miR-215 overexpression distinguishes ampullary carcinomas from pancreatic carcinomas
2015-02-06
Seoul, Korea
miR-215 overexpression distinguishes ampullary carcinomas from pancreatic carcinomas
Dong Ho Choi, Sang Jae Park and Hark Kyun Kim
Seoul, Korea
Distinguishing ampullary carcinoma from pancreatic carcinoma is important because of their different prognoses. microRNAs are differentially expressed according to the tissue of origin. However, there is rare research on the differential diagnosis between the two types of cancers by microRNA in periampullary cancers. The present study was undertaken to compare microRNA profles between ampullary and pancreatic carcinomas using microarrays. miR-215 was most signifcantly overexpressed in ampullary carcinomas; whereas the expressions of miR-134 and miR-214 were signifcantly lower in ampullary carcinomas than in pancreatic carcinomas. When these discriminatory microRNAs were applied to liver metastases, they were correctly predicted for the tissue of origin. Although this study is limited by small sample size, striking difference in microRNA expression and concordant expression of discriminating microRNAs in primary tumors and metastases suggest that these novel discriminatory microRNAs warrant future validation.
ampulla of Vater; pancreatic cancer; gene expression
Introduction
Distinguishing between ampullary carcinomas and pancreatic cancers is important due to their different prognoses, but neither tumor types have tissuespecifc immunohistochemistry markers.[1,2]Therefore, it is crucial to identify novel tissue-specifc biomarkers that differentiate periampullary cancers. microRNAs are important in tissue development, and they can be used to classify tumors according to tissue origin.[3]However, only a few studies using microRNAs as a tool to differentiate periampullary cancers. Schultz et al[4]compared 664 microRNAs expressions in benign lesions and ampullary and pancreatic carcinomas. However, the aforementioned study focused on microRNAs distinguishing between periampullary cancers and benign lesions, not on tissue-specifc microRNAs, partly because ampullary and pancreatic carcinomas were found to be similar in microRNA profles.[4]The current study investigated microRNAs differentially expressed between the two tumor types using a more comprehensive microRNA microarray approach and frozen tissue samples of Korean ampullary and pancreatic carcinomas. As a result, we report a novel set of microRNAs differentially expressed in ampullary and pancreatic carcinomas, which was validated in liver metastases in the proof-of-concept validation set.
Methods
microRNA microarray
Samples were collected at the time of surgery from cancer patients. Clinical diagnosis of all study samples (ampullary vs pancreatic carcinomas) was unequivocal based on the location of the epicenter of primary tumors and consistent histopathologic fndings. Specimens were collected with the approval of the Institutional Review Board (IRB) and all patients gave informed consent, signing IRB-approved forms. A 10-µm thick top slide was stained with hematoxylin and eosin. Guided by this top slide, the remaining tissue was macro-dissected to trim non-tumorous stromal components so that tumor-rich (>50%) area may be subjected to RNA isolation. Macrodissected, frozen tissue sample was mechanically crushed in liquid nitrogen, homogenized, and subjected to RNA isolation using TRI reagent (Invitrogen, Carlsbad, CA,USA), according to the manufacturer's instructions. Total RNA was then subjected to DNAse I treatment. Subsequently, 500 ng RNA was poly-A tailed, and the FlashTag Biotin HSR Labeling Kit (Genisphere LLC, Hatfeld, PA, USA) was used to join a proprietary biotin-labeled dendrimer molecule to the 3′ end using DNA ligase. Labeled samples were hybridized to GeneChip miRNA 2.0 microarrays (Affymetrix, Santa Clara, CA, USA) at 48 ℃ for 16 hours, washed, stained with a streptavidin-PE solution, and scanned. GeneChip miRNA 2.0 microarrays were based on miRbase version 15 and contained 15644 mature microRNA probe sets of 131 organisms including 1100 human microRNAs. All cell fles were robust multiarray average (RMA)-normalized. Only human microRNAs were subjected to further analyses for this study.
Validation microRNA quantitative RT-PCR (qRT-PCR)
The cycle threshold (CT) value of each microRNA was normalized to GAPDH by subtraction. DNAse I (QIAGEN, Valencia, CA, USA)-treated total RNA (500 ng) was used for reverse transcription, and 2.5% of the reverse transcription product was used for each qRTPCR reaction. The miScript PCR system (QIAGEN) was used for qRT-PCR, and reactions were performed in duplicate in 96-well plates, using a LightCycler 480 Instrument II (Roche, Basel, Switzerland).
Statistical analysis
BRB-ArrayTools software (version 4.1, NCI) was used to carry out unsupervised and supervised analyses. Principal component analyses (PCA) were performed using 1-correlation as the distance metric. To evaluate whether the sets had different microRNA profles, we analyzed class prediction using all samples as a training set. The crossvalidated misclassifcation rate was computed for all BRBArrayTools classifer functions [compound covariate predictor (CCP), linear discriminant analysis (LDA), 1-nearest neighbor (1-NN), 3-nearest neighbors (3-NN), nearest centroid (NC) and support vector machines (SVM)] in the training set. Then, class labels were randomly shuffed and the cross-validated misclassifcation rate was computed for each random dataset. The permutationPvalue, which is defned as the proportion of random datasets that give a small misclassifcation rate as that obtained with actual class labels, was then calculated. microRNA profles of the classes were considered signifcantly different if the permutationPvalue was less than 0.05.
Discriminatory microRNAs differentially expressed in primary periampullary cancers were applied to predict the class labels of two liver metastases. The best predictor, selected for having the lowest misclassifcation rate in the analysis of primary tumors in the training set, was applied to predict the tissue origin of two metastasis samples in the proof-of-concept validation set.
Results
Primary tumors used for the training set of this study were 4 ampullary carcinomas and 18 pancreatic carcinomas. Table 1 summarizes clinicopathological characteristics of the tissue samples. According to a PCA plot, ampullary carcinomas and pancreatic carcinomas clustered separately (Fig. 1A). Class prediction analysis indicatedthat the permutationPvalues for the misclassifcation rate (at feature selectionP<0.001) were <0.001, 0.001, <0.001, <0.001, <0.001 and 0.003, with CCP, LDA, 1-NN, 3-NN, NC and SVM, respectively. This suggests that the microRNA profles of ampullary and pancreatic carcinomas were different.
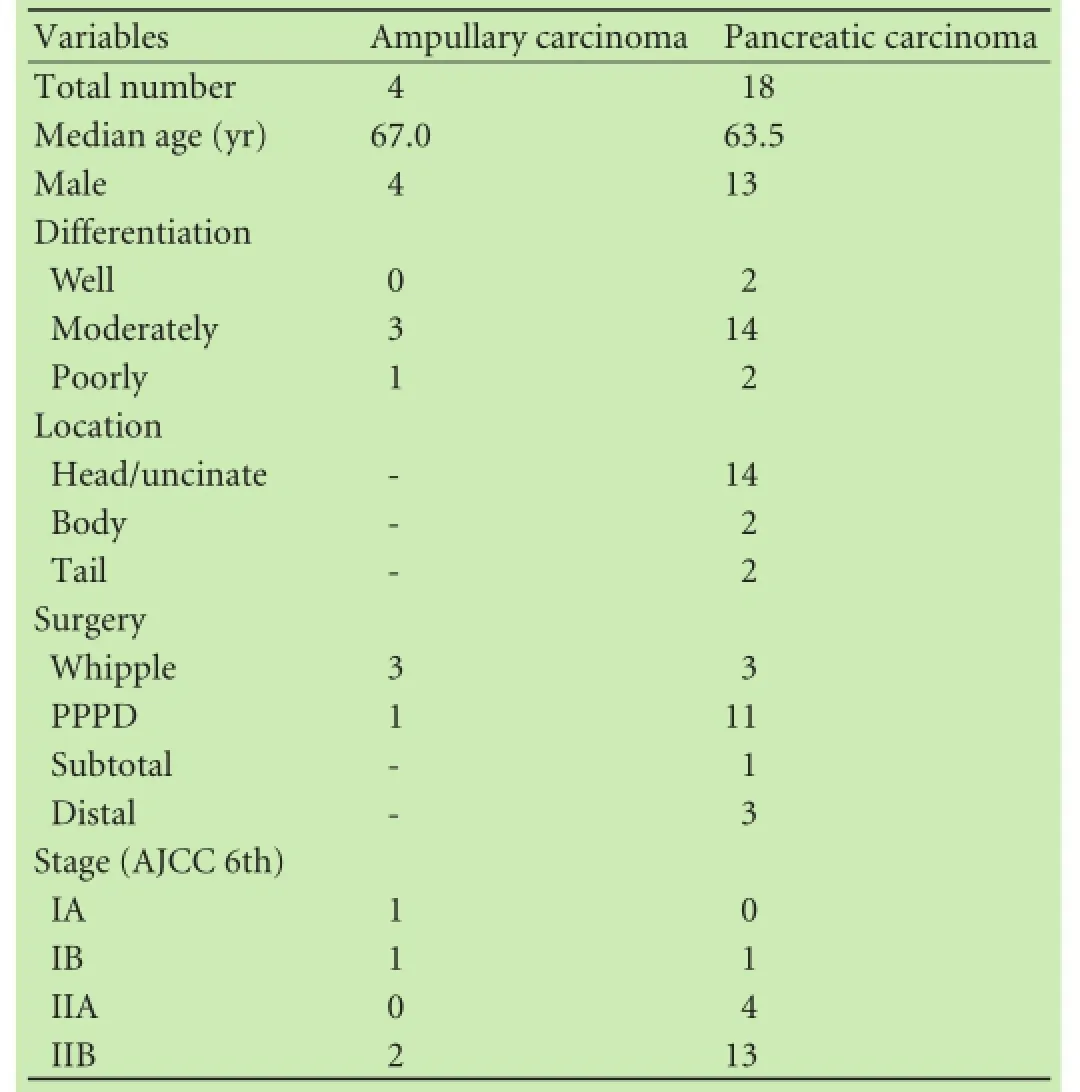
Table 1.Clinicopathologic characteristics of primary tumors
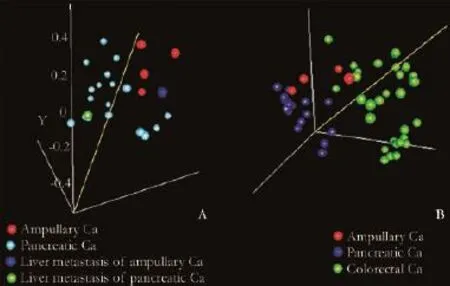
Fig. 1.Principal component analysis. Each sphere represents each sample. 1-correlation is used as the distance metric. Samples clustered according to the tissue of origin.
The CCP algorithm was found to be the best predictor for tissue of origin in the training set, and employed to predict the tissue origin of two liver metastases (an ampullary carcinoma and a pancreas carcinoma). When CCP and discriminatory microRNAs were applied to the metastatic lesions, the tissue of origin was predicted correctly in both cases. This result is consistent with a PCA plot showing that each of the liver metastases clustered with respective primary tumors of the same tissue of origin (Fig. 1A).
We then applied the CCP and discriminatory microRNAs to histologically-normal pancreatic tissues collected from three additional patients who underwent surgery. There were two females (66.7%) and the median age was 73.0 years (range 62-75). The tissue of origin of all these three samples was correctly predicted as the pancreas, by microRNA profles that were differentially expressed between 4 ampullary carcinomas and 18 pancreatic carcinomas.
Table 2 summarizes microRNAs differentially expressed between 4 ampullary carcinomas and 18 pancreatic carcinomas in the training set. miR-215 was most signifcantly overexpressed in ampullary carcinomas. In contrast to the overexpression of miR-215, the expression levels of miR-134 and miR-214 were signifcantly lower in ampullary carcinomas than in pancreatic carcinomas (Fig. 2). For these selected discriminatory microRNAs, microarray data were compared with qRT-PCR data (Fig. 3). The overexpression of miR-215 and underexpression of miR-134/miR-214 in ampullary vs pancreatic carcinomas were validated by qRT-PCR (Fig. 3). Pearson's product-moment correlation coeffcients between microarrayand qRT-PCR data were 0.58, 0.37 and 0.53, for miR-215, miR-134 and miR-214, respectively.
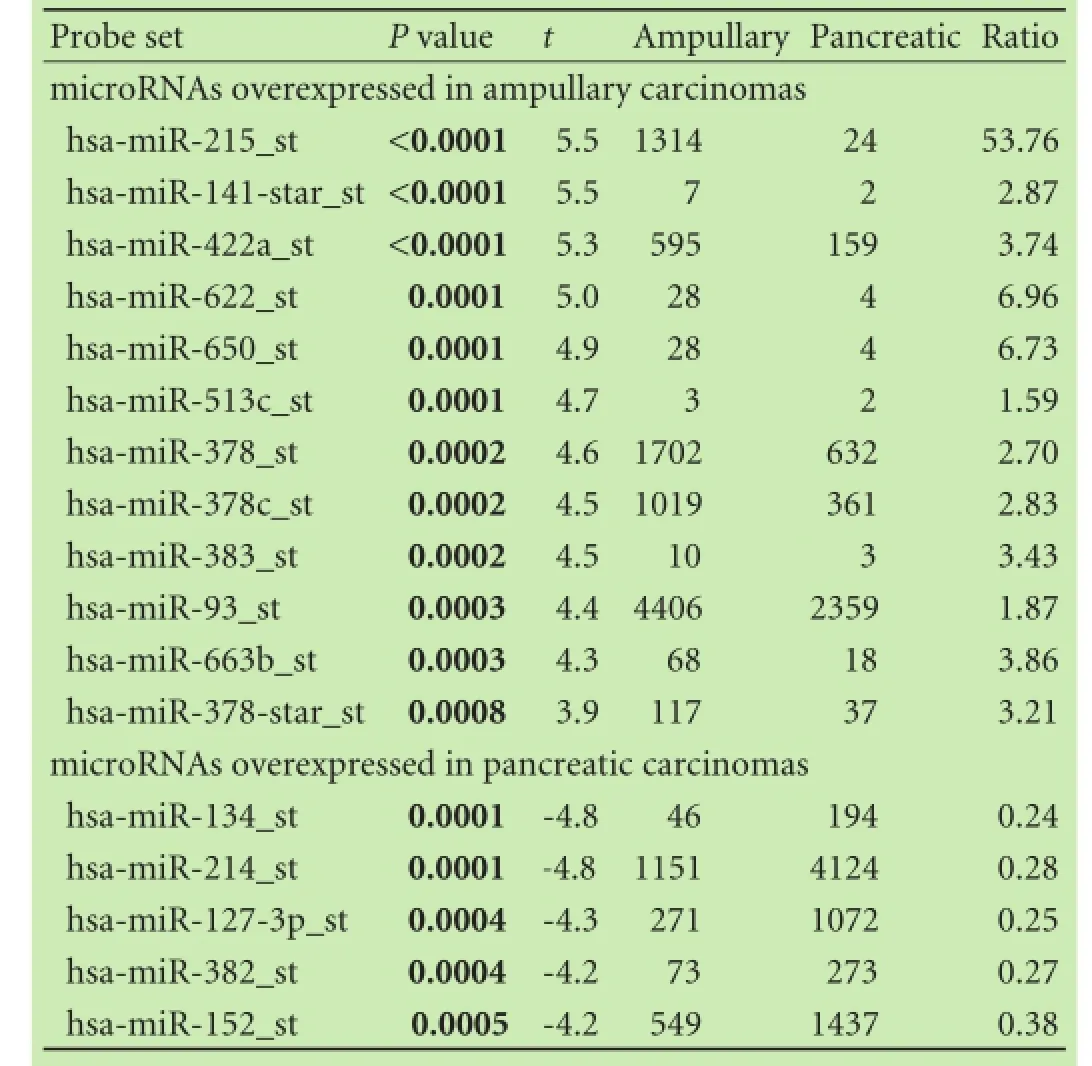
Table 2.microRNAs that are differentially expressed between ampullary carcinomas and pancreatic carcinomas
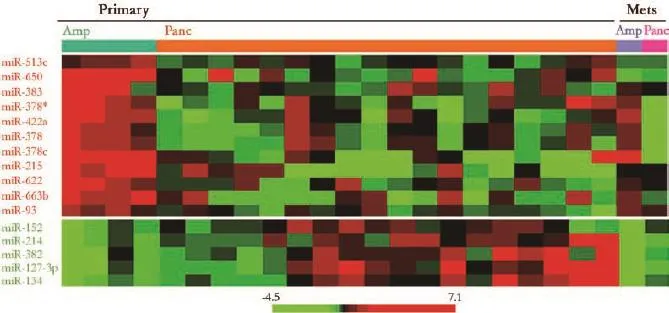
Fig. 2.Pattern of expression of microRNAs overexpressed in ampullary carcinomas (upper panel, shown in red) and microRNAs overexpressed in pancreatic carcinomas (lower panel, shown in green). Heatmap generated using a log2-pseudocolor image with microRNA centering. Red and green colors denote high and low expression of microRNA, respectively. Mets: metastases in the liver; Amp: ampullary carcinoma; Panc: pancreatic carcinoma.
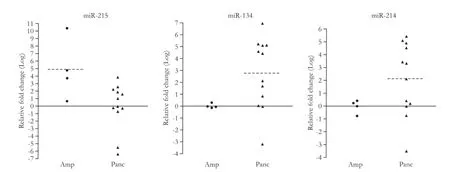
Fig. 3.qRT-PCR for miR-215, miR-134 and miR-214. The qRT-PCR expression value (Y-axis) represents -ΔΔCTtest to control. GAPDH was used as a reference. Dotted line represents the average qRT-PCR expression value. Amp: ampullary carcinoma; Panc: pancreatic carcinoma.
Discussion
This study demonstrated that microRNA profles of ampullary and pancreatic carcinomas were signifcantly different. We were also able to show that differentially expressed microRNAs predicted the tissue of origin of a small number of metastatic lesions, providing proof-ofconcept data for the robustness of the microRNA profles presented. To our knowledge, this study is the frst to report the overexpression of miR-215 in ampullary carcinomas. miR-215 is abundant in the gastrointestinal tract, and induces growth arrest and decreases cell proliferation in response to p53 and TGF-β.[5,6]Our own cancer tissue microRNA database also indicated that the expression of miR-215 was higher in the gastrointestinal tract than in the hepatobiliary system. We performed PCA analyses after 29 primary colorectal tumors in our cancer tissue microRNA database. According to the PCA analyses, colorectal tumors were more similar to our four ampullary carcinomas than to 18 pancreatic carcinomas (Fig. 1B). Thus, miR-215 overexpression in ampullary carcinoma may refect the global similarity in microRNA profles between colorectal and ampullary carcinomas. Notably, however, the median miR-215 expression level was even higher (9.7-fold) in 4 ampullary carcinomas than in 29 colorectal cancers of our tissue microRNA database, suggesting the possible unique role of miR-215 in the development of ampullary carcinomas.
In contrast to the overexpression of miR-215, the expression levels of miR-134 and miR-214 were signifcantly lower in ampullary carcinoma than in pancreatic carcinoma. Data in the literature suggested that these microRNAs are highly expressed in the pancreas.[7,8]miR-214 is important in the development of the pancreas,[7]and high levels of miR-134 have been reported in the serum and tumor tissue of patients with pancreatic carcinoma.[8]Differential expression of miR-215, miR-134 and miR-214 between ampullary and pancreatic carcinomas has not been previously reported. Schultz et al[4]did not identify any of these microRNAs as being differentially expressed between ampullary and pancreatic tissue. Their fndings may differ from ours due to differences in ethnicity, the cutoffPvalues used, the type of tissue samples studied (FFPE vs frozen), and the analytic platforms employed (qRT-PCR vs microarray).[4]Since no other microRNA microarray data for ampullary carcinoma are currently available in the literature, we await future studies to validate differential expression of these microRNAs. Distinguishing between ampullary carcinomas and pancreatic carcinomas is important due to their different prognoses. This study is limited by the fact that only two metastases were used to validate microRNAs differentially expressed between primary ampullary and pancreatic carcinomas, which should be regarded as descriptive results rather than a proof-of-concept validation set. Nonetheless, the striking differences in microRNA expression and the concordant expression of discriminating microRNAs in primary tumors and metastases suggest that these novel discriminatory microRNAs warrant future validation.
Contributors:CDH designed the study and provided samples. PSJ designed the study. KHK collected the data and wrote the frst draft. All authors contributed to the design and interpretation of the study and to further drafts. KHK is the guarantor.
Funding:The study was supported by grants from the Proteoge-nomic Research Program through the National Research Foundation of Korea; and the Converging Research Center Program (2013K000429) funded by the Korean Ministry of Science, ICT, and Future Planning.
Ethical approval:This study was approved by the Institutional Review Board.
Competing interest:No benefts in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
1 Fischer HP, Zhou H. Pathogenesis of carcinoma of the papilla of Vater. J Hepatobiliary Pancreat Surg 2004;11:301-309.
2 Morini S, Perrone G, Borzomati D, Vincenzi B, Rabitti C, Righi D, et al. Carcinoma of the ampulla of Vater: morphological and immunophenotypical classifcation predicts overall survival. Pancreas 2013;42:60-66.
3 Lu J, Getz G, Miska EA, Alvarez-Saavedra E, Lamb J, Peck D, et al. MicroRNA expression profles classify human cancers. Nature 2005;435:834-838.
4 Schultz NA, Werner J, Willenbrock H, Roslind A, Giese N, Horn T, et al. MicroRNA expression profles associated with pancreatic adenocarcinoma and ampullary adenocarcinoma. Mod Pathol 2012;25:1609-1622.
5 Davis BN, Hilyard AC, Nguyen PH, Lagna G, Hata A. Smad proteins bind a conserved RNA sequence to promote microRNA maturation by Drosha. Mol Cell 2010;39:373-384.
6 Braun CJ, Zhang X, Savelyeva I, Wolff S, Moll UM, Schepeler T, et al. p53-Responsive micrornas 192 and 215 are capable of inducing cell cycle arrest. Cancer Res 2008;68:10094-10104.
7 Joglekar MV, Parekh VS, Hardikar AA. New pancreas from old:microregulators of pancreas regeneration. Trends Endocrinol Metab 2007;18:393-400.
8 Li A, Yu J, Kim H, Wolfgang CL, Canto MI, Hruban RH, et al. MicroRNA array analysis fnds elevated serum miR-1290 accurately distinguishes patients with low-stage pancreatic cancer from healthy and disease controls. Clin Cancer Res 2013;19:3600-3610.
Received April 8, 2014
Accepted after revision November 5, 2014
(Hepatobiliary Pancreat Dis Int 2015;14:325-329)
AuthorAffliations:Department of Surgery, Hanyang University School of Medicine, 222 Wangsimni-ro, Seongdong, Seoul, 133-791, Korea (Choi DH); Center for Liver Cancer (Park SJ) and Biomolecular Function Research Branch (Kim HK), National Cancer Center, 323 Ilsan-ro, Goyang, Gyeonggi, 410-769, Korea
Hark Kyun Kim, MD, PhD, Biomolecular Function Research Branch, Research Institute, National Cancer Center, 323 Ilsanro, Goyang, Gyeonggi, 410-769, Republic of Korea (Tel: +82-31-9202238; Fax: +82-31-9202006; Email: hkim@ncc.re.kr)
© 2015, Hepatobiliary Pancreat Dis Int. All rights reserved.
10.1016/S1499-3872(15)60368-X
Published online May 6, 2015.
杂志排行
Hepatobiliary & Pancreatic Diseases International的其它文章
- Meetings and Courses
- Adjuvant chemotherapy after liver transplantation for hepatocellular carcinoma
- Letters to the Editor
- Combined right hemicolectomy and pancreaticoduodenectomy for locally advanced right hemicolon cancer
- Endoscopic ultrasound-guided fne-needle aspiration cytology in pancreaticobiliary carcinomas: diagnostic effcacy of cell-block immunocytochemistry
- Omental faps reduces complications after pancreaticoduodenectomy
