The role of the Rho/ROCK signaling pathway in inhibiting axonal regeneration in the central nervous system
2015-02-07JingLiuHongyanGaoXiaofengWang
Jing Liu, Hong-yan Gao Xiao-feng Wang
1 Department of Neonatology & NICU of Bayi Children’s Hospital, General Hospital of Beijing Military Command of Chinese PLA, Beijing, China
2 Department of Neonatology, People’s Hospital of Rizhao, Rizhao, Shangdong Province, China
The role of the Rho/ROCK signaling pathway in inhibiting axonal regeneration in the central nervous system
Jing Liu1,*, Hong-yan Gao1, Xiao-feng Wang1,2
1 Department of Neonatology & NICU of Bayi Children’s Hospital, General Hospital of Beijing Military Command of Chinese PLA, Beijing, China
2 Department of Neonatology, People’s Hospital of Rizhao, Rizhao, Shangdong Province, China
The Rho/Rho-associated coiled-coil containing protein kinase (Rho/ROCK) pathway is a major signaling pathway in the central nervous system, transducing inhibitory signals to block regeneration. After central nervous system damage, the main cause of impaired regeneration is the presence of factors that strongly inhibit regeneration in the surrounding microenvironment. These factors signal through the Rho/ROCK signaling pathway to inhibit regeneration. Therefore, a thorough understanding of the Rho/ROCK signaling pathway is crucial for advancing studies on regeneration and repair of the injured central nervous system.
nerve regeneration; Rho/Rho-associated coiled-coil containing protein kinase; signaling pathway; axonal regeneration; central nervous system; microenvironment; reviews; NSFC grant; neural regeneration
Funding: This research was supported by a grant from the National Natural Science Foundation of China, No. 81471087, 81170577.
Liu J, Gao HY, Wang XF (2015) The role of the Rho/ROCK signaling pathway in inhibiting axonal regeneration in the central nervous system. Neural Regen Res 10(11):1892-1896.
Introduction
Neurite growth, neurogenesis and inhibition are in a relatively balanced state during the development of the central nervous system (Gu et al., 2013). This balance is perturbed after central nervous system damage, resulting in degeneration (Gu et al., 2013). A lack of growth stimulating factors and the presence of a variety of inhibitory molecules in microenvironment block axonal regeneration in the central nervous system and weaken the capacity for neuronal regeneration after injury (Cao et al., 2010). The Rho/Rho-associated coiled-coil containing protein kinase (Rho/ROCK) signaling pathway is an important signal transduction system within the central nervous system, and is critically involved in cell growth, diff erentiation, migration and development (Chen et al., 2013). A large number of inhibitory factors signal via axonal surface receptors, thereby activating the Rho/ ROCK signaling pathway, which in turn impacts actin cytoskeletal dynamics, leading to collapse and retraction of the growth cone. This aff ects axon projections, guidance, extension and nerve regeneration (Menendez-Castro et al., 2011; Mishra et al., 2011; Adelson et al., 2012; Liu, 2012; Teramura et al., 2012; Frisca et al., 2013). Thus, researchers have targeted the Rho/ROCK signaling pathway in attempts to promote neural regeneration (Fujimura et al., 2011; Tan et al., 2011). In the present study, we review the basic characteristics and regulation of the Rho/ROCK signaling pathway, and we discuss strategies that have targeted this pathway to promote regeneration.
The Rho/ROCK signaling pathway
The Rho/ROCK signaling pathway consists of (1) suppressor proteins (growth inhibitors): myelin-associated glycoprotein, neuronal growth inhibitory factor (Nogo-A, Nogo-B and Nogo-C) and oligodendrocyte myelin glycoprotein; (2) receptors for these inhibitory proteins: Nogo receptor, paired immunoglobulin-like receptor B (PIR-B) and p75 neurotrophin receptor; (3) Rho; (4) ROCK; (5) ROCK eff ector molecule (Fujimura et al., 2011; Tan et al., 2011; Liu, 2012; Frisca et al., 2013).
Biological characteristics of the Rho/ROCK signaling pathway
Rho
Rho is a member of the Rho subfamily of GTPases, with a relatively small molecular mass, and belongs to the Ras superfamily (Cui et al., 2013). Molecular weight is 20–30 kDa. Rho proteins include RhoA, B, C, D and E. RhoA, B and C are highly homologous at the amino acid level (Fujimura et al., 2011; Tan et al., 2011; Liu, 2012; Frisca et al., 2013). RhoA expression is higher than that of the other subtypes in neurons (Fujimura et al., 2011; Tan et al., 2011; Liu, 2012; Frisca et al., 2013). Rho in the cytoplasm is mainly in two
distinct states: the active GTP-bound form and the inactive GDP-bound form. Rho proteins are regulated by a variety of factors. Guanine nucleotide exchange factor, a Rho activator, induces Rho to release GDP and bind GTP. GTPase activating protein and nucleotide dissociation inhibitor serve as inactivating agents for Rho. GTPase activating protein can activate the GTPase activity of Rho itself and promote GTP hydrolysis into GDP. Nucleotide dissociation inhibitor prevents nucleotide exchange, maintaining Rho in the inactive state. Under the regulation of these factors, Rho functions as a “molecular switch” during signal transduction (Fujimura et al., 2011; Tan et al., 2011; Liu, 2012; Frisca et al., 2013).
ROCK
ROCK belongs to the serine/threonine protein kinase family. ROCK is the most important downstream target eff ector molecule of Rho, presents as the highly homologous isomers ROCK1 and ROCK2. ROCK2 is mainly expressed in the central nervous system, including hippocampal pyramidal neurons, cerebral cortex and cerebellar Purkinje cells. ROCK1 is mainly expressed in non-neural tissues, such as lung, kidney and skeletal muscle. The ROCK polypeptide includes an N-terminal kinase domain, an α-helical coiledcoil domain containing a Rho binding site, and a cysteine-rich domain at the C-terminal. Activated Rho binds to the α-helical coiled-coil domain of ROCK. This removes the autoinhibition of ROCK, thereby activating the protein. Activated ROCK then activates its substrate (Schmandke et al., 2007; Fujimura et al., 2011; Tan et al., 2011; Liu, 2012; Frisca et al., 2013).
Upstream regulation of the Rho/ROCK signaling pathway The Rho/ROCK signaling pathway can be activated by various activated membrane receptors, such as G protein-coupled receptors, tyrosine kinase receptors and intracellular receptors. There are at least three major myelin-associated inhibitory factors in the central nervous system, myelin-associated glycoprotein, oligodendrocyte myelin glycoprotein and Nogo (Tan et al., 2011; Wälchli et al., 2013). These inhibitory factors signal by binding to membrane receptors such as the Nogo receptor/Lingo-1/p75 receptor complex, thereby activating intracellular GTPases, including the Rho/ ROCK cascade. This leads to changes in actin cytoskeletal dynamics, resulting in growth cone collapse, and the suppression of neurite growth. TROY/TAJ, a member of the tumor necrosis factor receptor family, can replace the p75 neurotrophin receptor to form functional membrane receptor complexes with Nogo receptor and Lingo-1. TROY/ TAJ can activate the Rho/ROCK signaling pathway and mediate inhibitory signaling by myelin-associated inhibitory molecules (Mi, 2008). PIR-B is a newly discovered high affi nity receptor for myelin-associated inhibitory factors. Nogo, myelin-associated glycoprotein and oligodendrocyte myelin glycoprotein combined with PIR-B exert inhibitory effects on neurite growth (Atwal et al., 2008; Filbin, 2008; Cao et al., 2010; Adelson et al., 2012). The p75 receptor can transduce signals in association with PIR-B (Fujita et al., 2011). Blocking PIR-B activity can partially abrogate the inhibitory eff ects ofmyelin-associated inhibitory factors on nerve regeneration in vitro (Atwal et al., 2008). However, PIR-B gene knockout cannot improve axonal plasticity or functional recovery after trauma-induced brain damage in vivo (Fujita et al., 2011). Nonetheless, the contribution of PIR-B in inhibitory signal transduction may be stronger than that of Nogo receptor. Indeed, previous studies have demonstrated that blocking PIR-B has a greater positive effect on regeneration than blocking Nogo receptor (Chivatakarn et al., 2007; Omoto et al., 2010). In addition to the myelin-associated inhibitory factors, repulsive molecules can also inhibit axonal regeneration, especially repulsive guidance molecules. Repulsive guidance molecules interact with transmembrane proteins (e.g., neogenin) to induce growth cone collapse and inhibit axonal regeneration. This inhibition is mediated by the Rho/ ROCK signaling pathway (Conrad et al., 2007; Jiang et al., 2012; Li et al., 2012; Bell et al., 2013) (Figure 1).
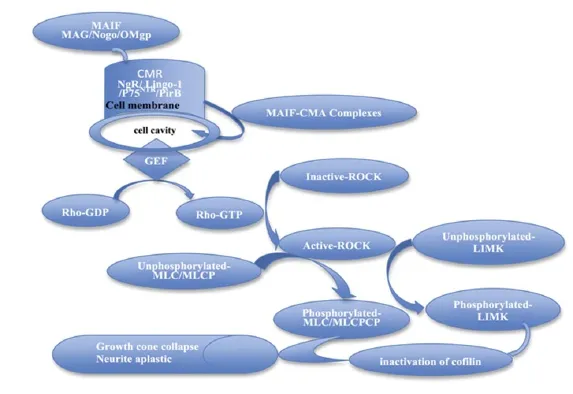
Figure 1 The Rho/ROCK signaling pathway.
Rho can be activated by a variety of cytokines and infl ammatory mediators. Interleukin-4 and interleukin-13 can upregulate RhoA in smooth muscle via activator transcription factor 6 (Chiba et al., 2009; Chiba et al., 2010). Angiotensin II can activate the Rho/ROCK signaling pathway and is involved in MPTP-induced dopamine neuron degeneration and microglial activation. The ROCK inhibitor Y-27632 and angiotensin type 1 receptor antagonists can abrogate these eff ects (Ichikawa et al., 2012; Villar-Cheda et al., 2012; Xue et al., 2012).
Downstream regulation of the Rho/ROCK signaling pathway
Normally, ROCK is inactive in the cytoplasm, but can be activated by Rho and arachidonic acid. Activated Rho /ROCK interacts with downstream eff ector molecules, and triggers specifi c signaling cascades. ROCK regulates the phosphorylation of myosin light chain, which is a classical ROCK signaling pathway (Tan et al., 2011; Liu, 2012; Frisca et al., 2013). Activated ROCK can directly phosphorylate myosin light chain, and further phosphorylate myosin light chain phosphatase. Inactive myosin light chain phosphatase cannot dephosphorylate myosin light chain. The direct and indirect pathways increase cytoplasmic phosphorylated myosin light chain levels, thereby increasing the interaction of actin and myosin to induce cytoskeletal reorganization, resulting in growth cone collapse and neurite retraction (Narumiya et al., 2009; Zhou, 2011).
Collapsin response mediator protein-2 is another downstream effector of ROCK, and regulates microtubule assembly mainly by binding to tubulin during axonal development. The amino terminal residues 480–509 of human collapsin response mediator protein-2 are essential for stimulating tubulin GTPase activity. A human collapsin response mediator protein-2 mutant that cannot activate GTPase activity can suppress microtubule assembly and neurite formation (Chae et al., 2009; Yoneda et al., 2012). LIM kinase is a ROCK downstream effector substrate. The activated actin depolymerizing factor cofi lin can sever myofi laments, depolymerize actin chains, and promote neurite outgrowth. When LIM kinase is phosphorylated by ROCK, cofilin is inactivated, which contributes to actin fi lament elongation and formation of dendritic spines (Bernstein and Bamburg, 2010; Morin et al., 2011; Piccioli and Littleton, 2014; Wen et al., 2014) (Figure 1).
Targeting the Rho/ROCK pathway to promote regeneration
Y-27632 is the most common ROCK inhibitor, and can be internalized by cells by carrier-mediated facilitated diff usion. Y-27632 acts on the ATP-binding site of the ROCK catalytic domain, thereby inhibiting ROCK activity (Zohrabian et al., 2009). Fasudil (HA-1077) is a selective inhibitor of ROCK, and is an isoquinoline sulfonamide. Hydroxy fasudil (H-1152) is an active metabolite of fasudil, and a specifi c inhibitor of ROCK with strong eff ectiveness and selectivity (Lie et al., 2010; Gurpinar and Gok, 2012). Lingor et al. (2007) showed that Y-27632, fasudil and hydroxyfasudil promote neuronal regeneration and block chondroitin sulfate proteoglycan, suggesting that they can be used as candidate drugs to treat neurodegenerative diseases (Li et al., 2013). In an in vitro model of optic nerve damage, Y-27632 promotes the regeneration of retinal ganglion cell axons in a dose-dependent manner (Lingor et al., 2008). The combination of Y-27632 and ciliary neurotrophic factor (another ROCK inhibitor) is more conducive to neurite growth and regeneration (Lingor et al., 2008). In models of aluminun maltolate-induced cortical neuron neurotoxicity, Y-27632 can not only maintain cell viability, but also prevent the formation of amyloid fi laments, and reduce levels of soluble amyloid-beta precursor protein fragments (Chen et al., 2010). In models of cerebral ischemia, fasudil improves hemodynamics by inhibiting the Rho/ROCK pathway, inhibiting infl ammation, reducing infarct area and promoting recovery of neurological function, reducing neuronal apoptosis in the ischemic area, and modulating neuronal actin cytoskeletal polymerization. It also had a wide therapeutic time window for ischemic brain injury (Satoh et al., 2008; Gisselsson et al., 2010; Wu et al., 2012). C3 exoenzyme is a Rho inhibitor. In models of end-to-side peroneal/tibial nerve injury, C3 promotes myelination and axonal sprouting, but does not impact nerve conduction or motor function (Penna et al., 2012). In rat models of spinal cord injury in vitro, C3 alone can promote axon extension at the injury site and increase the density of axons in the surrounding area, but does not aff ect myelination (Boomkamp et al., 2012). Y-27632 alone can signifi cantly increase myelination in the contiguous zone of damage foci, but does not signifi -cantly promote axonal growth (Boomkamp et al., 2012). The combination of C3 enzyme and Y-27632 can promote axonal growth and improve myelination (Boomkamp et al., 2012). Moreover, the role of these two inhibitors is not synergistic (Boomkamp et al., 2012). Yang et al. (2010) showed that in a microenvironment simulating central nervous system damage, dominant negative ROCK inhibits the formation of stress fibers in NIH3T3 cells, and promotes the growth of neurites. Different types of Rho/ROCK blockers have diff erent degrees of protective eff ects on neurons. Wang etal. (2009a) demonstrated that the application of a ROCK inhibitor (Y-27632, fasudil incubation) before and after mouse cortical neuron hypoxia can prevent or signifi cantly mitigate F-actin cytoskeletal changes induced by hypoxia. Moreover, neuronal viability did not apparently decrease. However, the application of C3a cannot prevent these changes.
Numerous recent studies have focused on non-steroidal anti-inflammatory drugs (Butterton, 2013; Day and Graham, 2013; Gurpinar et al., 2014; Lehrer, 2014; Thompson, 2014). In the pediatric fi eld, ibuprofen and indomethacin are mainly used for the treatment of preterm children with patent ductus arteriosus (Jones et al., 2011;Heo et al., 2012; Neumann et al., 2012). Studies have shown that ibuprofen may protect nerve tissue, stimulate axonal growth and promote spinal cord regeneration, and enhance functional recovery after spinal cord injury (Wang et al., 2009b; Ribeiro et al., 2011). Xing et al. (2011) showed that ibuprofen and indomethacin reduce glial cell death in vitro and cell death in white matter nerve bundles in models of spinal cord injury, and they demonstrated that non-steroidal anti-infl ammatory drugs that inhibit RhoA promote axonal myelination in white matter nerve bundles after spinal cord contusion. Non-steroidal anti-infl ammatory drugs that do not inhibit RhoA did not have these eff ects. Thus, non-steroidal anti-infl ammatory drugs that inhibit RhoA have therapeutic potential for axonal injury in the adult central nervous system.
Conclusion
The Rho/ROCK signaling pathway is a major pathway transducing inhibitory signals, and it suppresses central nervous system regeneration in vivo. The Rho/ROCK signaling pathway is a promising target for axonal regeneration in the central nervous system.
Author contributions: JL wrote the paper, was in charge of paper authorization, and obtained the funding. XFW and HYG participated in data collection and collation and critical revision. All authors approved the fi nal version of the paper.
Confl icts of interest: None declared.
Plagiarism check: This paper was screened twice using Cross-Check to verify originality before publication.
Peer review: This paper was double-blinded, stringently reviewed by international expert reviewers.
Adelson JD, Barreto GE, Xu L, Kim T, Brott BK, Ouyang YB, Naserke T, Djurisic M, Xiong X, Shatz CJ, Giff ard RG (2012) Neuroprotection from stroke in the absence of MHCI or PirB. Neuron 73:1100-1107.
Atwal JK, Pinkston-Gosse J, Syken J, Stawicki S, Wu Y, Shatz C, Tessier-Lavigne M (2008) PirB is a functional receptor for myelin inhibitors of axonal regeneration. Science 322:967-970.
Bell CH, Healey E, van Erp S, Bishop B, Tang C, Gilbert RJ, Aricescu AR, Pasterkamp RJ, Siebold C (2013) Structure of the repulsive guidance molecule (RGM)-neogenin signaling hub. Science 341:77-80.
Bernstein BW, Bamburg JR (2010) ADF/Cofi lin: a functional node in cell biology. Trends Cell Biol 20:187-195.
Boomkamp SD, Riehle MO, Wood J, Olson MF, Barnett SC (2012) The development of a rat in vitro model of spinal cord injury demonstrating the additive eff ects of rho and ROCK inhibitors on neurite outgrowth and myelination. Glia 60:441-456.
Butterton S (2013) NSAIDs in the treatment of polyuria and polydipsia in dogs. Vet Rec 173:352.
Cao Z, Gao Y, Deng K, Williams G, Doherty P, Walsh FS (2010) Receptors for myelin inhibitors: Structures and therapeutic opportunities. Mol Cell Neurosci 43:1-14.
Chae YC, Lee S, Heo K, Ha SH, Jung Y, Kim JH, Ihara Y, Suh PG, Ryu SH (2009) Collapsin response mediator protein-2 regulates neurite formation by modulating tubulin GTPase activity. Cell Signal 21:1818-1826.
Chen K, Zhang W, Chen J, Li S, Guo G (2013) Rho-associated protein kinase modulates neurite extension by regulating microtubule remodeling and vinculin distribution. Neural Regen Res 8:3027-3035.
Chen TJ, Hung HS, Wang DC, Chen SS (2010) The protective eff ect of Rho-associated kinase inhibitor on aluminum-induced neurotoxicity in rat cortical neurons. Toxicol Sci 116:264-272.
Chiba Y, Goto K, Momata M, Kobayashi T, Misawa M (2010) Induction of RhoA gene expression by interleukin-4 in cultured human bronchial smooth muscle cells. J Smooth Muscle Res 46:217-224.
Chiba Y, Nakazawa S, Todoroki M, Shinozaki K, Sakai H, Misawa M (2009) Interleukin-13 augments bronchial smooth muscle contractility with an up-regulation of RhoA protein. Am J Respir Cell Mol Biol 40:159-167.
Chivatakarn O, Kaneko S, He Z, Tessier-Lavigne M, Giger RJ (2007) The Nogo-66 receptor NgR1 is required only for the acute growth cone-collapsing but not the chronic growth-inhibitory actions of myelin inhibitors. J Neurosci 27:7117-7124.
Conrad S, Genth H, Hofmann F, Just I, Skutella T (2007) Neogenin-RGMa Signaling at the Growth Cone Is Bone Morphogenetic Protein-independent and Involves RhoA, ROCK, and PKC. J Biol Chem 282:16423-16433.
Cui Q, Zhang Y, Chen H, Li J (2013) Rho kinase: A new target for treatment of cerebral ischemia/reperfusion injury. Neural Regen Res 8:1180-1189.
Day RO, Graham GG (2013) Republished research: non-steroidal anti-infl ammatory drugs (NSAIDs). Br J Sports Med 47:1127.
Filbin MT (2008) PirB, a second receptor for the myelin inhibitors of axonal regeneration nogo66, MAG, and OMgp: implications for regeneration in vivo. Neuron 60:740-742.
Frisca F, Crombie DE, Dottori M, Goldshmit Y, Pébay A (2013) Rho/ ROCK pathway is essential to the expansion, diff erentiation, and morphological rearrangements of human neural stem/progenitor cells induced by lysophosphatidic acid. J Lipid Res 54:1192-1206.
Fujimura M, Usuki F, Kawamura M, Izumo S (2011) Inhibition of the Rho/ROCK pathway prevents neuronal degeneration in vitro and in vivo following methylmercury exposure. Toxicol Appl Pharmacol 250:1-9.
Fujita Y, Takashima R, Endo S, Takai T, Yamashita T (2011) The p75 receptor mediates axon growth inhibition through an association with PIR-B. Cell Death and Dis 2:e198.
Gisselsson L, Toresson H, Ruscher K, Wieloch T (2010) Rho kinase inhibition protects CA1 cells in organotypic hippocampal slices during in vitro ischemia. Brain Res 1316:92-100.
Gu H, Yu SP, Gutekunst CA, Gross RE, Wei L (2013) Inhibition of the Rho signaling pathway improves neurite outgrowth and neuronal diff erentiation of mouse neural stem cells. Int J Physiol Pathophysiol Pharmacol 5:11-20.
Gurpinar E, Grizzle WE, Piazza GA (2014) NSAIDs inhibit tumorigenesis, but how? Clin Cancer Res 20:1104-1113.
Gurpinar T, Gok S (2012) Vasodilator effects of cromakalim and HA 1077 in diabetic rat aorta. Swiss Med Wkly 142:w13558.
Heo M, Lee O, Lim S (2012) Comparative evaluation for the use of oral ibuprofen and intravenous indomethacin in Korean infants with patent ductus. Arch Pharm Res 35:1673-1683.
Ichikawa H, Nakata N, Abo Y, Shirasawa S, Yokoyama T, Yoshie S, Yue F, Tomotsune D, Sasaki K (2012) Gene pathway analysis of the mechanism by which the Rho-associated kinase inhibitor Y-27632 inhibits apoptosis in isolated thawed human embryonic stem cells. Cryobiology 64:12-22.
Jiang F, Yin H, Qin X (2012) Fastigial nucleus electrostimulation reduces the expression of repulsive guidance molecule, improves axonal growth following focal cerebral ischemia. Neurochem Res 37:1906-1914.
Jones LJ, Craven PD, Attia J, Thakkinstian A, Wright I (2011) Network meta-analysis of indomethacin versus ibuprofen versus placebo for PDA in preterm infants. Arch Dis Child Fetal Neonatal Ed 96:F45-F52.
Lehrer S (2014) Nasal NSAIDs for Alzheimer’s disease. Am J Alzheimers Dis Other Demen 29:401-403.
Li J, Ye L, Sanders AJ, Jiang WG (2012) Repulsive guidance molecule B (RGMB) plays negative roles in breast cancer by coordinating BMP signaling. J Cell Biochem 113:2523-2531.
Li M, Yasumura D, Ma AAK, Matthes MT, Yang H, Nielson G, Huang Y, Szoka FC, LaVail MM, Diamond MI (2013) Intravitreal administration of HA-1077, a ROCK inhibitor, improves retinal function in a mouse model of huntington disease. PLoS One 8:e56026.
Lie M, Grover M, Whitlon DS (2010) Accelerated neurite growth from spiral ganglion neurons exposed to the Rho kinase inhibitor H-1152. Neuroscience 169:855-862.
Lingor P, Teusch N, Schwarz K, Mueller R, Mack H, Bähr M, Mueller BK (2007) Inhibition of Rho kinase (ROCK) increases neurite outgrowth on chondroitin sulphate proteoglycan in vitro and axonal regeneration in the adult optic nerve in vivo. J Neurochem 103:181-189.
Lingor P, Tönges L, Pieper N, Bermel C, Barski E, Planchamp V, Bähr M (2008) ROCK inhibition and CNTF interact on intrinsic signalling pathways and diff erentially regulate survival and regeneration in retinal ganglion cells. Brain 131:250-263.
Liu Y (2012) Rho-ROCK signal pathway. Zhongguo Ertong Baojian Za Zhi 20:822-825.
Menendez-Castro C, Fahlbusch F, Cordasic N, Amann K, Münzel K, Plank C, Wachtveitl R, Rascher W, Hilgers KF, Hartner A (2011) Early and late postnatal myocardial and vascular changes in a protein restriction rat model of intrauterine growth restriction. PLoS One 6:e20369.
Mi S (2008) Troy/Taj and its role in CNS axon regeneration. Cytokine Growth Factor Rev 19:245-251.
Mishra M, Akatsu H, Heese K (2011) The novel protein MANI modulates neurogenesis and neurite-cone growth. J Cell Mol Med 15:1713-1725.
Morin P, Wickman G, Munro J, Inman GJ, Olson MF (2011) Diff ering contributions of LIMK and ROCK to TGFβ-induced transcription, motility and invasion. Eur J Cell Biol 90:13-25.
Narumiya S, Tanji M, Ishizaki T (2009) Rho signaling, ROCK and mDia1, in transformation, metastasis and invasion. Cancer Metast Rev 28:65-76.
Neumann R, Schulzke SM, Bührer C (2012) Oral ibuprofen versus intravenous ibuprofen or intravenous indomethacin for the treatment of patent ductus arteriosus in preterm infants: a systematic review and meta-analysis. Neonatology 102:9-15.
Omoto S, Ueno M, Mochio S, Takai T, Yamashita T (2010) Genetic deletion of PIR-B does not promote axonal plasticity or functional recovery after traumatic brain injury. J Neurosci 30:13045-13052.
Penna V, Bjoern Stark G, Leibig N, Boyle V, Sakalidou M (2012) Rho-inhibition by local application of c3-toxin for enhancement of axonal sprouting in a rat end-to-side nerve repair model. Microsurgery 32:207-212.
Piccioli ZD, Littleton JT (2014) Retrograde BMP signaling modulates rapid activity-dependent synaptic growth via presynaptic LIM kinase regulation of cofi lin. J Neurosci 34:4371-4381.
Ribeiro MM, Pinto AR, Domingues MM, Serrano I, Heras M, Bardaji ER, Tavares I, Castanho MA (2011) Chemical conjugation of the neuropeptide kyotorphin and ibuprofen enhances brain targeting and analgesia. Mol Pharm 8:1929-1940.
Satoh S, Toshima Y, Hitomi A, Ikegaki I, Seto M, Asano T (2008) Wide therapeutic time window for Rho-kinase inhibition therapy in ischemic brain damage in a rat cerebral thrombosis model. Brain Res 1193:102-108.
Schmandke A, Schmandke A, Strittmatter SM (2007) ROCK and Rho: biochemistry and neuronal functions of Rho-associated protein kinases. Neuroscientist 13:454-469.
Tan HB, Zhong YS, Cheng Y, Shen X (2011) Rho/ROCK pathway and neural regeneration: a potential therapeutic target for central nervous system and optic nerve damage. Int J Ophthalmol 4:652-657.
Teramura T, Takehara T, Onodera Y, Nakagawa K, Hamanishi C, Fukuda K (2012) Mechanical stimulation of cyclic tensile strain induces reduction of pluripotent related gene expressions via activation of Rho/ROCK and subsequent decreasing of AKT phosphorylation in human induced pluripotent stem cells. Biochem Biophys Res Commun 417:836-841.
Thompson CA (2014) More information emerging on NSAIDs’ potential cardiovascular risks. Am J Health Syst Pharm 71:442-444.
Villar-Cheda B, Dominguez-Meijide A, Joglar B, Rodriguez-Perez AI, Guerra MJ, Labandeira-Garcia JL (2012) Involvement of microglial RhoA/Rho-Kinase pathway activation in the dopaminergic neuron death. Role of angiotensin via angiotensin type 1 receptors. Neurobiol Dis 47:268-279.
Wälchli T, Pernet V, Weinmann O, Shiu JY, Guzik-Kornacka A, Decrey G, Yüksel D, Schneider H, Vogel J, Ingber DE, Vogel V, Frei K, Schwab ME (2013) Nogo-A is a negative regulator of CNS angiogenesis. Proc Natl Acad Sci U S A 110:E1943-1952.
Wang H, Chen AM, Guo FJ, Zhang DD, Yi CL (2009a) Eff ects of Rho-ROK inhibitors on axon regeneration and viability of mouse neuron induced by hypoxia in vitro. Shenjing Sunshang yu Gongneng Chongjian 4:98-101.
Wang X, Budel S, Baughman K, Gould G, Song K-H, Strittmatter SM (2009b) Ibuprofen enhances recovery from spinal cord injury by limiting tissue loss and stimulating axonal growth. J Neurotrauma 26:81-95.
Wen X, Ding L, Wang JJ, Qi M, Hammonds J, Chu H, Chen X, Hunter E, Spearman P (2014) ROCK1 and LIM kinase modulate retrovirus particle release and cell-cell transmission events. J Virol 88:6906-6921.
Wu J, Li J, Hu H, Liu P, Fang Y, Wu D (2012) Rho-kinase inhibitor, fasudil, preventsneuronal apoptosis via the Akt activation and PTEN inactivation in the ischemic penumbra of rat brain. Cell Mol Neurobiol 32:1187-1197.
Xing B, Li H, Wang H, Mukhopadhyay D, Fisher D, Gilpin CJ, Li S (2011) RhoA-inhibiting NSAIDs promote axonal myelination after spinal cord injury. Exp Neurol 231:247-260.
Xue ZW, Shang XM, Xu H, Lü SH, Dong TW, Liang CH, Yuan Y (2012) Rho-associated coiled kinase inhibitor Y-27632 promotes neuronal-like diff erentiation of adult human adipose tissue-derived stem cells. Chin Med J (Engl) 125:3332-3335.
Yang P, Wen HZ, Zhang JH (2010) Expression of a dominant-negative Rho-kinase promotes neurite outgrowth in a microenvironment mimicking injured central nervous system. Acta Pharmacol Sin 31:531-539.
Yoneda A, Morgan-Fisher M, Wait R, Couchman JR, Wewer UM (2012) A collapsin response mediator protein 2 isoform controls myosin II-mediated cell migration and matrix assembly by trapping ROCK II. Mol Cell Biol 32:1788-1804.
Zhou GP (2011) The structural determinations of the leucine zipper coiled-coil domains of the cGMP-dependent protein kinase Iα and its interaction with the myosin binding subunit of the myosin light chains phosphase. Protein Pept Lett 18:966-978.
Zohrabian VM, Forzani B, Chau Z, Murali R, Jhanwar-Uniyal M (2009) Rho/ROCK and MAPK signaling pathways are involved in glioblastoma cell migration and proliferation. Anticancer Res 29:119-123.
Copyedited by Patel B, Robens J, Yu J, Qiu Y, Li CH, Song LP, Zhao M
*Correspondence to: Jing Liu, M.D., liujingbj@live.cn.
orcid: 0000-0001-8356-9515 (Jing Liu)
10.4103/1673-5374.170325 http://www.nrronline.org/
Accepted: 2015-06-17
杂志排行
中国神经再生研究(英文版)的其它文章
- Targeting brain microvascular endothelial cells: a therapeutic approach to neuroprotection against stroke
- Severe bilateral anterior cingulum injury in patients with mild traumatic brain injury
- Injury of corticoreticular pathway and corticospinal tract caused by ventriculoperitoneal shunting
- Susceptibility weighted imaging in the evaluation of hemorrhagic dif use axonal injury
- Mechanical properties of nerve roots and rami radiculares isolated from fresh pig spinal cords
- Endogenous neurotrophin-3 promotes neuronal sprouting from dorsal root ganglia
