Ef cacy of glucagon-like peptide-1 mimetics for neural regeneration
2015-02-07
Ef cacy of glucagon-like peptide-1 mimetics for neural regeneration
Glucagon-like peptide 1 (GLP-1) is secreted from enteroendocrine L cells in response to nutrient ingestion and exhibits insulinotropic properties by stimulating specif c G protein-linked receptors (GLP-1Rs) on pancreatic β cells. Several GLP-1 mimetics, such as exenatide (exendin-4 (Ex-4)), liraglutide, and lixisenatide, have been developed and approved as treatments for patients with type 2 diabetes. These peptides show bioactivities almost identical to those of GLP-1 and have a substantially longer plasma half-life than GLP-1 because of their resistance to dipeptidyl peptidase-4, a GLP-1 degrading enzyme. GLP-1Rs are found in not only the pancreas but also the extrapancreatic tissues, including the nervous tissues (Harkavyi and Whitton, 2010). It is important to note that GLP-1 mimetics can cross the blood brain barrier and directly act on neurons in the central nervous system. In addition to the inhibition of appetite, the neuroprotective properties of GLP-1 have been receiving increasing attention. Recent studies have suggested that GLP-1 mimetics confer benef cial ef ects in neurodegenerative disorders, such as Parkinson’s disease (PD), Alzheimer’s disease, amyotrophic lateral sclerosis, ischemia and stroke, and multiple sclerosis (Holscher, 2014). In particular, the neuroprotective properties of Ex-4 have been demonstrated in animal and cell culture models of PD. A single-blinded clinical trial with 45 PD patients revealed that the treatment with Ex-4 signif cantly improved the cognition and memory of patients (Aviles-Olmos et al., 2013). The benef cial ef ects of GLP-1 mimetics on the peripheral nervous system (PNS) have also been reported. Both GLP-1 and Ex-4 delivered via osmotic minipumps prevented axonal degeneration in a rat model of pyridoxine-induced neuropathy (Perry et al., 2007). Treatment of streptozotocin (STZ)-induced diabetic mice with Ex-4 for 4 weeks restored motor and sensory nerve conduction velocities and hypoalgesia without normalizing blood glucose levels (Himeno et al., 2011). In addition, repeated intraperitoneal injections of Ex-4 signif -cantly promoted axonal regeneration and functional recovery following sciatic nerve crush injury in normal adult rats (Yamamoto et al., 2013). These f ndings are in agreement with in vitro studies that revealed that GLP-1 and Ex-4 promoted neurite outgrowth of rat pheochromocytoma-derived PC12 cells (Perry et al., 2002) and adult mouse dorsal root ganglion (DRG) neurons (Himeno et al., 2011). Together these results provide further evidence of the direct actions of Ex-4 on the PNS; however, the underlying mechanisms remain unclear. Our recent study (Tsukamoto et al., 2015) aimed to elucidate the precise localization of GLP-1R in adult rat DRG in vivo and in vitro as well as to determine the neurotrophic and neuroprotective properties of Ex-4 in adult rat DRG neurons.
Double immunof uorescence histochemistry was performed using anti-GLP-1R antibody and specif c neuron markers (anti-200 kDa neurof lament (NF200) and anti-calcitonin gene-related peptide (CGRP) antibodies and Griffonia simplicifolia isolectin B4 (IB4)). Staining results revealed that GLP-1R was predominantly found in NF200-immunoreactive large neurons and CGRP-immunoreactive small peptidergic neurons rather than IB4-binding small non-peptidergic neurons. It is generally accepted that large sensory neurons transmit proprioception and vibration and that small peptidergic and non-peptidergic neurons transmit nociception and thermoreception; whether these two groups of small neurons have distinct functions has been the subject of controversy (Takaku et al., 2013). This distribution pattern of GLP-1R agrees with previous f ndings that GLP-1 and Ex-4 restores pyridoxine-induced large f ber neuropathy (Perry et al., 2007) and diabetes-induced large and small f ber dysfunction (Himeno et al., 2011; Jolivalt et al., 2011).
We maintained adult rat DRG neurons in serum-free culture conditions with dif erent concentrations of Ex-4 (0, 1, 10, and 100 nM) in the presence or absence of insulin, and evaluated neurite outgrowth at 2 days and neuronal survival at 7 days in culture, respectively. For the neurite outgrowth assay, DRG neurons were immunostained with the anti-βIII tubulin antibody and the number of neurite-bearing cells was expressed as a relative value wherein the total number of neurons per well was assumed to be 100. The length of the neurites (in μm) was measured from digital images of the stained neurites, and expressed as the average value calculated from the measurements of about 60 neurites in each experimental group. For the survival assay, dead neurons were detected by positive trypan blue staining. The number of viable neurons at 7 days was expressed as a relative value when the original number measured 16 hours after seeding was assumed to be 100. Ex-4 promoted neurite outgrowth and survival of DRG neurons in a dose-dependent manner (1 nM < 10 nM < 100 nM), and effects of Ex-4 were more noticeable in the absence of insulin than in its presence. Treatment with 100 nM Ex-4 almost completely restored the reduced neurite outgrowth and viability of DRG neurons caused by insulin removal from the medium. Insulin is recognized as a neuroprotective molecule, and the predominant distribution of insulin receptors in small peptidergic and non-peptidergic DRG neurons has been documented (Baiou et al., 2007). Insulin and GLP-1 have been suggested to activate common signaling pathways, such as the phosphatidylinositol 3´-phosphate kinase (PI3K) and Ras/Raf/mitogen-activated protein kinase (MAPK) pathways (Holscher, 2014). Thus, the f ndings of our study suggest that GLP-1 mimetics confer a neuroprotective function, at least partly, by compensating for the absence of insulin receptor activation. Considering the dif erences in the distribution patterns between GLP-1R and insulin receptor described above, it seems plausible that Ex-4 and insulin exhibit synergistic ef ects on the small peptidergic neurons but complementary ef ects on the other subtypes of neurons. To further conf rm this theory,we are currently investigating the colocalization of GLP-1R with insulin receptor in DRG and the possible association between insulin and GLP-1 signaling pathways in DRG and other neurons (Figure 1).
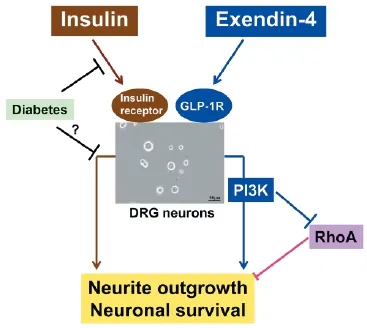
Figure 1 Possible signaling pathways mediating the ef ects of exendin-4 (Ex-4) on cultured dorsal root ganglion (DRG) neurons.
GLP-1 mimetics have been shown to activate several signaling pathways in the nervous system, including cyclic AMP (cAMP)/ protein kinase A/nuclear transcription activator CREB, PI3K/ Akt, and MAPK/ERK (Harkavyi and Whitton, 2010; Holscher, 2014). The upregulation of cAMP (Liu et al., 2011) and activation of ERK signaling (Jolivalt et al., 2011) were suggested to play a role in the restoring ef ects of GLP-1 mimetics against peripheral nerve dysfunction in STZ-diabetic rats. In our study, the promoting ef ects of Ex-4 on the neurite outgrowth and survival of DRG neurons were abolished by PI3K inhibitor LY294002. Furthermore, pretreatment with LY294002 in PC12 cells canceled Ex-4-induced inactivation of RhoA, an inhibitory regulator for peripheral nerve regeneration.These f ndings suggest that Ex-4 exhibits neurotrophic and neuroprotective activities through the activation of PI3K signaling pathway, which negatively regulates RhoA activity (Figure 1). The implication of RhoA was conf rmed in PC12 cells but not in DRG neurons because insuf cient amount of protein was obtained from the latter to measure RhoA activity. Therefore, we cannot def nitely state that this hypothesis holds true in DRG neurons. However, on the basis of the evidence of the inverse relation between the RhoA activity/expression and the neurite outgrowth activity and viability of DRG neurons, the inhibition of RhoA activity appears to be one of the mechanisms accounting for the neuroprotective ef ects of GLP-1 mimetics. In agreement with our study, Wang et al. (2013) reported the involvement of RhoA inhibition in the cytoprotective actions of GLP-1 in cultured cardiac microvascular endothelial cells under diabetic conditions. The possible association between PI3K and the other signaling pathways described above and the target genes upregulated through PI3K-RhoA signaling remain to be determined.
In summary, the neurotrophic and neuroprotective properties of Ex-4 illustrated in our study (Tsukamoto et al., 2015) imply their ef cacy for the acceleration of axonal regeneration and functional repair following peripheral nerve injury. Impaired axonal regeneration and remyelination are the characteristic features in the pathobiology of diabetic neuropathy; therefore, therapeutic approaches of Ex-4 and other GLP-1 mimetics against diabetic neuropathy can be expected. Classical neurotrophic factors, such as nerve growth factor (NGF) and glial cell line-derived neurotrophic factor (GDNF), exhibited more potent bioactivities on the neurite outgrowth of DRG neurons as compared with Ex-4 (Tsukamoto et al., personal data), but clinical trials with NGF resulted in painful side ef ects and failed to conf rm its ef cacy for diabetic neuropathy. Although Ex-4 has milder neurotrophic ef ects than NGF, Ex-4 and other GLP-1 mimetics are now available to patients with type 2 diabetes and may be benef cial for both stable glycemic control and the restoration of peripheral nerve function in patients with diabetic neuropathy. Moreover, the direct actions of GLP-1 mimetics on PNS suggest their therapeutic utility against the neuropathy caused by type 1 diabetes. In addition to its ef cacy for neurite outgrowth and neuronal survival, we are currently investigating whether Ex-4 promotes myelin formation in cocultured DRG neurons and immortalized adult rat Schwann cells. The growing evidence that GLP-1 mimetics accelerate peripheral nerve regeneration and remyelination in preclinical studies will encourage us to consider the clinical trials with the drugs for diabetic and other peripheral neuropathies as well as traumatic nerve injury. Because the safety of GLP-1 mimetics in long-term use for patients with type 2 diabetes has been proved and their clinical trials for PD and Alzheimer’s disease are in progress, we believe that their clinical applications for the PNS disorders described above will launch in the near future.
This study was supported by a Grant-in-aid for Scientif c Research from the Ministry of Education, Science, Sports and Culture of Japan (grant number: 25430056) and the fund from Nukada Institute for Medical and Biological Research, Chiba, Japan.
Kazunori Sango*, Kazunori Utsunomiya
Diabetic Neuropathy Project (Former ALS/Neuropathy project), Department of Sensory and Motor Systems, Tokyo Metropolitan Institute of Medical Science, Setagaya-ku, Tokyo, Japan (Sango K) Division of Diabetes, Metabolism & Endocrinology, Department of Internal Medicine, Jikei University School of Medicine, Minato-ku, Tokyo, Japan (Utsunomiya K)
*Correspondence to: Kazunori Sango, M.D., Ph.D., sango-kz@igakuken.or.jp.
Accepted: 2015-08-15
orcid: 0000-0002-9750-9596 (Kazunori Sango)
Aviles-Olmos I, Dickson J, Kefalopoulou Z, Djamshidian A, Ell P, Soderlund T, Whitton P, Wyse R, Isaacs T, Lees A, Limousin P, Foltynie T (2013) Exenatide and the treatment of patients with Parkinson’s disease. J Clin Invest 123:2730-2736.
Baiou D, Santha P, Avelino A, Charrua A, Bacskai T, Matesz K, Cruz F, Nagy I (2007) Neurochemical characterization of insulin receptor-expressing primary sensory neurons in wild-type and vanilloid type 1 transient receptor potential receptor knockout mice. J Comp Neurol 503:334-347.
Harkavyi A, Whitton PS (2010) Glucagon-like peptide 1 receptor stimulation as a means of neuroprotection. Br J Pharmacol 159:495-501.
Himeno T, Kamiya H, Naruse K, Harada N, Ozaki N, Seino Y, Shibata T, Kondo M, Kato J, Okawa T, Fukami A, Hamada Y, Inagaki N, Seino Y, Drucker DJ, Oiso Y, Nakamura J (2011) Benef cial ef ects of exendin-4 on experimental polyneuropathy in diabetic mice. Diabetes 60:2397-2406.
Holscher C (2014) Insulin, incretins and other growth factors as potential novel treatments for Alzheimer’s and Parkinson’s diseases. Biochem Soc Trans 42:593-599.
Jolivalt CG, Fineman M, Deacon CF, Carr RD, Calcutt NA (2011) GLP-1 signals via ERK in peripheral nerve and prevents nerve dysfunction in diabetic mice. Diabetes Obes Metab 13:990-1000.
Perry T, Lahiri DK, Chen D, Zhou J, Shaw KT, Egan JM, Greig NH (2002) A novel neurotrophic property of glucagon-like peptide 1: a promoter of nerve growth factor-mediated dif erentiation in PC12 cells. J Pharmacol Exp Ther 300:958-966.
Perry T, Holloway HW, Weerasuriya A, Mouton PR, Duffy K, Mattison JA, Greig NH (2007) Evidence of GLP-1-mediated neuroprotection in an animal model of pyridoxine-induced peripheral sensory neuropathy. Exp Neurol 203:293-301.
Takaku S, Yanagisawa H, Watabe K, Horie H, Kadoya T, Sakumi K, Nakabeppu Y, Poirier F, Sango K (2013) GDNF promotes neurite outgrowth and upregulates galectin-1 through the RET/PI3K signaling in cultured adult rat dorsal root ganglion neurons. Neurochem Int 62:330-339.
Tsukamoto M, Niimi N, Sango K, Takaku S, Kanazawa Y, Utsunomiya K (2015) Neurotrophic and neuroprotective properties of exendin-4 in adult rat dorsal root ganglion neurons: involvement of insulin and RhoA. Histochem Cell Biol 144:249-259.
Wang D, Luo P, Wang Y, Li W, Wang C, Sun D, Zhang R, Su T, Ma X, Zeng C, Wang H, Ren J, Cao F (2013) Glucagon-like peptide-1 protects against cardiac microvascular injury in diabetes via a cAMP/PKA/Rho-dependent mechanism. Diabetes 62:1697-1708.
Yamamoto K, Amako M, Yamamoto Y, Tsuchihara T, Nukada H, Yoshihara Y, Arino H, Fujita M, Uenoyama M, Tachibana S, Nemoto K (2013) Therapeutic ef ect of exendin-4, a long-acting analogue of glucagon-like peptide-1 receptor agonist, on nerve regeneration after the crush nerve injury. Biomed Res Int 2013:315848.
Yamashita T, Higuchi H, Tohyama M (2002) The p75 receptor transduces the signal from myelin-associated glycoprotein to Rho. J Cell Biol 157:565-570.
10.4103/1673-5374.169611 http://www.nrronline.org/
Sango K, Utsunomiya K (2015) Ef cacy of glucagon-like peptide-1 mimetics for neural regeneration. Neural Regen Res 10(11):1723-1724.
杂志排行
中国神经再生研究(英文版)的其它文章
- Brain protein oxidation: what does it refl ect?
- The role of the Rho/ROCK signaling pathway in inhibiting axonal regeneration in the central nervous system
- Intracellular sorting pathways of the amyloid precursor protein provide novel neuroprotective strategies
- VEGF in the nervous system: an important target for research in neurodevelopmental and regenerative medicine
- Compliant semiconductor scaf olds: building blocks for advanced neural interfaces
- Use of sensory substitution devices as a model system for investigating cross-modal neuroplasticity in humans
