Novel approaches for the development of peripheral nerve regenerative therapies
2015-02-07FelixBeyer,PatrickKüry
Novel approaches for the development of peripheral nerve regenerative therapies
Schwann cells are the myelinating glial cells of the peripheral nervous system (PNS). By establishing lipid-rich myelin sheaths around large-caliber axons, they ensure that electrical signal transmission is accelerated–a process referred to as saltatory signal propagation. Apart from this prominent physiological function, these cells also exert important pathophysiological roles in PNS injuries or diseases. In contrast to the central nervous system (CNS), the adult PNS retains a remarkably high degree of intrinsic regeneration. As a consequence, transected axons and damaged myelin sheaths can be repaired and nerve functionality can be restored. This spontaneous regenerative capacity depends on (inter) actions of macrophages, neurons, and Schwann cells. Although highly specialized and tightly interacting with axons, Schwann cells can revert upon nerve injury or disease to an immature and repair-mediating phenotype (Arthur-Farraj et al., 2012). Dedifferentiated Schwann cells participate in myelin clearance and attract macrophages for further clearance, enabling Wallerian degeneration of distal nerve stumps to proceed. They were also shown to positively infl uence injured axons and to stimulate regrowth of their tips toward their target cells in the periphery. Finally, re-established axons can be wrapped up again by rediff erentiating Schwann cells, thereby generating new isolating myelin sheaths. Thus, spontaneous peripheral nerve regeneration can be mainly attributed to Schwann cells and their particular and specifi c responses to trauma and disease. This is remarkable cell behavior and implies that these cells have a large capacity to switch and adjust their transcriptional programs, most likely by means of epigenetic controls (Jacob et al., 2011; Heinen et al., 2012). Moreover, multiple interactions with cells and components of the immune system were recently revealed (Tzekova et al., 2014).
Despite this well-developed intrinsic repair function, the overall capacity of peripheral nerves to heal and functionally restore remains limited, particularly in pathological conditions such as inherited, toxic, infl ammatory, and diabetic neuropathies, as well as after deep traumatic lesions. The underlying reasons for this impairment remain to be fully elucidated, but it is likely that regenerative Schwann cell functions are defective, either due to immunological processes or due to kinetic aspects, i.e., nerve restoration cannot cope with recurring degeneration. Given that neuropathies of different etiologies are quite common, it is remarkable that no therapeutic approaches exist to promote, stabilize, accelerate, or even trigger peripheral nerve repair. This unmet therapeutic need related to axonal and myelin regeneration therefore requires increased attention to develop novel treatment strategies. In addition, because nerve regeneration, which includes remyelination of spared axons, myelination of regenerated axons, and axonal regeneration, is time-consuming, long-lasting processes need to be supported.
Two recent preclinical investigations from our laboratory addressed to what degree well-described pharmacological treatments could aff ect Schwann cells and their potential to adopt a repair-mediating phenotype, and thus serve as future medications for patients with PNS injuries or diseases.
In the fi rst study, we investigated glial responses upon exposure to Fingolimod/FTY720P (Heinen et al., 2015) (Figure 1). Fingolimod is a sphingosine-1-phosphate (S1P) receptor agonist and is an approved treatment for relapse-remitting multiple sclerosis (Gilenya) (Ingwersen et al., 2012). Currently, it is undergoing a clinical trial for the treatment of chronic infl ammatory demyelinating polyneuropathies (CIDP; ClinicalTrials.gov Identifier: NCT01625182). Fingolimod modulates S1P signaling and prevents immune cells from exiting lymphoid tissues (Ingwersen et al., 2012). Previous studies have indicated that Schwann cells express all fi ve S1P receptors aff ecting glial migration and cytoskeletal dynamics and interfering with myelination in vitro [see Heinen et al. (2015) for further references]. Moreover, in experimental autoimmune neuritis (EAN), the rodent model of PNS Guillain-Barré syndrome (GBS), FTY720P application led to substantial amelioration of the disease course (Zhang et al., 2008), most likely due to its immunomodulatory action. Possible direct FTY720P-related neuroregenerative eff ects have not yet been investigated. In our study we stimulated primary neonatal and adult rat Schwann cells with Fingolimod/FTY 720P and investigated its impact on the regeneration-promoting phenotype. We found that this treatment resulted in the activation of a number of dediff erentiation markers, including the transcription factor cJun, which was recently described to reprogram Schwann cells to act as repair-mediating cells (Arthur-Farraj et al., 2012). While it interfered with the expression of mature markers and myelin, Fingolimod also negatively aff ected intracellular Akt signaling, which is known to be critically involved in Schwann cell maturation (Heinen et al., 2015). Besides this shift toward a dediff erentiated cellular state, FTY720P-treated Schwann cells also increased growth factor expression, which in turn rendered these cells more potent in enhancing neurite outgrowth–even on inhibitory substrates, as evidenced by dorsal root ganglion neuron stimulation by conditioned media of FTY720P-treated Schwann cells. Therefore, these fi ndings provide strong evidence that S1P receptor stimulation supports the generation of a repair-promoting cellular phenotype, suggesting that Fingolimod/Gilenya should be further investigated for PNS regenerative treatments. Currently, it is not clear which of the fi ve S1P receptors initially described on Schwann cells [Heinen et al. (2015) and references therein] are responsible for Fingolimod’s promotion of cellular dediff erentiation. A more detailed description of involved receptor subtypes and further signaling cascades is currently being undertaken, along with a translation towards clinically relevant in vivo models.
In the second study we examined whether immunoglobulins can aff ect glial cell homeostasis, diff erentiation, or Schwann cell-dependent nerve regenerative processes (Tzekova et al., 2015) (Figure 1). Intravenous immunoglobulins(IVIG) mainly consist of polyclonal human immunoglobulin G (IgG). They are generally used for the treatment of immune defi ciencies, but they are also given to patients suff ering from polyneuropathies. IVIG most likely act on several immunomodulatory mechanisms, and positive eff ects on disease severity and recovery have been reported for EAN (Tzekova et al., 2015). Their underlying mode of action is not well understood, but it certainly includes Fc-dependent and F(ab’)2-dependent mechanisms blocking cellular receptors, neutralizing cytokines, complement and autoantibodies, as well as modulation of activating and inhibitory FcγR expression on immune cells (Lunemann et al., 2015). Of note, recent data indicate that endogenous antibodies also participate in myelin clearance and axon regeneration after peripheral nerve injury (Vargas et al., 2010), suggesting an intrinsic contribution of immune/neural (cell) interactions to spontaneous nerve repair processes.
We discovered that IVIG specifically bind to Schwann cells, including interactions via the high-affinity 1a Fc receptor (Fcgr1a/CD64) expressed on their surface. On stimulation with IVIG, non-diff erentiating Schwann cells reduced proliferation rates, accelerated growth of cellular protrusions, and transiently increased myelin gene expression as well as myelination-related signaling pathways. Myelin expression of diff erentiation-competent Schwann cells was enhanced in the long-term and in vitro myelination was improved. Importantly, myelin responses could not be detected when IgG1 control antibodies were applied. Moreover, we were able to demonstrate that IVIG stimulate interleukin-18 production by Schwann cells and that this cytokine instructs them to promote axonal growth from sensory neurons ex vivo. We therefore concluded that polyvalent immunoglobulin preparations can positively infl uence the Schwann cell diff erentiation process and that it enhances their regenerative potential. Currently, it is not known how IVIG-dependent signals act on Schwann cells or how they can stimulate maturation pathways and gene expression. Based on our fi ndings, it is conceivable that, similar to immune cells, Fc-dependent and F(ab’)2-dependent mechanisms account for this process.
Given that Schwann cells are central components of the peripheral nerve repair process, and given that this activity critically depends on their plastic differentiation potential, our fi ndings using two approved medications for the treatment of (among others) infl ammatory demyelinating diseases of the CNS are of particular interest. While the establishment of a dediff erentiation process can be enforced via S1P receptor activation, the subsequent rediff erentiation stage was clearly promoted in the presence of and upon interaction with IVIG. Although these are preclinical fi ndings mainly drawn from observations ex vivo, they strongly indicate that Schwann cell differentiation can indeed be modulated specifi cally and that these treatments could be used to overcome regeneration restrictions imposed by multiple pathological conditions of the PNS. Several polyvalent immunoglobulin preparations are approved and on the market, and besides Gilenya/Fingolimod several novel S1P receptor agonists have been developed – some of them are already evaluated in clinical trials (for example, Siponimod; ClinicalTrials.gov Identifier: NCT01665144). Of note, as discussed in our articles (Heinen et al., 2015) (Tzekova et al., 2015), similar treatments have also been shown to aff ect oligodendroglial precursor cells (OPCs), which are immature glial cells of the CNS that can eventually give rise to new oligodendrocytes. Specifi c OPC responses ex vivo or in experimental demyelination conditions were reported upon FTY720P stimulation, and immunoglobulin M (IgM) was discovered as a potent OPC diff erentiation inducer that led to a clinical trial on hIgM22 (ClinicalTrials.gov Identifier: NCT01803867).
Moreover, recent findings revealed that Herceptin, a monoclonal antibody directed against human epidermal growth factor receptor 2 (erbB2), promotes axonal outgrowth after peripheral nerve transection (Placheta et al., 2014) (Figure 1). The underlying mechanism awaits future analyses because Herceptin’s actions could not be attributed to altered neuregulin/erbB2 signaling. Because this antibody was not found to be eff ective in our experiments and served (together with Avastin and Synagis) as a control, this might be of interest considering potential combinatory treatments (e.g., IVIG). In addition to testing immunoglobulins and S1P receptor agonists in suitable in vivo paradigms, optimal windows of opportunity need to be established and parallel overlapping or counteracting eff ects on the immune system need to be explored.
The search for active ingredients in polyvalent immunoglobulin preparations (Fc receptor or Schwann cell antigen–directed) as well as studies of S1P receptor activation by means of more specifi c ligands might further pave the way for novel repair therapies for patients with different peripheral nerve conditions. The studies presented here also demonstrate that Schwann cells exert a high degree of immunocompetence and that multiple signaling interfaces between immune and Schwann cells exist, and these could be explored for pharmacological modulation.
Our research on Schwann cell differentiation was supported by grants from the DFG (German Research Council), Novartis Pharma GmbH (Nürnberg, Germany), and Baxter Innovations GmbH (Vienna, Germany).
Felix Beyer, Patrick Küry*
Department of Neurology, Medical Faculty, Heinrich-Heine-University, Düsseldorf, Germany
*Correspondence to: Patrick Küry, Ph.D., kuery@uni-duesseldorf.de.
Accepted: 2015-10-12
orcid: 0000-0002-3329-0249 (Felix Beyer) 0000-0002-2654-1126 (Patrick Küry)
Arthur-Farraj PJ, Latouche M, Wilton DK, Quintes S, Chabrol E, Banerjee A, Woodhoo A, Jenkins B, Rahman M, Turmaine M, Wicher GK, Mitter R, Greensmith L, Behrens A, Raivich G, Mirsky R, Jessen KR (2012) c-Jun reprograms Schwann cells of injured nerves to generate a repair cell essential for regeneration. Neuron 75:633-647.
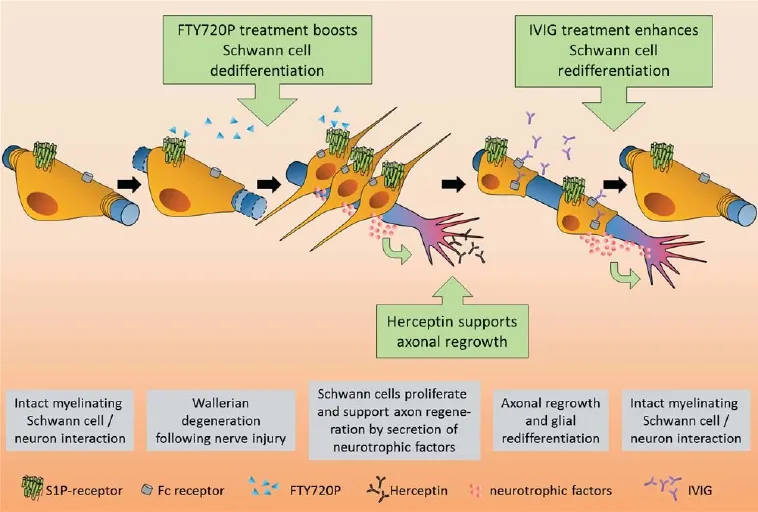
Figure 1 Schwann cell responses and degeneration- and regeneration-related subprocesses following peripheral nerve injury.
Heinen A, Beyer F, Tzekova N, Hartung HP, Küry P (2015) Fingolimod induces the transition to a nerve regeneration promoting Schwann cell phenotype. Exp Neurol 271:25-35.
Heinen A, Tzekova N, Graff mann N, Torres KJ, Uhrberg M, Hartung HP, Küry P (2012) Histone methyltransferase enhancer of zeste homolog 2 regulates Schwann cell diff erentiation. Glia 60:1696-1708.
Ingwersen J, Aktas O, Kuery P, Kieseier B, Boyko A, Hartung HP (2012) Fingolimod in multiple sclerosis: mechanisms of action and clinical effi cacy. Clin Immunol 142:15-24.
Jacob C, Christen CN, Pereira JA, Somandin C, Baggiolini A, Lotscher P, Ozcelik M, Tricaud N, Meijer D, Yamaguchi T, Matthias P, Suter U (2011) HDAC1 and HDAC2 control the transcriptional program of myelination and the survival of Schwann cells. Nat Neurosci 14:429-436.
Lunemann JD, Nimmerjahn F, Dalakas MC (2015) Intravenous immunoglobulin in neurology–mode of action and clinical effi cacy. Nat Rev Neurol 11:80-89.
Placheta E, Hendry JM, Wood MD, Lafontaine CW, Liu EH, Cecilia Alvarez Veronesi M, Frey M, Gordon T, Borschel GH (2014) The ErbB2 inhibitor Herceptin (Trastuzumab) promotes axonal outgrowth four weeks after acute nerve transection and repair. Neurosci Lett 582:81-86.
Tzekova N, Heinen A, Küry P (2014) Molecules involved in the crosstalk between immune- and peripheral nerve Schwann cells. J Clin Immunol 34:S86-104.
Tzekova N, Heinen A, Bunk S, Hermann C, Hartung HP, Reipert B, Küry P (2015) Immunoglobulins stimulate cultured Schwann cell maturation and promote their potential to induce axonal outgrowth. J Neuroinfl ammation 12:107.
Vargas ME, Watanabe J, Singh SJ, Robinson WH, Barres BA (2010) Endogenous antibodies promote rapid myelin clearance and eff ective axon regeneration after nerve injury. Proc Natl Acad Sci U S A 107:11993-11998.
Zhang Z, Zhang ZY, Fauser U, Schluesener HJ (2008) FTY720 ameliorates experimental autoimmune neuritis by inhibition of lymphocyte and monocyte infi ltration into peripheral nerves. Exp Neurol 210:681-690.
10.4103/1673-5374.170298 http://www.nrronline.org/
Beyer F, Küry P (2015) Novel approaches for the development of peripheral nerve regenerative therapies. Neural Regen Res 10(11):1743-1745.
杂志排行
中国神经再生研究(英文版)的其它文章
- Intracellular sorting pathways of the amyloid precursor protein provide novel neuroprotective strategies
- The role of the Rho/ROCK signaling pathway in inhibiting axonal regeneration in the central nervous system
- VEGF in the nervous system: an important target for research in neurodevelopmental and regenerative medicine
- Studying neurological disorders using induced pluripotent stem cells and optogenetics
- Ef cacy of glucagon-like peptide-1 mimetics for neural regeneration
- Compliant semiconductor scaf olds: building blocks for advanced neural interfaces
