Genotype 3 is the predominant hepatitis C genotype in a multi-ethnic Asian population in Malaysia
2015-02-06
Kuala Lumpur, Malaysia
Genotype 3 is the predominant hepatitis C genotype in a multi-ethnic Asian population in Malaysia
Shiaw-Hooi Ho, Kee-Peng Ng, Harvinder Kaur and Khean-Lee Goh
Kuala Lumpur, Malaysia
BACKGROUND: Genotypes of hepatitis C virus (HCV) are distributed differently across the world. There is a paucity of such data in a multi-ethnic Asian population like Malaysia. The objectives of this study were to determine the distribution of HCV genotypes between major ethnic groups and to ascertain their association with basic demographic variables like age and gender.
METHODS: This was a cross-sectional prospective study conducted from September 2007 to September 2013. Consecutive patients who were detected to have anti-HCV antibodies in the University of Malaya Medical Centre were included and tested for the presence of HCV RNA using Roche Cobas Amplicor Analyzer and HCV genotype using Roche single Linear Array HCV Genotyping strip.
RESULTS: Five hundred and ninety-six subjects were found to have positive anti-HCV antibodies during this period of time. However, only 396 (66.4%) were HCV RNA positive and included in the fnal analysis. Our results showed that HCV genotype 3 was the predominant genotype with overall frequency of 61.9% followed by genotypes 1 (35.9%), 2 (1.8%) and 6 (0.5%). There was a slightly higher prevalence of HCV genotype 3 among the Malays when compared to the Chinese (P=0.043). No other statistical signifcant differences were observed in the distribution of HCV genotypes among the major ethnic groups. There was also no association between the predominant genotypes and basic demographic variables.
CONCLUSIONS: In a multi-ethnic Asian society in Malaysia, genotype 3 is the predominant genotype among all the majorethnic groups with genotype 1 as the second commonest genotype. Both genotypes 2 and 6 are uncommon. Neither genotype 4 nor 5 was detected. There is no identifcation of HCV genotype according to ethnic origin, age and gender.
(Hepatobiliary Pancreat Dis Int 2015;14:281-286)
Asian;genotype; hepatitis C; Malaysia; multi-ethnic
Introduction
Hepatitis C infection is a global disease. The pathogen, hepatitis C virus (HCV), with its vast genetic diversity, shows distinct genotypic distribution according to geographical region. About 60%-80% of acute HCV infections fail to resolve leading to chronic hepatitis, frequently progressing to cirrhosis and hepatocellular carcinoma (HCC).[1]It has been estimated that 20% of patients with chronic hepatitis C progress to cirrhosis within 10 to 20 years and in this group of cirrhotic patients, 1% to 4% develop HCC per year.[1,2]In our local setting, Qua and Goh reported that up to 18.5% of patients with cirrhosis were attributable to chronic hepatitis C.[3]In Western countries and Japan, hepatitis C is known as the most common cause of cirrhosis and liver cancer.[4-6]So, being a common cause of chronic liver disease worldwide, it is not surprising that HCV infection accounts for most of patients with HCC and liver transplantation.
Fortunately, hepatitis C infection can be effectively treated with pegylated interferon and oral ribavirin combination which was frst approved for use by FDA in 2001. However, its effcacy varies considerably between different genotypes of the virus, which is now considered the strongest predictive factor for sustained viral response (SVR) after treatment.[7-9]Other important predictive factors are host factors such as IL28B allele, liverfbrosis stage, and age of the patient, as well as viral factors such as baseline viral load.[7-11]
Genotypes are major genetic groups in HCV defned by phylogenetic analysis with a nucleotide sequence similarity of 65.7% to 68.9% in the non-structural 5 (NS5) coding region and are numbered in Arabic numeral according to the order of their discovery. Subtypes are closely matched HCV strains within genotype with a nucleotide similarity of 76.9% to 80.1% in the NS5 coding region and are given lower case letters according to the order of their discovery.[12,13]Based on the consensus proposal for HCV classifcation in 2005, all HCV, including the newly detected variants, are categorized into six genotypes (1 to 6) and numerous subtypes as defned by phylogenetic analysis. This consensus ensured consistency in nomenclature and provided solid framework for future studies.[14]Since then, data set on HCV sequences has greatly expanded and over 1300 near-complete genome sequences were obtained by May 2013. Latest analysis from these sequences indicated the presence of 7 genotypes and 67 confrmed subtypes and this was just recently reported by Smith et al in 2014.[15]
Genotypes 2 and 3 are generally considered favorable response genotypes with SVR rates of over 70% while genotypes 1, 4, 5 and 6 generally respond much more poorly and require a longer duration of treatment.[16-19]This variation in treatment response appears to be consistent over different populations and geographical regions. Hence, accurate genotyping is important in the prediction of treatment response and in determining the duration of treatment. We were not able to further subtype the HCV becasue of the availability of only the commercial hybridization method in our hospital. Furthermore, classifying HCV at the genotype level was proven to be suffcient for prognostication and treatment planning in our local setting. However, with emerging new treatment for HCV infection, subtyping may be necessary in the future to ascertain the genetic factors that contribute to drug resistance in different subtypes.[20]
Lack of treatment response in genotype 1 was addressed with the introduction of second-generation NS3/ 4A serine protease inhibitors, NS5A replication complex inhibitors and NS5B RNA-dependent RNA polymerase inhibitors. Despite the shorter course of treatment (12 weeks) and better side-effect profle, these new combinations of oral direct antiviral agents (DAA) improve SVR tremendously. It is thus said that treatment of hepatitis C is entering an exciting new era, an era with interferonfree regimens which have long been the holy grail of hepatitis C treatment.[21]And for the frst time, treatment response for genotype 1 seems to be better than that of genotype 3.[22,23]
Recognizing the importance of HCV genotype as well as a paucity of data on HCV genotypes in Malaysia, we performed this study to determine the distribution of HCV genotypes among patients seen for hepatitis C in our hospital and to look for any differences between different ethnic groups, older age group and gender.
Methods
The study protocol was approved by the University of Malaya Medical Centre (UMMC) Ethics Committee. Consecutive patients who had been detected to have anti-HCV antibodies from September 2007 to September 2013 in UMMC were recruited in the study. These patients comprised those who came to the hospital for primary consultation or who were referred from community doctors or asymptomatic blood donors from the catchment area that it serves. UMMC is a major general hospital serving a large stable suburban population of approximately 2 million in Petaling Jaya which is adjacent to the capital city of Kuala Lumpur.
During this period of time, 596 subjects were detected to have positive anti-HCV antibodies and were subjected for further analysis. Using the concept of reverse transcription and amplifcation, a Cobas Amplicor Analyzer (Roche Diagnostic) was used to detect HCV RNA from the extracted samples.
In this study, 396 subjects were detected to have positive qualitative results for HCV RNA and were subjected to HCV genotyping using single Linear Array HCV Genotyping strip (Roche Diagnostic). In this process, denatured amplicons were hybridized to the genotype specifc oligonucleotide probe. With the addition of streptavidin-horseradish peroxidase conjugate and 3, 3', 5, 5'-tetramethylbenzidine, oxidation process occurred after several washes and resulted in the formation of bluecolored complex which precipitated at the probe position where hybridization occurred. The pattern of blue bands on the strip was then read visually and the genotyping was achieved by comparing to a reference table of known genotype pattern.
The results of the HCV genotyping were then tabulated into SPSS version 16.0 and baseline characteristics were analyzed. The relationship between ethnic groups and HCV genotypes was assessed using two by two tables to generate risk andPvalue. For each genotype, interracial risk (i.e. between Malay and Chinese, Malay and Indian & Chinese and Indian) was assessed to ascertain racial predilection in certain genotype. Similar analysis was carried out to assess the association between major genotypes (i.e. genotypes 1 and 3) and basic demographic variables such as older age group (i.e. age equalor more than 50-year-old) and gender.Pvalues were generated using the Chi-square test when the number of subjects involved was more than 5, and when the number was less than 5, Fisher's exact test was used. APvalue <0.05 was considered statistically signifcant.
Results
A total of 396 subjects were found to have HCV RNA and were further analyzed for genotype. In these subjects, 268 were males and 128 females; Malay and Chinese subjects each made up 42.4% (168) followed by Indian, 15.2% (60). Totally 198 (50.0%) of the subjects were equal or more than 50 years old, 176 (44.4%) were between 30 to 49 years old, and 22 (5.6%) were less than 30 years old. The baseline characteristics of the subjects are summarized in Table 1.
Four HCV genotypes namely genotypes 1, 2, 3 and 6 were detected among different ethnic groups (Table 2). HCV genotype 3 accounted for 61.9% (245) of the study population. This genotype was followed by genotype 1, 35.9% (142), genotype 2, 1.8% (7) and genotype 6, 0.5% (2). HCV genotype 3 was predominant among the major ethnic groups. It was seen in up to 67.9% in the Malays, 57.1% in the Chinese and 58.3% in the Indians. The proportion of patients with genotype 3 in all 3 ethnic groupswas signifcantly higher than that of patients with other genotypes.
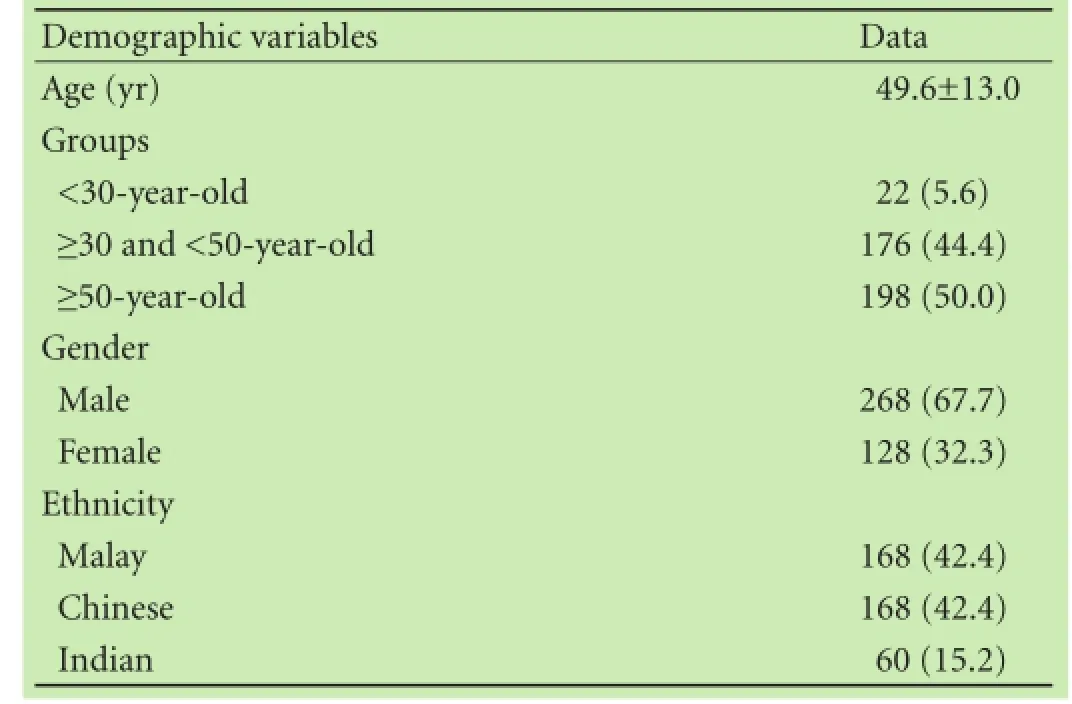
Table 1.Demographic and baseline characteristics (n, %)
Genotype 1 was the second commonest genotype in this region with a frequency of 31.5% in the Malays, 38.7% in the Chinese and 40.0% in the Indians. Genotype 2 was seen in only 7 subjects, 5 of whom were Chinese (71.4%), 1 (14.3%) Malay and 1 (14.3%) Indian. Genotype 6 was the least prevalent genotype here and was seen in only 2 Chinese subjects.
The difference in proportion of patients with genotype 3 was signifcantly higher in the Malays compared with the Chinese (P=0.043), but there was no difference between the Malays with the Indians and the Chinese with the Indians. Other two by two analyses did not yield any statistical signifcant differences in the distribution of other HCV genotypes among the major ethnic groups (Table 2). Predominant genotypes i.e. genotypes 1 and 3 also did not show any association with demographic variables such as older age (age equal or more than 50-yearold) and gender (Table 3). Genotypes 2 and 6 were not analyzed because of small sample size.
Discussion
The pattern of distribution of HCV genotypes varies throughout the world. Genotypes 1, 2 and 3 are widely spread all over the world.[24]One population-based study[25]in the United States identifed genotype 1a as the predominant genotype which was seen in 56.7% of those with positive HCV RNA; whereas genotype 1b in 17%, 2a in 3.5%, 2b in 11.4%, 3a in 7.4%, 4 in 0.9% and 6 in 3.2%.HCV genotype 1 is also common in Australia, McCaw et al[26]reported that the 55% of HCV isolates were genotype 1, 38% were genotype 3 with only 7% contributed by genotype 2. In Europe, HCV genotypes 1b and 2 are the predominant subtypes, this is followed by genotypes 3a, 1a, 4 and 5.[27]In the Middle East, 2 main patterns of HCV genotype distributions are identifed. The frst pattern is seen in most of the Arab countries (Egypt, Gaza, Jordan, Kuwait, Lebanon, Saudi Arabia and Syria) with genotype 4 being the predominant genotype. The second pattern is seen in other non-Arab countries (Iran, Israel and Turkey) in the Middle East where genotypes 1a and 1b are most frequently seen.[24,28-32]In South Africa, genotype 5 is still the predominant genotype. Gededzha et al[33]reported recently that genotype 5 was seen in 54% of the isolate, genotype 1 in 19%, genotype 4 in 19% and genotype 3 in 2%. There was also multi-genotypes coinfection seen in the remaining 5%.
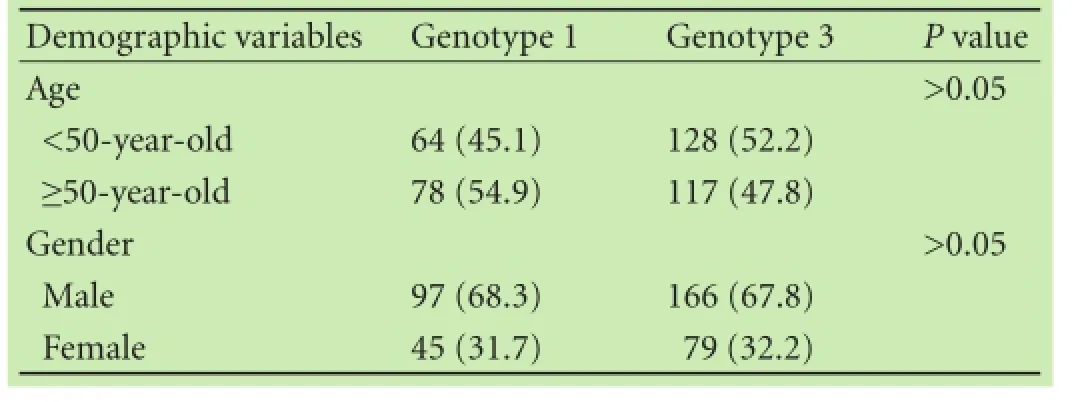
Table 3.Analysis on association of basic demographic variables with major predominant HCV genotypes (n, %)
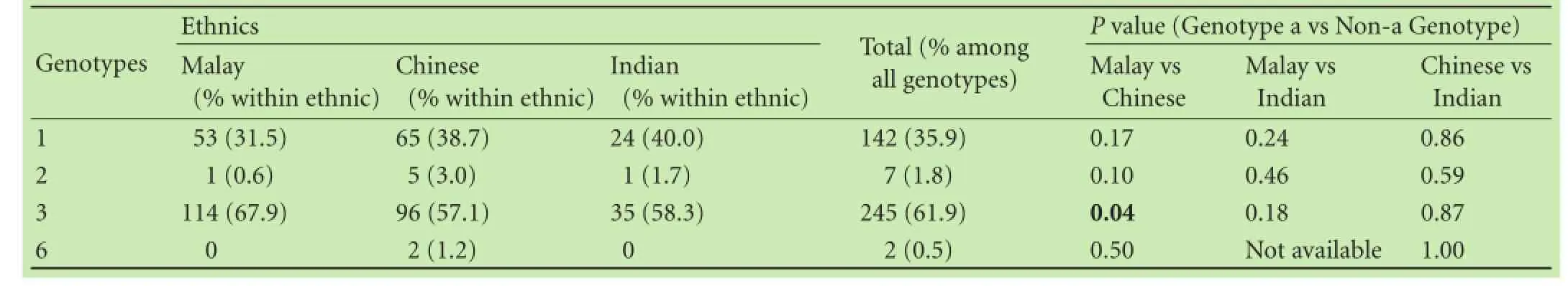
Table 2.Distribution of HCV genotypes among major ethnic groups in Malaysia (n, %)
HCV genotype 3, being the commonest genotype in India, is generally found across the different regions in India although genotype 1 is also found in high frequency in individuals from south India, followed closely behind genotype 3.[34-36]In Hong Kong, Leung et al[37]reported in 2006 a prevalence of 27% of genotype 6a among blood donors and this was second only to genotype 1b (58.8%). However, in most areas of China, subtype 1b surpasses the others to be the most prevalent subtype followed by subtype 2a. In the southern region of Pearl River Delta (including Foshan, Guangzhou, Macau, etc.), subtype 6a is the second most prevalent genotype instead of 2a. But in the southwest China (Kunming), subtypes 3b and 6a are noted to be second and third most common subtypes respectively following subtype 1b.[38,39]
An early survey[40]conducted in South Korea and a few other South East Asian countries namely Singapore, Thailand, Indonesia and Philippines revealed a prevalence of genotype 1 of 71%, genotype 3, 19% and genotype 2, 9% in 1995. Further analysis showed that, overall, subtype 1b was more common with a prevalence of 58% compared with subtype 1a at only 13%. However, subtype 1a which was the main HCV subtype in the United States was also the predominant subtype in Philippines where it was seen in up to 54.5% and 1b was only 36.4%. This pattern of subtype 1a dominancy was not seen in Indonesia, Thailand, Singapore or South Korea, where the predominant genotype 1 subtype was 1b with no reported 1a subtype. On the other hand, subtype 3a was the predominant subtype in Thailand and was seen in up to 45.5% of the subjects. However, in this study, serum samples of only 67 subjects were pooled from Singapore (11 subjects), Thailand (22), Indonesia (13), Philippines (11) and South Korea (10) but none from Malaysia. Owing to the small sample size from each country, the data were prone to sampling error and bias and can be considered unrealistic and not representative of the actual situation in those countries.[40]Greene's fndings were in consistent with those of Ng et al who reported in the same year that genotype 1 was the commonest genotype in Singapore and Indonesia.[41]More recently, core sequence analysis revealed that genotype 1b was the predominant subtype (47.3%) in Indonesia followed by subtypes 1c (18.7%), 3k (10.7%), 2a (10.0%), 1a (6.7%), 2e (5.3%), 3a (0.7%) and 2f (0.7%).[42]Similar to Greene's report, genotype 3a was also the most predominant genotype across different regions in Thailand in various other studies. It was seen in up to 38.5% in one study[43]and 51.1% in another HCV positive populationbased study.[44]
In Malaysia, Duraisamy et al[45]and Ng et al[46]reported an anti-HCV antibodies prevalence of 1.5% and 1.9% respectively among blood donors in mid-1990s. However, this sero-prevalence rate was signifcantly decreased over the last 1 to 2 decades and was reported in 2012 to be as low as 0.14% among blood donors in the north-eastern population in Malaysia.[47]In another earlier multi-center study which involved Malaysia as one of the participating countries, Davidson et al[48]documented, in Malaysia, a prevalence of genotype 1 of 64% and genotype 3, 36%. However, the number of the Malaysian subjects in this study was very small, i.e. 25 persons.
We acknowledge that our study does not necessarily refect the actual distribution of hepatitis C in the population. However, it does refect accurately the pattern of genotype in patients seeking treatment in a major general hospital in the country. Our hospital which is located adjacent to the capital city of Kuala Lumpur serves a large suburban population and has a large catchment area of approximately 2 million people. This is perhaps more important as the genotype pattern infuences key treatment decisions.
Owing to the epidemiology and nature of transmission of the infection, where hepatitis C may be confned to a community or ethnic group, we also wanted to explore if there were any particular distribution of genotype between races in our local population. Although a multi-ethnic country for more than 2 generations now, there have been little inter-ethnic marriages and the races in general remain distinct in Malaysia. A previous study involving genotypic distribution in Malaysia was not representative of local situation due to very small sample size. Our study is the frst large scale study on hepatitis C genotypes.
In conclusion, genotype 3 was the predominant genotype followed by genotype 1 among all the major ethnicgroups in Malaysia. Both genotypes 2 and 6 were uncommon genotypes. Neither genotype 4 nor 5 was seen in this study. This fnding is of particular importance since genotype 3 confers better treatment response with a shorter duration of standard treatment with pegylated interferon and ribavirin. However, genotype 3 is not better than genotype 1 in terms of treatment response in the era of interferon-free regimen. Apart from the slightly higher prevalence of genotype 3 in the Malays compared with the Chinese, no other signifcant differences were observed in the distribution of genotypes according to ethnic origin, age and gender in this study.
Acknowledgments:We would like to thank Ms. Shirley Liew from the Department of Microbiology for her assistance in data collection.
Contributors:NKP and GKL proposed the study. HSH and KH performed the data collection and analysis. HSH wrote the frst draft. All authors contributed to the design and interpretation of the data and drafting of this manuscript. HSH is the guarantor.
Funding:This study was supported by University of Malaya High Impact Research grant (UM.C/625/1/HIR/MOHE/MED/31).
Ethical approval:The study protocol was approved by the University of Malaya Medical Centre Ethics Committee.
Competing interest:No benefts in any form have been or will be received from a commercial party related directly or indirectly to the subject of this article.
1 Giannini C, Bréchot C. Hepatitis C virus biology. Cell Death Differ 2003;10:S27-38.
2 Lauer GM, Walker BD. Hepatitis C virus infection. N Engl J Med 2001;345:41-52.
3 Qua CS, Goh KL. Liver cirrhosis in Malaysia: peculiar epidemiology in a multiracial Asian country. J Gastroenterol Hepatol 2011;26:1333-1337.
4 Di Bisceglie AM. Natural history of hepatitis C: its impact on clinical management. Hepatology 2000;31:1014-1018.
5 Grant BF, Dawson DA, Stinson FS, Chou SP, Dufour MC, Pickering RP. The 12-month prevalence and trends in DSM-IV alcohol abuse and dependence: United States, 1991-1992 and 2001-2002. Drug Alcohol Depend 2004;74:223-234.
6 Michitaka K, Nishiguchi S, Aoyagi Y, Hiasa Y, Tokumoto Y, Onji M, et al. Etiology of liver cirrhosis in Japan: a nationwide survey. J Gastroenterol 2010;45:86-94.
7 Jacobson IM, Brown RS Jr, Freilich B, Afdhal N, Kwo PY, Santoro J, et al. Peginterferon alfa-2b and weight-based or fatdose ribavirin in chronic hepatitis C patients: a randomized trial. Hepatology 2007;46:971-981.
8 Manns MP, McHutchison JG, Gordon SC, Rustgi VK, Shiffman M, Reindollar R, et al. Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for initial treatment of chronic hepatitis C: a randomised trial. Lancet 2001;358:958-965.
9 Poynard T, McHutchison J, Goodman Z, Ling MH, Albrecht J. Is an “a la carte” combination interferon alfa-2b plus ribavirin regimen possible for the frst line treatment in patients with chronic hepatitis C? The ALGOVIRC Project Group. Hepatology 2000;31:211-218.
10 O’Brien TR, Everhart JE, Morgan TR, Lok AS, Chung RT, Shao Y, et al. An IL28B genotype-based clinical prediction model for treatment of chronic hepatitis C. PLoS One 2011;6:e20904.
11 Zeuzem S, Buti M, Ferenci P, Sperl J, Horsmans Y, Cianciara J, et al. Effcacy of 24 weeks treatment with peginterferon alfa-2b plus ribavirin in patients with chronic hepatitis C infected with genotype 1 and low pretreatment viremia. J Hepatol 2006;44:97-103.
12 Simmonds P, Holmes EC, Cha TA, Chan SW, McOmish F, Irvine B, et al. Classifcation of hepatitis C virus into six major genotypes and a series of subtypes by phylogenetic analysis of the NS-5 region. J Gen Virol 1993;74:2391-2399.
13 Zein NN. Clinical signifcance of hepatitis C virus genotypes. Clin Microbiol Rev 2000;13:223-235.
14 Simmonds P, Bukh J, Combet C, Deléage G, Enomoto N, Feinstone S, et al. Consensus proposals for a unifed system of nomenclature of hepatitis C virus genotypes. Hepatology 2005;42:962-973.
15 Smith DB, Bukh J, Kuiken C, Muerhoff AS, Rice CM, Stapleton JT, et al. Expanded classifcation of hepatitis C virus into 7 genotypes and 67 subtypes: updated criteria and genotype assignment web resource. Hepatology 2014;59:318-327.
16 Kim KT, Han SY, Kim JH, Yoon HA, Baek YH, Kim MJ, et al. Clinical outcome of pegylated interferon and ribavirin therapy for chronic hepatitis C. Korean J Hepatol 2008;14:36-45.
17 Lee H, Choi MS, Paik SW, Kim JH, Kim DY, Lee JH, et al. Peginterferon alfa-2a plus ribavirin for initial treatment of chronic hepatitis C in Korea. Korean J Hepatol 2006;12:31-40.
18 Lee HJ, Eun JR, Choi JW, Kim KO, Moon HJ. Comparison of therapeutic results between combination therapy of peginterferon alpha-2a plus ribavirin and interferon alpha-2b plus ribavirin according to treatment duration in patients with chronic hepatitis C. Korean J Hepatol 2008;14:46-57.
19 Liu CJ, Chuang WL, Lee CM, Yu ML, Lu SN, Wu SS, et al. Peginterferon alfa-2a plus ribavirin for the treatment of dual chronic infection with hepatitis B and C viruses. Gastroenterology 2009;136:496-504.e3.
20 Chevaliez S, Bouvier-Alias M, Brillet R, Pawlotsky JM. Hepatitis C virus (HCV) genotype 1 subtype identifcation in new HCV drug development and future clinical practice. PLoS One 2009;4:e8209.
21 Cartwright EJ, Miller L. Novel drugs in the management of diffcult-to-treat hepatitis C genotypes. Hepat Med 2013;5:53-61.
22 Jacobson IM, Gordon SC, Kowdley KV, Yoshida EM, Rodriguez-Torres M, Sulkowski MS, et al. Sofosbuvir for hepatitis C genotype 2 or 3 in patients without treatment options. N Engl J Med 2013;368:1867-1877.
23 Lawitz E, Mangia A, Wyles D, Rodriguez-Torres M, Hassanein T, Gordon SC, et al. Sofosbuvir for previously untreated chronic hepatitis C infection. N Engl J Med 2013;368:1878-1887.
24 Zarkesh-Esfahani SH, Kardi MT, Edalati M. Hepatitis C virus genotype frequency in Isfahan province of Iran: a descriptive cross-sectional study. Virol J 2010;7:69.
25 Alter MJ, Kruszon-Moran D, Nainan OV, McQuillan GM, Gao F, Moyer LA, et al. The prevalence of hepatitis C virus infection in the United States, 1988 through 1994. N Engl J Med 1999;341:556-562.
26 McCaw R, Moaven L, Locarnini SA, Bowden DS. Hepatitis C virus genotypes in Australia. J Viral Hepat 1997;4:351-357.
27 Fattovich G, Ribero ML, Pantalena M, Diodati G, Almasio P, Nevens F, et al. Hepatitis C virus genotypes: distribution and clinical signifcance in patients with cirrhosis type C seen at tertiary referral centres in Europe. J Viral Hepat 2001;8:206-216.
28 Ramia S, Eid-Fares J. Distribution of hepatitis C virus genotypes in the Middle East. Int J Infect Dis 2006;10:272-277.
29 Fallahian F, Najaf A. Epidemiology of hepatitis C in the Middle East. Saudi J Kidney Dis Transpl 2011;22:1-9.
30 Samimi-Rad K, Nategh R, Malekzadeh R, Norder H, Magnius L. Molecular epidemiology of hepatitis C virus in Iran as refected by phylogenetic analysis of the NS5B region. J Med Virol 2004;74:246-252.
31 Osoba AO, Ibrahim M, Abdelaal MA, Al-Mowallad A, Al Shareef B, Hussein BA. Hepatitis C virus genotyping by polymerase chain reaction and DNA enzyme immunoassay among Saudi patients in the Western Province, Saudi Arabia. Ann Saudi Med 2000;20:394-397.
32 Al Ashgar HI, Khan MQ, Al-Ahdal M, Al Thawadi S, Helmy AS, Al Qahtani A, et al. Hepatitis C genotype 4: genotypic diversity, epidemiological profle, and clinical relevance of subtypes in Saudi Arabia. Saudi J Gastroenterol 2013;19:28-33.
33 Gededzha MP, Selabe SG, Kyaw T, Rakgole JN, Blackard JT, Mphahlele MJ. Introduction of new subtypes and variants of hepatitis C virus genotype 4 in South Africa. J Med Virol 2012;84:601-607.
34 Chowdhury A, Santra A, Chaudhuri S, Dhali GK, Chaudhuri S, Maity SG, et al. Hepatitis C virus infection in the general population: a community-based study in West Bengal, India. Hepatology. 2003 Apr;37:802-809.
35 Raghuraman S, Shaji RV, Sridharan G, Radhakrishnan S, Chandy G, Ramakrishna BS, et al. Distribution of the different genotypes of HCV among patients attending a tertiary care hospital in south India. J Clin Virol 2003;26:61-69.
36 Verma V, Chakravarti A, Kar P. Genotypic characterization of hepatitis C virus and its signifcance in patients with chronic liver disease from Northern India. Diagn Microbiol Infect Dis 2008;61:408-414.
37 Leung N, Chu C, Tam JS. Viral hepatitis C in Hong Kong. Intervirology 2006;49:23-27.
38 Lu L, Nakano T, He Y, Fu Y, Hagedorn CH, Robertson BH. Hepatitis C virus genotype distribution in China: predominance of closely related subtype 1b isolates and existence of new genotype 6 variants. J Med Virol 2005;75:538-549.
39 Yan Z, Fan K, Wang Y, Fan Y, Tan Z, Deng G. Changing pattern of clinical epidemiology on hepatitis C virus infection in southwest china. Hepat Mon 2012;12:196-204.
40 Greene WK, Cheong MK, Ng V, Yap KW. Prevalence of hepatitis C virus sequence variants in South-East Asia. J Gen Virol 1995;76:211-215.
41 Ng WC, Guan R, Tan MF, Seet BL, Lim CA, Ngiam CM, et al. Hepatitis C virus genotypes in Singapore and Indonesia. J Viral Hepat 1995;2:203-209.
42 Utama A, Tania NP, Dhenni R, Gani RA, Hasan I, Sanityoso A, et al. Genotype diversity of hepatitis C virus (HCV) in HCV-associated liver disease patients in Indonesia. Liver Int 2010;30:1152-1160.
43 Akkarathamrongsin S, Hacharoen P, Tangkijvanich P, Theamboonlers A, Tanaka Y, Mizokami M, et al. Molecular epidemiology and genetic history of hepatitis C virus subtype 3a infection in Thailand. Intervirology 2013;56:284-294.
44 Sunanchaikarn S, Theamboonlers A, Chongsrisawat V, Yoocharoen P, Tharmaphornpilas P, Warinsathien P, et al. Seroepidemiology and genotypes of hepatitis C virus in Thailand. Asian Pac J Allergy Immunol 2007;25:175-182.
45 Duraisamy G, Zuridah H, Ariffn MY. Prevalence of hepatitis C virus antibodies in blood donors in Malaysia. Med J Malaysia 1993;48:313-316.
46 Ng KP, Saw TL, Wong NW, Goh KL, Chuah SY, Nagaratnam M. The prevalence of anti-HCV antibody in risk groups and blood donors. Med J Malaysia 1995;50:302-305.
47 Haslina MN, Khairiah Y, Zainy DZ, Shafni MY, Rosnah B, Marini R. Seroprevalence of hepatitis C virus infection among blood donors in a teaching hospital in northeastern Malaysia. Southeast Asian J Trop Med Public Health 2012;43:668-673.
48 Davidson F, Simmonds P, Ferguson JC, Jarvis LM, Dow BC, Follett EA, et al. Survey of major genotypes and subtypes of hepatitis C virus using RFLP of sequences amplifed from the 5’non-coding region. J Gen Virol 1995;76:1197-1204.
Received August 19, 2014
Accepted after revision January 9, 2015
AuthorAffliations:Gastroenterology and Hepatology Unit, Department of Internal Medicine (Ho SH and Goh KL); and Department of Medical Microbiology (Ng KP and Kaur H), University of Malaya, Kuala Lumpur 50603, Malaysia
Shiaw-Hooi Ho, MD, Department of Medicine, Faculty of Medicine, University of Malaya, Kuala Lumpur 50603, Malaysia (Tel: +60379492965, +60379492299; Fax: +60379604190; Email: shooiho@ yahoo.com)
© 2015, Hepatobiliary Pancreat Dis Int. All rights reserved.
10.1016/S1499-3872(15)60363-0
Published online May 6, 2015.
杂志排行
Hepatobiliary & Pancreatic Diseases International的其它文章
- Combined right hemicolectomy and pancreaticoduodenectomy for locally advanced right hemicolon cancer
- Histological examination of frozen sections for patients with acute cholecystitis during cholecystectomy
- Omental faps reduces complications after pancreaticoduodenectomy
- Endoscopic ultrasound-guided fne-needle aspiration cytology in pancreaticobiliary carcinomas: diagnostic effcacy of cell-block immunocytochemistry
- Fast magnetic reconstruction of the portal vein with allogeneic blood vessels in canines
- miR-215 overexpression distinguishes ampullary carcinomas from pancreatic carcinomas
