VEGF in the nervous system: an important target for research in neurodevelopmental and regenerative medicine
2015-02-07MatthiasDumpich,CarstenTheiss
VEGF in the nervous system: an important target for research in neurodevelopmental and regenerative medicine
Vascular endothelial growth factor (VEGF) in neurodevelopment and regeneration: VEGF is a well-known factor that promotes vascularization and angiogenesis. Besides this it participates in the pathogenesis of several diseases, such as colorectal carcinoma, lung cancer or diabetic retinopathy. Within the last decade, VEGF has been successfully integrated into the treatment of such diseases, for example as a therapy for colorectal cancer with the VEGF-receptor (VEGFR)-inhibitor axitinib. VEGF effects in those diseases are primarily exerted via neovascularization and angiogenesis, which are mainly initiated by hypoxia to support tumor growth. VEGF is expressed by a high number of diff erent cells, amongst others its expression was confi rmed in diff erent tumor cell-lines as well as in common, physiological cells. A well known initiator of VEGF (over-) expression is the hypoxia inducible factor (HIF), which is a transcriptional factor, leading to an enhanced VEGF expression.
In recent years, VEGF has become the focus of investigations with regards to eff ects within the neuronal system. At this juncture, data about the role of VEGF within the nervous system is rapidly accumulating, clearly showing that VEGF belongs to the long list of classical neuro-stimulative factors such as nerve growth factor (NGF), Slit/Robo or netrin-1. Some eff ects of VEGF in neurodevelopment and in diff erent neurological diseases, such as glioblastoma or stroke have been shown, so that a deeper focus on the role of VEGF in neuronal structures seems to be a good foundation for new approaches to treat neurological diseases. Findings in diverse neuronal structures of both the central, and peripheral nervous systems underline the importance of VEGF in neurogenesis, and in the development of proper working neuronal networks (revised by Dumpich et al., 2015). An example for the multiplicity of cells giving response to VEGF stimulation are astrocytes. VEGF supports an increase in cell proliferation, gap junctional intercellular communication and cell motility (Wuestefeld et al., 2012). Besides this, there are currently many examples of neurons and glial cells of the peripheral and central nervous systems, such as dorsal root ganglia cells, hippocampal neurons, oligodendrocytes, schwann cells, granule cells or Purkinje cells which are positively aff ected by VEGF (Dumpich et al., 2015). For instance, it was shown in vivo that VEGF is indispensable for the development of the optic chiasm. Secreted by the fl oor plate, VEGF helps the axon to accomplish the complex process of commissural crossing to reach their fi nal destination (Figure 1). VEGF120/120mice, which are unable to express the most abundant VEGF-A-isoforms: VEGF-164 and VEGF-188, displayed a range of growth-cone pathfi nding errors with defasciculated ipsilateral and contralateral optic tracts. The axons were organized into two discrete bundles with an increase of axons that grew in an ipsilateral direction (Erskine et al., 2011). It can be concluded that the expression of VEGF is indispensable for the development of proper working neuronal networks and that VEGF plays a big role in important milestones of neurodevelopment.
In a recent study, the positive eff ect of VEGF-B in neuroregeneration was shown. Studies revealed in vivo, that VEGF-B is necessary for nerve regeneration. Neuroregeneration of VEGF-B deficient mice was decreased compared to wild-type mice. The cornea of the diff erent mice models were injured and observed in regard of the rate of nerve regeneration into the injured area after one week. Wild-type mice did show a greater number of growing nerve endings into the injured zone than VEGF-B defi cient mice. VEGF which was applied exogenously into adjacent subconjuntival space induced stronger nerve regeneration in both mice. The regeneration-range of VEGF-B defi cient mice was confi ned to peripheral areas, while wild-type mice did show a larger area of nerve regeneration (Guaiquil et al., 2014).
VEGF also plays an important role in several brain diseases. In brain tumors for example, it was shown that hypoxia induced up-regulation of VEGF enhanced tumor growth (Neurath et al., 2006). These eff ects are mainly mediated by angiogenesis within the tumor tissue, but there are also hints that VEGF has a direct impact on different tumor cells, for example by enhancement of gap junctional cell communication within neoplastic neuronal cells (Zhang et al., 2003). This example of VEGF’s effects in diseases makes it clear why the role of VEGF in the nervous system is of high importance. There are also several other diseases associated with VEGF, such as Alzheimer’s disease or cerebral stroke. A better understanding of the role of VEGF within those diseases is important to establish new therapeutical strategies, such as the treatment of glioblastoma with bevacizumab.
The role of VEGF in the axonal growth cone: A structure of upmost importance for axonal growth and neurodevelopment is the growth cone, which is a highly motile structure at the tip of growing axons that lead growing axons to their fi nal destination to form synapses. It was shown that growth cones of chicken dorsal root ganglion (DRG) neurons rapidly respond to VEGF stimulation and that VEGF acts as an attractant for growth cones, leading to directed growth. Furthermore, it was shown that VEGF-stimulation resulted in growth cones with larger circumferences and areas compared to control growth cones (Figure 1). The measured sizes were comparable to results of NGF-stimulated growth cones. The combination of VEGF and NGF even potentiated these eff ects, leading to very large growth cones (Olbrich et al., 2013). As the growing velocity of growth cones is related to the size of the growth cone, bigger growth cones grow faster, smaller growth cones grow slower (Argiro et al., 1984). Hence it was shown that VEGF attracts growth cones and enhances the speed of growth. These effects are mediated via different VEGF receptors. For example, NRP1 is the corresponding receptor that is indispensable for the development of the optic chiasm, where it directs growth and axon crossing (Erskine et al., 2011). Other studies have revealed that VEGFR2 is responsible for directed growth cone guidance in chicken DRG growth cones (Foehring et al., 2012; Olbrich et al., 2013). Just recently, VEGFR3 has been discussed to be important during brain development, as this receptor is highly expressed during early developmental stages in rat neurons of the forebrain, however the receptor’s expression then decreases throughout development (Ward et al., 2015). Because of the high diversity of expressed receptors in diff erent neuronal tissues, cooperating receptors and even alternations in the expression levels of these diff erent receptors at diff erent stages of development is important to understand the cellular mechanisms of VEGF stimulation downstream of the receptors.
Neurological diseases and VEGF: VEGF is suspected to play a role in diff erent neurological diseases such as Alzheimer’s disease, amyotrophic lateral sclerosis or multiple sclerosis. It also participates in the development of brain tumors such as glioblastoma, by supporting tumor growth. Initial studies have shown that the inhibition of VEGF has positive eff ects against glioblastoma cells, but successful integration into the clinical treatment procedures of those tumors has not yet been possible with such positive eff ects as suspected. In clinical studies with Bevacizumab treatment, to block VEGF in glioblastoma tissue, patients with glioblastoma did not show any increase in the overall survival, compared to patients who received a placebo. The progression-free interval of glioblastoma was increased after VEGF treatment (Chinot et al., 2014). In recent studies, VEGF was also shown to participate highly in stroke. In ischemic hippocampal neurons, VEGF was able to attenuate the increase of outwardly delayed potassium currents, which support neuronal survival. Besides stroke, those currents also play a role in Alzheimer’s disease and seizures (Wu et al., 2015). This underlines the role of VEGF in neuroprotection under unfavorable conditions, such as hypoxia. Therefore, VEGF might be an option for the treatment of neurodegenerative diseases.
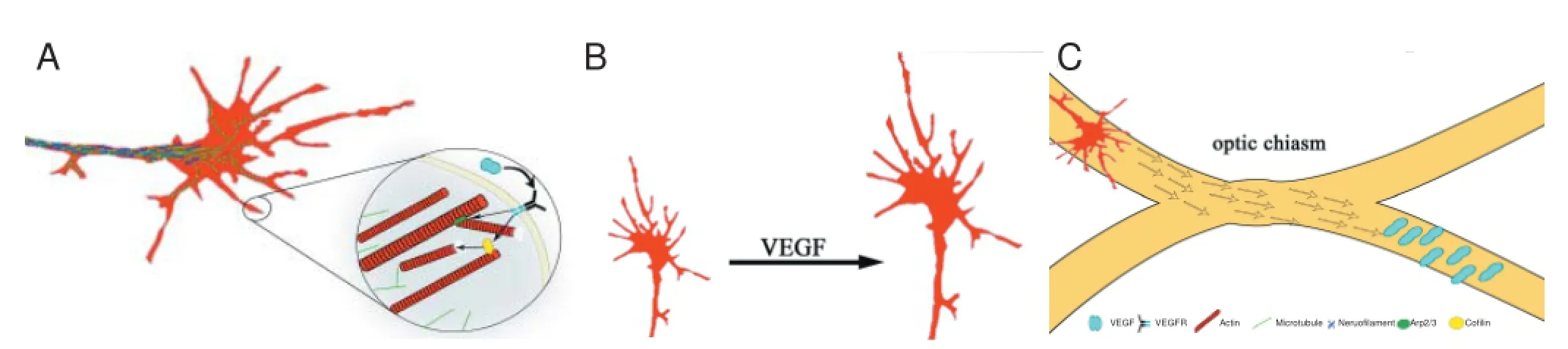
Figure 1 VEGF eff ects on the axonal grouth cone.
VEGF-effects in actin-signaling: Time-lapse imaging of growth cones revealed that VEGF stimulation provokes rapid morphological changes of growth cones from chicken DRG neurons. Stimulated cells were microinjected with plasmids encoding for RFP-actin and GFP-NF-M (neurofi lament). It was observed that growth cones, which were stimulated with VEGF, showed high fi lopodia and lamellipodia turnover rates and directional growth. Unstimulated control cells just scanned their environment and did not show signifi cant growth, which underlines the strong stimulating eff ect of VEGF (Olbrich et al., 2013). Furthermore, it was observed that actin motility was higher than the motility of neurofi laments, and that growth was mainly driven by alterations in the actin distribution (Figure 1). Immunohistochemistry supported those observations even more. Growth cones stained with antibodies against neurofi lament, microtubules and phalloidin-rhodamine displayed an expression pattern typical of these cytoskeletal proteins. Microtubules and neurofi laments were mainly found in the central regions of growth cones, whereas actin was highly expressed in the peripheral region. VEGF-stimulation mainly affected the peripheral region, resulting in high actin-turnover (Olbrich et al., 2013). As such, actin seems to be of great importance for cytoskeletal reorganization downstream of VEGF-stimulation. Put another way, this means that VEGF aff ects signaling cascades that result in actin-reorganization. According to those observations, further investigations must be undertaken to fi gure out how VEGF infl uences the actin-cytoskeleton. There are well known models of actin signaling which involve Cdc42-dependent pathways, leading to actin reorganization. Those pathways have been shown in non-neuronal tissue downstream of VEGF, and in neuronal tissue downstream of other guidance cues, such as NGF, netrin-1 or brain morphogenetic protein 7 (BMP7). There are many hints that especially the actin-supporting proteins, cofi lin and the Arp2/3-complex, are highly involved in VEGF dependent actin signaling pathways. Because of their properties, they seem to be at least partly responsible for the rapid morphological changes of growth cones after stimulation. Cofi lin for example is regulated by diff erent upstream signaling proteins, which are either able to activate or inactivate cofi lin. These activity states are mediated via diff erent proteins. It is not quite clear how the protein activation is organized. Additionally, spatiotemporal activation within neuronal structures plays an important role for directed growth. The regulation of the Arp2/3-complex is also of comparable complexity. Interestingly, the diff erent proteins even interact with each other, which make actin-signaling a very versatile process in many ways (revised by Dumpich et al., 2015). These various observations open up questions concerning actin signaling, which is why it is of the highest importance to fi gure out how VEGF regulates actin-reorganization in different neuronal tissues. Furthermore, it might be of interest to fi nd out how it can be integrated into other modes of regulation, and how it interacts with other growth-stimulating factors. The high number of actin-supporting proteins, the way they cooperate, their up-stream signals and their diff erent modes of regulation, together enable a high number of potential targets that could interfere with actin-signaling. Additionally, the diversity of the responses of those proteins towards diff erent growth factors, such as NGF, VEGF, Sema-3a or BMP7 must be investigated more thoroughly. This could allow us to fi gure out whether the suppression or over expression of certain factors supports or suppresses diff erent eff ects on cells.
Conclusion: VEGF is highly involved in axonal growth, neurodevelopment and in the pathogenesis of diff erent neurological disorders. It enhances neuroprotection under unfavorable conditions and supports the growth of cerebral tumor tissue. The eff ects of VEGF in axon guidance are primarily mediated via reorganization of the actin cytoskeleton, but the exact downstream signaling of VEGF signaling is not clear yet. All of those aspects lead us to suspect that VEGF will play a big role in upcoming neurological investigations and clinical treatments. It is conceivable that stimulation of axonal growth via VEGF will be used to support rehabilitation or regenerative processes after spinal cord injuries or axonal damage. Furthermore, the therapies of cerebral tumors by inhibition of VEGF, or the support of neuroprotective mechanisms after ischemic insults by up-regulation of VEGF, are therapeutical options that might be of interest to future investigations.
Matthias Dumpich would like to thank the Heinrich and Alma Vogelsang Foundation for fi nancial support in the form of a graduation scholarship. We would also like to acknowledge D. Terheyden-Keighley for the critical reading of this paper.
Matthias Dumpich, Carsten Theiss*
Faculty of Medicine, Institute of Anatomy, Department of Cytology Ruhr-University Bochum, Bochum, Germany
*Correspondence to: Carsten Theiss, Ph.D., carsten.theiss@rub.de.
Accepted: 2015-10-08
Argiro V, Bunge MB, Johnson MI (1984) Correlation between growth form and movement and their dependence on neuronal age. J Neurosci 4:3051-3062.
Chinot OL, Wick W, Mason W, Henriksson R, Saran F, Nishikawa R, Carpentier AF, Hoang-Xuan K, Kavan P, Cernea D, Brandes AA, Hilton M, Abrey L, Cloughesy T (2014) Bevacizumab plus radiotherapy-temozolomide for newly diagnosed glioblastoma. N Engl J Med 370:709-722.
Dumpich M, Mannherz HG, Theiss C (2015) VEGF signaling regulates cofi lin and the Arp2/3-complex within the axonal growth cone. Curr Neurovasc Res 12:293-307.
Erskine L, Reijntjes S, Pratt T, Denti L, Schwarz Q, Vieira JM, Alakakone B, Shewan D, Ruhrberg C (2011) VEGF signaling through neuropilin 1 guides commissural axon crossing at the optic chiasm. Neuron 70:951-965.
Foehring D, Brand-Saberi B, Theiss C (2012) VEGF-induced growth cone enhancement is diminished by inhibiting tyrosine-residue 1214 of VEGFR-2. Cells Tissues Organs 196:195-205.
Guaiquil VH, Pan Z, Karagianni N, Fukuoka S, Alegre G, Rosenblatt MI (2014) VEGF-B selectively regenerates injured peripheral neurons and restores sensory and trophic functions. Proc Natl Acad Sci U S A 111:17272-17277.
Neurath KM, Keough MP, Mikkelsen T, Claffey KP (2006) AMP-dependent protein kinase alpha 2 isoform promotes hypoxia-induced VEGF expression in human glioblastoma. Glia 53:733-743.
Olbrich L, Foehring D, Happel P, Brand-Saberi B, Theiss C (2013) Fast rearrangement of the neuronal growth cone’s actin cytoskeleton following VEGF stimulation. Histochem Cell Biol 139:431-445.
Ward MC, Cunningham AM (2015) Developmental expression of vascular endothelial growth factor receptor 3 and vascular endothelial growth factor C in forebrain. Neuroscience 303:544-557.
Wu KW, Yang P, Li SS, Liu CW, Sun FY (2015) VEGF attenuated increase of outward delayed-rectifi er potassium currents in hippocampal neurons induced by focal ischemia via PI3-K pathway. Neuroscience 298:94-101.
Wuestefeld R, Chen J, Meller K, Brand-Saberi B, Theiss C (2012) Impact of vegf on astrocytes: analysis of gap junctional intercellular communication, proliferation, and motility. Glia 60:936-947.
Zhang W, DeMattia JA, Song H, Couldwell WT (2003) Communication between malignant glioma cells and vascular endothelial cells through gap junctions. J Neurosurg 98:846-853.
10.4103/1673-5374.170287 http://www.nrronline.org/
Dumpich M, Theiss C (2015) VEGF in the nervous system: an important target for research in neurodevelopmental and regenerative medicine. Neural Regen Res 10(11):1725-1726.
杂志排行
中国神经再生研究(英文版)的其它文章
- Brain protein oxidation: what does it refl ect?
- The role of the Rho/ROCK signaling pathway in inhibiting axonal regeneration in the central nervous system
- Intracellular sorting pathways of the amyloid precursor protein provide novel neuroprotective strategies
- Studying neurological disorders using induced pluripotent stem cells and optogenetics
- Ef cacy of glucagon-like peptide-1 mimetics for neural regeneration
- Compliant semiconductor scaf olds: building blocks for advanced neural interfaces
