pFTAA: a high af nity oligothiophene probe that detects f lamentous tau in vivo and in cultured neurons
2015-02-07
pFTAA: a high af nity oligothiophene probe that detects f lamentous tau in vivo and in cultured neurons
Tauopathies describe a group of neurodegenerative diseases in which the protein tau, encoded by the gene MAPT, is aberrantly misfolded, leading to tau aggregation, neural dysfunction, and cell death (Spillantini and Goedert, 2013). In Alzheimer’s disease (AD), tau forms the characteristic intracellular neurofibrillary tangles (NFTs), which are thought to be the major cause of neurodegeneration (Bloom, 2014). In other tauopathies, including frontotemporal dementia with Parkinsonism linked to chromosome 17 (FTDP-17T), corticobasal degeneration and progressive supranuclear palsy, there are specif c forms of tau aggregates and f laments without any amyloid pathology, demonstrating tau’s potent disease-causing potential (Spillantini and Goedert, 2013). Tau is a microtubule (MT) binding protein, which becomes abnormally hyperphosphorylated on several residues prior/during the process of aggregation, thereby causing loss of its MT binding activity (Mandelkow and Mandelkow, 2012). The importance of tau as a single cause of disease is reinforced by the identif cation of numerous (> 55) mutations in autosomal-dominant forms of familial tauopathies, many of which occur in the MT binding domain. Indeed, more and more diseases are being uncovered in which aberrant phosphorylation and folding of tau is implicated, not least in Huntington’s disease (HD), while MAPT is a genetic risk factor for Parkinson’s disease (PD), so it is of major interest to understand how it leads to cell dysfunction and death.
Despite the propensity of tau to misfold, it has been dif cult to demonstrate the presence of insoluble f brils of tau in animal and living cell models. Indeed, even in the test tube, f bril formation of pure tau requires addition of polyanionic polymers, such as heparin (Goedert et al., 1996). Many studies of tau have therefore relied on non-neuronal cell models where mutant tau is over-expressed to understand the causes of tau-related cell death. However, not only is the cellular milieu unlikely to be similar to that of neurons, but many cell lines express immortalising genes, making these cells prone to die by apoptosis whereas death by apoptosis in neurodegeneration is rather rare. In most studies, even those involving primary central nervous system (CNS) neurons transfected with tau, cell death is only studied over a few days, unlike the case in human disease where neuronal death extends over decades.
To address this problem, we sought a robust primary neuronal model of tauopathy, leading us to develop a culture system of dorsal root ganglion (DRG) neurons from a transgenic mouse expressing human mutant P301S tau (Mellone et al., 2013). The P301S tau mice develop gradual degeneration in the CNS over 5-6 months that is accompanied by tau hyperphosphorylation, f bril formation with identical structures to those found typically in FTDP-17T with a P301L mutation, and cell death. The advantage of this culture system is that these neurons can be cultured from adult mice at any age, and cultures can be maintained for months, during which time one can study how tau becomes hyperphosphorylated, how it misfolds, how pathology develops and how the neurons die.
However, until recently, we have found it impossible to track which living DRG neurons develop filamentous tau because we lacked a dye that penetrates living neurons and binds to f lamentous tau in a reliable and specif c manner without toxicity or perturbing cell physiology. The problem arises because less than 20% of the neurons express transgenic tau, and of these less than 50% (so overall around 10%) develop full advanced tau pathology at any one time. Moreover, as some neurons develop tau f laments, others that had already developed f laments will have died. Hence, we could only verify the presence of tau f brils in f xed cultures, which had been traced after studies on living neurons, by returning to the same f eld of view, a very laborious process. We tried several dyes on the neurons – including thiof avin T and S, 2-(4′-Methylaminophenyl) benzothiazole (BTA)-1, and congo red (for structures see Figure 1), but either the dyes did not penetrate the neurons (congo red, BTA-1) or the background was too high (Thiof avin T/S), leading to a poor signal to noise ratio.
In our paper published in Frontiers in Neuroscience (Brelstaf et al., 2015), we demonstrate the use of the pentameric form of formyl thiophene acetic acid (pFTAA) as a vital f uorescent dye that stains specif cally f lamentous tau in living neurons. We use several criteria to demonstrate that pFTAA binds to f lamentous tau in the neurons:
1) Positive staining coincides only with those neurons that express markers of f lamentous tau, the anti phospho-T212/S214/ T217 antibody AT100 and MC1, a conformational antibody that detects a compacted ‘paper-clip’ conformation of tau that is found in AD brains with tau pathology. pFTAA+ve neurons were also positive for the hyperphosphorylated epitopes pS202/pT205 (AT8) and pS396/pS404 (PHF-1 or AD2). However, although AT8 is widely used to diagnose NFTs in AD and other tauopathies, and the name PHF-1 implies that the antibody stains paired helical f laments (e.g., NFTs), in fact, these antibodies stain both phosphorylated soluble and f lamentous tau so cannot be used as sole markers for f laments.
2) Neurons that stained with pFTAA and MC1 can no longer be stained after treating the cells with formic acid, which solublises f lamentous tau by changing its conformation, although they do stain again with an antibody that detects total human P301S tau.
3) pFTAA did not stain neurons cultured from 2-month-old P301S tau mice, which express as much total P301S tau as that found in more mature neurons, but have marginal, if any filamentous tau, nor did it stain neurons cultured from a 5 monthold transgenic mouse known as Alz17 (Probst et al., 2000), which expresses the longest human tau isoform under the same Thy1.2 promoter as that used to generate P301S tau mice.
We demonstrate the utility of the dye by showing that we can now follow the same set of pFTAA-stained neurons in culture up to 25 days. After scoring for survival we can clearly conclude that DRG neurons expressing pFTAA+ve f lamentous tau die faster than neurons that are tau+ve but pFTAA-ve present in the same culture.
The reason that pFTAA performs so well is that it binds tightly to tau f laments, with a half saturation constant (EC50) of 142 nM. This likely explains why it can be used to visualise f lamentous tau in live neurons, while other dyes give poor signal to noise. The dif erences in af nity can be seen in Figure 2, which compares the binding of pFTAA with that of heptameric FTAA (EC50, 600 nM) and Thiof avin T (EC50, 3.7 μM).
In keeping with its high af nity, staining is remarkably stable. We have inspected f xed brains from mice that were injected with pFTAA 6 months prior to observation, and still see a clear pattern of staining (an example shown in Brelstaf et al., 2015).
By distinguishing f lament+ve from f lament-ve neurons, both of which express tau, we are at last in a position to study the mechanism of neuronal cell death by interfering with critical steps in the process. For example, several reports suggest that autophagy enhancement may benef t neurons expressing toxic proteins such as HD, SOD1, and tau (Frake et al., 2015). There is also an argument as to whether tau aggregates are the toxic species (Kuchibhotla et al., 2014), a question we can now answer using pFTAA. Moreover, we are now in a position to select by FACS three populations of neurons – pFTAA+ve neurons, pFTAA-ve htau+ve neurons (that have not yet formed f laments), and unlabelled htau-ve neurons, and apply omic techniques to understand the changes wrought by f lamentous tau. And lastly, it has not escaped our notice that abundant neurons from P301S tau mice at advanced stages of pathology are also labeled in vivo with pFTAA after delivery via intravenousinjection (Brelstaf et al., 2015). We are now establishing cultures from these brains as deciphering the mechanism of neuronal death in CNS neurons is of prime importance.
Figure 1 The structure of compounds that bind to beta-sheet containing protein aggregates and f brils mentioned in this study.
Some remarks about the limitation of pFTAA should be heeded. First, the dye stains other f lamentous proteins, not least amyloid plaques in human AD brains, and prions that have converted to PrpSC(Aslund et al., 2009). The same is true in mouse models of these diseases. Although the spectral properties of pFTAA bound to these dif erent proteins may be dif erent, it may be dif cult to distinguish between beta-amyloid+ve plaques and tau f laments, especially if the latter are released from cells into the brain parenchyma and transmitted to other neurons (Clavaguera et al., 2015). As with all f uorescent compounds, the dye will bleach upon high illumination, although we did not notice any reduction in intensity when exposed to the same cumulative intensity we used to visualize the bound dye every day from day 7 to day 25 in culture.
Altogether, use of pFTAA has solved a major problem in trying to decipher mechanisms of cell death induced by f lamentous forms of tau, as we can now follow living neurons to their death. We also noted previously that DRG neurons with abnormal forms of tau regenerate abnormally, with splayed growth cones, spheroids (a typical feature of human tauopathies), and thick short axons, after they are severed prior to culture (Mellone et al., 2013). Given that we can identify pFTAA+ve neurons and axons, we can now approach the question whether tau f laments, or soluble abnormal tau, are responsible for inducing these aberrant retarded growth patterns. This will be especially interesting in studying the regeneration of DRG neurons (and motor neurons, which also express pFTAA+ve f lamentous tau (Klingstedt et al., 2013)) in vivo, and ultimately CNS neuronal axons and dendrites during synapse formation.
We thank our colleagues Michel Goedert, Thérese Klingstedt, and K. Peter Nilsson for critical reading of the paper. Our work is funded by grant NC/L000741/1 from the National Council of the 3Rs.
Jack Brelstaf , Maria Grazia Spillantini, Aviva M. Tolkovsky*
Department of Clinical Neurosciences, The Clif ord Allbutt Building,
University of Cambridge, Cambridge, CB2 0AH, UK
*Correspondence to: Aviva M. Tolkovsky, Ph.D., amt1004@cam.ac.uk.
Accepted: 2015-08-22
orcid: 0000-0003-1951-3175 (Aviva M. Tolkovsky)
Aslund A, Sigurdson CJ, Klingstedt T, Grathwohl S, Bolmont T, Dickstein DL, Glimsda E, Prokop S, Lindgren M, Konradsson P, Holtzman DM, Hof PR, Heppner FL, Gandy S, Jucker M, Aguzzi A, Hammarström P, Nilsson KP (2009) Novel pentameric thiophene derivatives for in vitro and in vivo optical imaging of a plethora of protein aggregates in cerebral amyloidoses. ACS Chem Biol 4:673-684.
Bloom GS (2014) Amyloid-beta and tau: the trigger and bullet in Alzheimer disease pathogenesis. JAMA Neurol 71:505-508.
Brelstaf J, Ossola B, Neher JJ, Klingstedt T, Nilsson KP, Goedert M, Spillantini MG, Tolkovsky AM (2015) The f uorescent pentameric oligothiophene pFTAA identif es f lamentous tau in live neurons cultured from adult P301S tau mice. Front Neurosci 9:184.
Clavaguera F, Hench J, Goedert M, Tolnay M (2015) Invited review: Prion-like transmission and spreading of tau pathology. Neuropathol Appl Neurobiol 41:47-58.
Frake RA, Ricketts T, Menzies FM, Rubinsztein DC (2015) Autophagy and neurodegeneration. J Clin Invest 125:65-74.
Goedert M, Jakes R, Spillantini MG, Hasegawa M, Smith MJ, Crowther RA (1996) Assembly of microtubule-associated protein tau into Alzheimer-like f laments induced by sulphated glycosaminoglycans. Nature 383:550-553.
Klingstedt T, Shirani H, Aslund KO, Cairns NJ, Sigurdson CJ, Goedert M, Nilsson KP (2013) The structural basis for optimal performance of oligothiophene-based f uorescent amyloid ligands: conformational f exibility is essential for spectral assignment of a diversity of protein aggregates. Chemistry 19:10179-10192.
Kuchibhotla KV, Wegmann S, Kopeikina KJ, Hawkes J, Rudinskiy N, Andermann ML, Spires-Jones TL, Bacskai BJ, Hyman BT (2014). Neurofibrillary tangle-bearing neurons are functionally integrated in cortical circuits in vivo. Proc Natl Acad Sci U S A 111:510-514.
Mandelkow EM, Mandelkow E (2012) Biochemistry and cell biology of tau protein in neurof brillary degeneration. Cold Spring Harb Perspect Med 2:a006247.
Mellone M, Kestoras D, Andrews MR, Dassie E, Crowther RA, Stokin GB, Tinsley J, Horne G, Goedert M, Tolkovsky AM, Spillantini MG (2013) Tau pathology is present in vivo and develops in vitro in sensory neurons from human P301S tau transgenic mice: a system for screening drugs against tauopathies. J Neurosci 33:18175-18189.
Probst A, Gotz J, Wiederhold KH, Tolnay M, Mistl C, Jaton AL, Hong M, Ishihara T, Lee VM, Trojanowski JQ, Jakes R, Crowther RA, Spillantini MG, Burki K, Goedert M (2000) Axonopathy and amyotrophy in mice transgenic for human four-repeat tau protein. Acta Neuropathol 99:469-481.
Spillantini MG, Goedert M (2013) Tau pathology and neurodegeneration. Lancet Neurol 12:609-622.
10.4103/1673-5374.165298 http://www.nrronline.org/
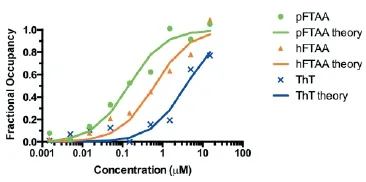
Figure 2 Comparison of binding af nities of pFTAA, hFTAA, and thiof avin T (ThT).
Dorsal root ganglion neurons cultured for 2 days from P301S tau transgenic mice were f xed for 10 minutes in 95% ethanol, rehydrated, and incubated for 30 minutes at the indicated concentrations of the respective compounds. Fluorescence intensity values from 15 neurons per dose were averaged and the results were submitted to a least square curve f tting analysis according to an equation that predicts a single, saturable binding site (lines, theory) from which half saturation constants of 142 (pFTAA), 600 (hFTAA), and 3,700 (ThT) nM were derived. hFTAA: Heptamer formyl thiophene acetic acid; pFTAA: pentameric form of formyl thiophene acetic acid.
Brelstaff J, Spillantini MG, Tolkovsky AM (2015) pFTAA: a high affinity oligothiophene probe that detects filamentous tau in vivo and in cultured neurons. Neural Regen Res 10(11):1746-1747.
杂志排行
中国神经再生研究(英文版)的其它文章
- The role of the Rho/ROCK signaling pathway in inhibiting axonal regeneration in the central nervous system
- Targeting brain microvascular endothelial cells: a therapeutic approach to neuroprotection against stroke
- Severe bilateral anterior cingulum injury in patients with mild traumatic brain injury
- Injury of corticoreticular pathway and corticospinal tract caused by ventriculoperitoneal shunting
- Susceptibility weighted imaging in the evaluation of hemorrhagic dif use axonal injury
- Mechanical properties of nerve roots and rami radiculares isolated from fresh pig spinal cords
