of the interrupted zone was stronger, and the continuity of the linear echo was improved. The dividing line between the nerve perilemma and the surrounding tissue was less obscure than that at 2 weeks within each group (Figure 3D). At 6 weeks, the continuity of the echo structure, the inner diameter of the nerve, the echogenicity of the interrupted zone, and the dividing line between the nerve perilemma and surrounding tissue were all greatly improved. Compared with that observed at 4 weeks, the interruptions were less pronounced, the inner diameter was smaller, and the dividing line was clearer (Figure 3E). At 8 weeks, the continuity of the linear echo structure was good, the interrupted zone was almost entirely repaired, the inner diameter recovered well and there was a clear dividing line between the nerve perilemma and the surrounding tissue (Figure 3F). The inner diameter of the damaged sciatic nerve was smallest in the I-HFU group and largest in the model group, and generally decreased with time after injury. In the I-HFU group, thickness was restored to control measurements by 8 weeks in the I-HFU group. Recovery was best in the I-HFU group, followed by the I-IM and 14-HFU groups, and lastly the 14-IM group (Figure 3G).
Electrophysiological function of the triceps surae
At 8 weeks after injury, the functional recovery of the triceps surae on the injured side was evaluated electrophysiologically. CMAP amplitude and latency represent the degree of recovery. The model group showed the lowest amplitude and the longest latency of all groups (P < 0.05). Among the treatment groups, I-HFU had the highest amplitude and the shortest latency (P < 0.05). The amplitude was higher and the latency shorter in both HFU-mediated treatment groups than in the groups that received i.m. NGF (P < 0.05; Figure 4).
Recovery rate of triceps surae
At 8 weeks after injury, the triceps surae wet weight recovery rate was lowest in the model group (P < 0.05) and highest in the I-HFU group (P < 0.05). Of the four treatment groups, recovery rate was highest in the I-HFU group (P < 0.05), then the I-IM and 14-HFU groups, and lowest in the 14-IM group (P < 0.05) (Figure 5).
Pathological changes in injured sciatic nerve
Nerve tissue in the control group showed neatly arranged f bers and a uniform myelin sheath structure (Figure 6A). In the model group, sciatic nerve f bers were arranged in a disorganized manner and had swollen or missing axons, and vacuolation. Most of the myelin had disintegrated and inf ammatory cell inf ltration was observed (Figure 6B). In the I-HFU group, compared with the model group, the nerve fibers showed a more orderly arrangement, and less axonal swelling and vacuolation. Part of the myelin had disintegrated and more Schwann cells and fewer inf ammatory cells were distributed between the f bers (Figure 6C). In the I-IM group, f bers were slightly disordered, axons were swollen, and there was less vacuolation than in the model group, but more than in the I-HFU group. Myelin was partially disintegrated. More Schwann cells and fewer inf ammatory cells were distributed between the f bers than observed in the I-HFU group (Figure 6D). The pathology of the 14-HFU and 14-IM groups was similar to that in the model group (Figure 6E, F).
At 4 weeks after injury, in the control group, the nerve f -bers had returned to the control state, showing normal, uniform myelin sheaths and a small number of Schwann cells between the f bers (Figure 6A). In the model group, multiple changes were observed in the myelin sheath, including uneven distribution, variable thickness, irregular f ber arrangement, f ber degeneration, and disintegration of axons and myelin. In addition, the normal structure of the myelin sheath had almost disappeared, leaving an irregular outline and notable edema of the nerve. Distribution was sparse, and more inf ammatory cells were observed between f bers (Figure 6B). Compared with the control group, the I-IM group showed disorganized f bers, less obvious Schwann cell proliferation, better directional arrangement, and less vacuolation (Figure 6E). In comparison, the I-HFU group showed a greater number of Schwann cells and even less vacuolation (Figure 6C). In the 14-HFU and 14-IM groups, the fibers were arranged in a disorderly manner but less so than in the model group; in addition, compared with the model group, a larger number of Schwann cells were observed between f bers, and more vacuolation was observed (Figure 6D, F). Compared with the 14-IM group, far less vacuolation was observed in the 14-HFU group (Figure 6D).
At 6 weeks, in the control group the tract and structure of the nerve were normal and uniform. Few Schwann cells were observed between the f bers (Figure 6A). In the model group, the distribution of f bers was uneven, the thickness variable, and the arrangement of the myelin sheath irregular. Nerve f ber degeneration was observed, and the disintegrated axons and myelin sheath were partially absorbed. The myelin sheath was sparse and misshapen, and there were few regenerating nerve f bers. Some f bers were pyknotic, and notable spongiform change was observed. There was no evidence of axonal regeneration (Figure 6B). In the I-HFU and I-IM groups, compared with the model group, Schwann cell proliferation was more obvious, cells were distributed more uniformly, and the arrangement of nerve f bers was also more uniform. There was considerably less spongiform change than in the model group. There was better nerve f ber recovery in the I-HFU group than in the I-IM group (Figure 6C, E). In the 14-HFU and 14-IM groups, arrangement of f bers was considerably more disordered but more regular than in the model group. Some of the disintegrated materialhad been absorbed, and more Schwann cells were observed between the f bers. The nerve f bers had regenerated but were fewer in number than in the I-HFU and I-IM groups, and the spongiform changes were more evident. More regenerated f bers and Schwann cells were observed in the I-HFU group than in the I-IM group (Figure 6C, E).
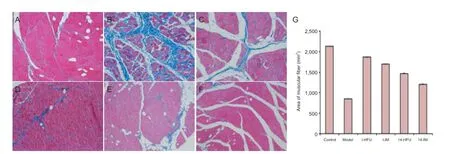
Figure 7 Muscle f bers of rabbits with sciatic nerve injury after high-frequency ultrasound (HFU)-guided nerve growth factor (NGF) injections.
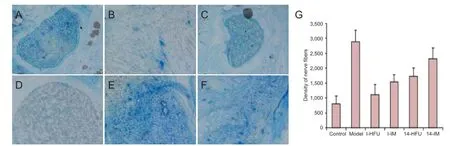
Figure 8 Ef ects of high-frequency ultrasound (HFU)-guided nerve growth factor (NGF) injection on injured sciatic nerve tissue.
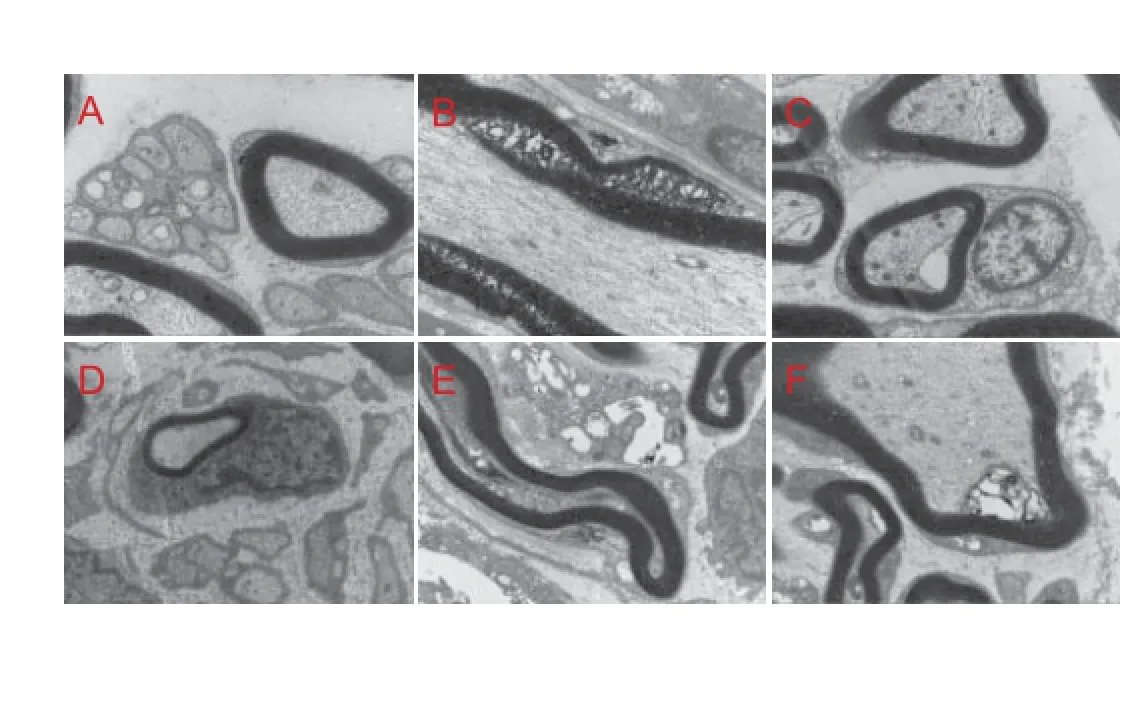
Figure 9 Sciatic nerve ultrastructure 8 weeks after injury and treatment with high-frequency ultrasound (HFU)-guided nerve growth factor (NGF) injections (transmisson electron microscope, × 1,500).
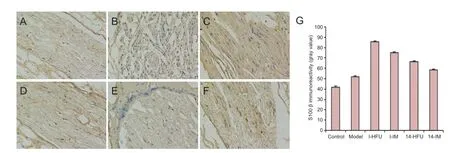
Figure 10 S100 β immunoreactivity in the sciatic nerve after injury and treatment with high-frequency ultrasound (HFU)-guided nerve growth factor (NGF) injection.
At 8 weeks after injury, in the control group, nerve f ber tracts were normal and uniform in structure. Axons were also uniform in diameter and arranged in a regular manner. Small Schwann cells were visible (Figure 6A). In the model group, the regenerating nerve f bers were sparse and dispersed, and with an uneven distribution. Myelin thickness was uniform, but the structure was loose and disorganized. Mesenchyme was evident. Spongiform changes were clear and inflammatory cell infiltration was observed (Figure 6B). In the I-HFU and I-IM groups, fibers were orderly. Compared with the model group, there were more regenerating f bers, and more evident proliferation and distribution of Schwann cells. Nerve f bers were arranged in a more orderly manner, and there was little spongiform change and few connective tissues between the f bers. Nerve f bers recovered better in the I-HFU group than in the model group (Figure 6C, E). In the 14-HFU and 14-IM groups, the disintegrated products were partially absorbed and Schwann cells were observed between the f bers. Fewer regenerating nerve fibers, more spongiform change and mesenchyme between f bers, and more inf ltrated inf ammatory cells, were observed in the 14-HFU and 14-IM groups than in the I-HFU and I-IM groups. The number of regenerated f bers was greater in both HFU groups than in both IM groups (Figure 6D, F).
Muscle f ber changes after sciatic nerve injury
At 8 weeks after injury, muscle f bers were plump on the control side but thin on the injured side (Figure 7A). In the model group, bonds between the muscle cells were loose and the structure was completely damaged (Figure 7B). In the I-HFU group the bonds between cells were relatively tight, with a regular structure (Figure 7C). In the I-IM group, the structure was not clear (Figure 7D). The recovery of the muscle f ber structure in the 14-HFU and 14-IM groups was better than in the model group, but not as good as in the I-HFU and I-IM groups (Figure 7E, F). Muscle fiber area was calculated on the cross-section of the triceps surae, and was greater in the four treatment groups than in the model group (P < 0.05). Muscle f ber area was greater in the I-HFU and I-IM groups than in the 14-HFU and 14-IM groups, greater in the HFU groups than in the IM groups, and greatest in the control group (Figure 7G).
The density of myelinated nerve f bers
In the control group, myelinated nerve f bers and myelin sheath thickness were uniform, and the arrangement was regular (Figure 8A). In the model group, myelinated f bers on the injured side were disorganized, with irregular form and sheath thickness (Figure 8B). In the I-HFU and 14-HFU groups, the myelinated nerve f bers were more regular than in the model group, but less so than in the control group. Early NGF administration, in particular with HFU guiding, resulted in more regular myelinated nerve f bers, and more uniform myelin sheath thickness, than later or i.m. administration (Figure 8C–F). The diameter of the myelinated nerve fibers and thickness of the myelin sheath showed greatest improvement in the I-HFU group (P < 0.05; data not shown). Nerve f ber density was highest in the model group and lowest in the control group. The density of myelinated nerve f bers was much lower if NGF was administered early, especially with HFU guiding (P < 0.05) (Figure 8G).
Ultrastructure of the injured sciatic nerve
Control tissue showed regular axonal arrangement, uniform myelin sheath thickness, and well-distributed staining, with oval Schwann cell nuclei (Figure 9A). In the model group, the thickness of the myelin sheath was not uniform; breakage and dispersion of the myelin layer was observed, staining was not well distributed, and Schwann cell nuclei were irregularly shaped (Figure 9B). Nerve f ber regeneration was better in the four treatment groups than in the model group. Immediate treatment resulted in good axonal regeneration, orderly axon arrangement, uniform axon diameters and myelin sheath thicknesses, and uniform dye uptake (Figure 9C, D). Schwann cell nuclei were oval in the I-HFU group but irregularly shaped in the I-IM group; similarly, myelin sheath thickness was uniform in the I-HFU group but varied in the I-IM group. In the 14-HFU and 14-IM group, axons were poorly developed and irregularly arranged, with variable myelin sheath thicknesses and irregularly shaped Schwann cell nuclei (Figure 9E, F).
S100 β immunoreactivity in the sciatic nerve
At 8 weeks after injury, the immunoreactivity of S100 β in the sciatic nerve varied with time after injury, and administration method. S100 β immunoreactivity was observed as brown granules in the cytoplasm. Some sporadic expression (+) was observed in the control group (Figure 10A). There was less S100 β immunoreactivity in the model group (+/-) than in the control group (Figure 10B). S100 β immunoreactivity was strongest in the I-HFU group (++++), weaker in the I-IM (++) and 14-HFU (++) groups, and weakest in the 14-IM group (++) (Figure 10C–F).
Gray value was used to quantify S100 β expression. Immediate treatment resulted in higher S100 β expression than delayed treatment (P < 0.05), and HFU-guided NGF injections resulted in higher NGF expression than i.m. injection (P< 0.05; Figure 10G).
Discussion
With advances in surgery, including microsurgery, treatment of peripheral nerve injury is becoming increasingly successful (Battiston et al., 2000; Martinez et al., 2014). Regeneration after peripheral nerve injury is af ected by many factors, and the prognosis depends on the location and extent of the damage. It is dif cult to improve treatment by modifying surgical techniques alone (Euler de Souza Lucena et al., 2014; Hsueh et al., 2014). Regeneration after peripheral nerve injury is greatly inf uenced by the microenvironment. Neurotrophic factors contribute to this microenvironment, and of these, NGF has been accepted as the most ef ective, with striking treatment ef ects obtained in clinical and preclinical studies (Wang et al., 2009). NGF promotes the survival of neurons and regeneration axons (Kaiser and Ianinec, 2012). Therefore, maintaining a microenvironment rich in NGF is key to repairing damaged nerves (Fumagalli et al., 2008). Treatment ef ect depends largely on the route and timing of administration. To date, studies of NGF administration have mainly focused on i.m., intravenous, or local administration. NGF cannot pass through the blood-brain barrier owing to its high molecular weight and short half-life. To maintain an ef ective concentration of NGF, repeated and long-term administration is needed, which limits its use in the clinic (Madduri et al., 2010). It therefore remains a priority to explore new methods of NGF use. Silicone tube implants have previously been used to infuse NGF continuously into the site of nerve damage, and the method can promote nerve regeneration (Scheidegger et al., 2011). However, the tubes cannot be implanted in the body for a long time because of histocompatibility problems. HFU can be used to detect peripheral nerve injury in the early period and accurately guide injection of the drug into the damaged area. This use of HFU has drawn considerable attention in the medical f eld, including a report of a clinical trial in which HFU was used in the treatment of Hansen’s disease (Bathala et al., 2012). In the present study, we used HFU to guide NGF injections into the damaged area to treat nerve injury. We compared two administration times and performed a series of experiments to determine a suitable administration schedule. Our results show that HFU-guided injection of NGF leads to better structural and functional outcomes in nerve and muscle recovery than i.m. injection.
Histological examinations and electron microscopy also revealed that HFU-guided NGF administration was more effective than i.m. injection in promoting recovery of the myelinated f bers and myelin sheath in the injured nerve, and in the surrounding muscle tissue. HFU-guided NGF injection was also more ef ective when administered immediately after injury than after 14 days.
In conclusion, HFU-guided injections of NGF, administered soon after the injury, leads to good recovery of nerve and muscle after nerve injury. Further studies exploring treatment schedules, doses, and administration sites are necessary to improve prospects for the clinical application of this promising technique.
Author contributions: HFL and YRW performed animal experiments. HFL wrote the paper. HPH analyzed the data. YXW designed the study and revised the paper. JT was responsible for fundraising and supervised the study. All authors approved the f nal version of this paper.
Conf icts of interest: None declared.
Plagiarism check: This paper was screened twice using Cross-Check to verify originality before publication.
Peer review: This paper was double-blinded, stringently reviewed by international expert reviewers.
Apfel SC (2002) Nerve growth factor for the treatment of diabetic neuropathy: what went wrong, what went right, and what does the future hold? Int Rev Neurobiol 50:393-413.
Barmpitsioti A, Konofaos P, Ignatiadis I, Papalois A, Zoubos AB, Soucacos PN (2011) Nerve growth factor combined with an epineural conduit for bridging a short nerve gap (10 mm). A study in rabbits. Microsurgery 31:545-550.
Bathala L, Kumar K, Pathapati R, Jain S, Visser LH (2012) Ulnar neuropathy in hansen disease: clinical, high-resolution ultrasound and electrophysiologic correlations. J Clin Neurophysiol 29:190-193.
Battiston B, Tos P, Cushway TR, Geuna S (2000) Nerve repair by means of vein filled with muscle grafts I. Clinical results. Microsurgery 20:32-36.
Dahlin LB (2008) Techniques of peripheral nerve repair. Scand J Surg 97:310-316.
Ebendal T (1992) Function and evolution in the NGF family and its receptors. J Neurosci Res 32:461-470.
Euler de Souza Lucena E, Guzen FP, Lopes de Paiva Cavalcanti JR, Galvão Barboza CA, Silva do Nascimento Júnior E, Cavalcante Jde S (2014) Experimental considerations concerning the use of stem cells and tissue engineering for facial nerve regeneration: a systematic review. J Oral Maxillofac Surg 72:1001-1012.
Fortun J, Hill CE, Bunge MB (2009) Combinatorial strategies with Schwann cell transplantation to improve repair of the injured spinal cord. Neurosci Lett 456:124-132.
Fumagalli F, Madaschi L, Brenna P, Caf no L, Marf a G, Di Giulio AM, Racagni G, Gorio A (2008) Single exposure to erythropoietin modulates Nerve Growth Factor expression in the spinal cord following traumatic injury: comparison with methylprednisolone. Eur J Pharmacol 578:19-27.
Hsueh YY, Chang YJ, Huang TC, Fan SC, Wang DH, Chen JJ, Wu CC, Lin SC (2014) Functional recoveries of sciatic nerve regeneration by combining chitosan-coated conduit and neurosphere cells induced from adipose-derived stem cells. Biomaterials 35:2234-2244.
Kaiser R, Haninec P (2012) Degeneration and regeneration of the peripheral nerve. Cesk Fysiol 61:9-14.
Kubo T, Hosokawa K, Haramoto U, Takagi S, Nakai K (2000) A simple technique for f brin glue application in skin grafting. Plast Reconstr Surg 105:1906-1907.
Liu F, Zhu J, Wei M, Bao Y, Hu B (2012) Preliminary evaluation of the sural nerve using 22-MHz ultrasound: a new approach for evaluation of diabetic cutaneous neuropathy. PLoS One 7:e32730.
Madduri S, Feldman K, Tervoort T, Papaloïzos M, Gander B (2010) Collagen nerve conduits releasing the neurotrophic factors GDNF and NGF. J Control Release 143:168-174.
Mandel RJ (2010) CERE-110, an adeno-associated virus-based gene delivery vector expressing human nerve growth factor for the treatment of Alzheimer’s disease. Curr Opin Mol Ther 12:240-247.
Martinez AM, Goulart CO, Ramalho Bdos S, Oliveira JT, Almeida FM (2014) Neurotrauma and mesenchymal stem cells treatment: From experimental studies to clinical trials. World J Stem Cells 6:179-194.
Matsuyama T, Mackay M, Midha R (2000) Peripheral nerve repair and grafting techniques: a review. Neurol Med Chir (Tokyo) 40:187-199.
Moges H, Wu X, McCoy J, Vasconcelos OM, Bryant H, Grunberg NE, Anders JJ (2011) Ef ect of 810 nm light on nerve regeneration after autograft repair of severely injured rat median nerve. Lasers Surg Med 43:901-906.
Olson L, Backlund EO, Ebendal T, Freedman R, Hamberger B, Hansson P, Hoffer B, Lindblom U, Meyerson B, Strömberg I, Olle Sydow O, Seiger A (1991) Intraputaminal infusion of nerve growth factor to support adrenal medullary autografts in Parkinson’s disease. Oneyear follow-up of f rst clinical trial. Arch Neurol 48:373-381.
Pettersson J, McGrath A, Kalbermatten DF, Novikova LN, Wiberg M, Kingham PJ, Novikov LN (2011) Muscle recovery after repair of short and long peripheral nerve gaps using f brin conduits. Neurosci Lett 500:41-46.
Scheidegger O, Küf er AF, Kamm CP, Rösler KM (2011) Reproducibility of sensory nerve conduction studies of the sural nerve using ultrasound-guided needle positioning. Muscle Nerve 44:873-876.
Schmitz HC, Beer GM (2001) The toe-spreading ref ex of the rabbit revisited--functional evaluation of complete peroneal nerve lesions. Lab Anim 35:340-345.
Shakhbazau A, Kawasoe J, Hoyng SA, Kumar R, van Minnen J, Verhaagen J, Midha R (2012) Early regenerative ef ects of NGF-transduced Schwann cells in peripheral nerve repair. Mol Cell Neurosci 50:103-112.
Shen H, Shen ZL, Zhang PH, Chen NL, Wang YC, Zhang ZF, Jin YQ (2010a) Ciliary neurotrophic factor-coated polylactic-polyglycolic acid chitosan nerve conduit promotes peripheral nerve regeneration in canine tibial nerve defect repair. J Biomed Mater Res B Appl Biomater 95:161-170.
Shen J, Zhou CP, Zhong XM, Guo RM, Griffith JF, Cheng LN, Duan XH, Liang BL (2010b) MR neurography: T1 and T2 measurements in acute peripheral nerve traction injury in rabbits. Radiology 254:729-738.
Siemionow M, Brzezicki G (2009) Current techniques and concepts in peripheral nerve repair. Int Rev Neurobiol 87:141-172.
Sunderland S (1951) A classif cation of peripheral nerve injuries producing loss of function. Brain 74:491-516.
Toros T, Karabay N, Ozaksar K, Sugun TS, Kayalar M, Bal E (2009) Evaluation of peripheral nerves of the upper limb with ultrasonography: a comparison of ultrasonographic examination and the intra-operative f ndings. J Bone Joint Surg Br 91:762-765.
Wahlberg LU, Lind G, Almqvist PM, Kusk P, Tornøe J, Juliusson B, Söderman M, Selldén E, Seiger Å, Eriksdotter-Jönhagen M, Linderoth B (2012) Targeted delivery of nerve growth factor via encapsulated cell biodelivery in Alzheimer disease: a technology platform for restorative neurosurgery. J Neurosurg 117:340-347.
Walshe P, Harkin C, Murphy S, Shah C, Curran A, McShane D (2001) The use of fibrin glue in refractory coagulopathic epistaxis. Clin Otolaryngol Allied Sci 26:284-285.
Wang H, Liu ZL, Zhuang XT, Wang MF, Xu L (2009) Neuroprotective ef ect of recombinant human erythropoietin on optic nerve injury in rats. Chin Med J (Engl) 122:2008-2012.
Wang Y, Tang P, Zhang L, Guo Y, Wan W (2010) Quantitative evaluation of the peripheral nerve blood perfusion with high frequency contrast-enhanced ultrasound. Acad Radiol 17:1492-1497.
Zochodne DW (2012) The challenges and beauty of peripheral nerve regrowth. J Peripher Nerv Syst 17:1-18.
Copyedited by Slone-Murphy J, Raye W, Li CH, Song LP, Zhao M
*Correspondence to: Jie Tang, M.D. or Yue-xiang Wang, M.D., txiner@vip.sina.com or wangyuexiang1999@sina.com.
orcid: 0000-0002-6882-4044 (Jie Tang) 0000-0003-4108-1161 (Yue-xiang Wang)
10.4103/1673-5374.170315 http://www.nrronline.org/
Accepted: 2015-07-20
杂志排行
中国神经再生研究(英文版)的其它文章
- Intracellular sorting pathways of the amyloid precursor protein provide novel neuroprotective strategies
- The role of the Rho/ROCK signaling pathway in inhibiting axonal regeneration in the central nervous system
- VEGF in the nervous system: an important target for research in neurodevelopmental and regenerative medicine
- Studying neurological disorders using induced pluripotent stem cells and optogenetics
- Ef cacy of glucagon-like peptide-1 mimetics for neural regeneration
- Compliant semiconductor scaf olds: building blocks for advanced neural interfaces
