Fast magnetic reconstruction of the portal vein with allogeneic blood vessels in canines
2015-02-06
Xi'an, China
Fast magnetic reconstruction of the portal vein with allogeneic blood vessels in canines
Shan-Pei Wang, Xiao-Peng Yan, Fei Xue, Ding-Hui Dong, Xu-Feng Zhang, Feng Ma, Hao-Hua Wang and Yi Lv
Xi'an, China
BACKGROUND: The resection and reconstruction of large vessels, including the portal vein, are frequently needed in tumor resection. Warm ischemia before reconstruction might have deleterious effects on the function of some vital organs and therefore, how to reconstruct the vessels quickly after resection is extremely important. The present study was to introduce a new type of magnetic compression anastomosis (MCA) device to establish a quick non-suture anastomosis of the portal vein after resection in canines.
METHODS: The new MCA device consists of a pair of titanium alloy and neodymium-ferrum-boron magnet (Ti-NdFeB) composite rings. The NdFeB magnetic ring as a core of the device was hermetically sealed inside the biomedical titanium alloy case. Twelve canines were divided into two groups: a MCA group in which the end-to-end anastomoses was made with a new device after resection in the portal vein and a traditional manual suture (TMS) group consisted of 6 canines. The anastomosis time, anastomotic patency and quality were investigated at week 24 postoperatively.
RESULTS: The portal vein was reconstructed successfully in all of the animals and they all survived. The duration of portal vein anastomosis was signifcantly shorter in the MCA group than in the TMS group (8.16±1.25 vs 36.24±2.17 min,P<0.05). Portography and ultrasound showed that the blood fow was normal without angiostenosis or thrombosis in all of the canines. Hematoxylin-eosin staining and electron microscopescanning showed in contrast to the TMS group, MCA anastomotic intimal was much smoother with more regularly arranged endothelial cells at week 24 postoperatively.
CONCLUSIONS: The Ti-NdFeB composite MCA device was applicable in reconstruction of large vessels after resection. This device was easy to use and the anastomosis was functionally better than the traditional sutured anastomosis.
(Hepatobiliary Pancreat Dis Int 2015;14:293-299)
magnetic compression anastomosis; portal vein reconstruction; non-suture anastomosis
Published online May 15, 2015.
Introduction
Large vessel diseases are common in vascular surgery, and most of these diseases need surgical replacement and require reconstruction after the resection. Fortner[1]in 1973 frst described portal vein resection (PVR) and reconstruction in patients who underwent pancreatectomy. This procedure is now widely performed in tumor patients with vascular invasion and thus, PVR extends the resectability of the tumors.
The surgical technique of PVR is a high demanding and challenging procedure in hepatobiliary surgery. The traditional manual suture anastomosis is a delicate procedure and time-consuming (30 minutes or longer) and therefore, places the patient at a substantial risk of vascular thrombosis or obstruction.[2]Many surgeons have attempted to develop techniques of non-suture vascular anastomoses; these techniques include mechanical devices (such as ring couplers, clips, and staples), biocompatible glues, laser welding, etc.[3]Among them, magnetic compression anastomosis (MCA) device is the most intensively investigated. MCA is easy to handle, quick and reliable and therefore widely used in clinical practice. The MCA based on magnetic felds for non-suture technique of vascular anastomoses was frst describedby Obora et al in 1978.[4]Our previous serious studies aimed to fnd an MCA device which is convenient to use and safe and shortens the time of vessel reconstruction as well as reduces the complications.[5-9]The present study was to investigate the feasibility, long-term patency, and tissue-healing characteristics of titanium alloy and neodymium-ferrum-boron magnet (Ti-NdFeB) composite MCA device for end-to-end anastomosis of the PVR with allogeneic vein graft in canines.
Methods
MCA devices
The new type of MCA device used for PVR consists of a pair of matching Ti-NdFeB composite rings (Fig. 1). The Ti-NdFeB composite ring was designed according to the parameters of canine portal vein (PV) with the inner diameter, outer diameter, thickness and weight of 11 mm, 21 mm, 5 mm and 5 g, respectively. The inner diameter of an MCA device was ten percent larger than the outside diameter of PV to avoid anastomotic stenosis. The magnetic fux density of the ring was approximately 140 mT. Each ring contains two parts: the biomedical titanium alloy case containing a magnetic core of neodymium-ferrumboron magnet (NdFeB) ring. The NdFeB magnetic ring was hermetically sealed inside the ultra-thin biomedical titanium shell by laser welding in helium gas protection atmosphere. The Ti-NdFeB composite rings were analyzed by helium mass spectrum leakage detector to make sure its sealability and sterilized with ethylene oxide.
There are six titanium pins and six holes (length, 3 mm; diameter, 0.5 mm; Northwest Institute for Nonferrous Metal Research) alternating evenly spaced on the anastomosis face of the Ti-NdFeB composite rings which fxed the everted edges of the vessels. The vessel stump is slipped through a ring and its edges are everted and immobilized by pins onto the face of the ring; the same operation was done to the other side of the vessel stump. Two rings were connected to establish anastomosis of vascular by means of the magnetic power.
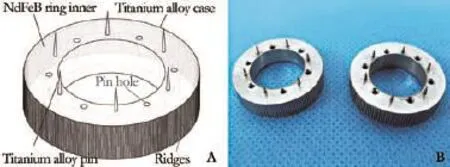
Fig. 1.A: Schematic illustration of the Ti-NdFeB composite MCA device;B: Real products of the Ti-NdFeB composite MCA device.
This type of MCA device guarantees the continuity of the severed conduit and, more importantly, intima-tointima vascular connection. The vessel edges are joined together layer by layer with the MCA device and no foreign material is in contact with the intima or with blood. The rings stay outside the vessel, the connection between the rings and the vessel is made by pins that penetrate within the vessel.
Animal model and grouping
This study was approved by the Ethics Committee of the Animal Experiments of Xi'an Jiaotong University (Permit Number: 2010-105). Twelve male mongrel canines (aged less than 1 year old and weighing 18-22 kg) were from the Experimental Animal Center, Medical School of Xi'an Jiaotong University (Xi'an, China). All the canines were received human care and vaccinated, surgery was performed under pentobarbital sodium anesthesia, and efforts were made to minimize suffering. They were randomly divided into two groups: MCA group (n=6) and traditional manual suture (TMS) group (n=6). After routine preoperative fasting for 12 hours and water deprivation for 6 hours, anesthesia was induced by intraperitoneal injection of 3% (m/v) pentobarbital sodium (dosage 30 mg/kg).
After the canine was placed in a supine position, the upper abdomen and right lateral crural region were shaved, disinfected with povidone iodine, and draped with sterile towels. The canine then had a saphenous vein cannulation, and sodium lactate Ringer's injection (SLRI) (dosage 500 mL) was infused through the right great saphenous vein.
Allograft
The allografts for PVR were obtained from the portal veins of adult canines with aseptic operation while they were sacrifced for other experiments. The allografts were sectioned into 4-cm segments. Connective tissue was removed and each segment was immediately placed in SLRI in a separate container and frozen at -80 ℃ until use.
Surgical procedure and technique
The abdomen was incised through a 10-cm longitudinal incision along the linea mediana ventralis. The PV trunk was fully exposed about a length of 6-7 cm (from the hepatic hilus to the splenic vein) and occluded temporarily with atraumatic vascular clamps at the proximal and distal end, respectively. Before the PV was clamped, 100 IU/kg of heparin was administered intravenously. Then a segment (about 3 cm long) of the PV was excised with vascular scissors, and the vessel stump lumen was washed with heparinized saline.
The proximal vessel stump wall edge was slipped through the internal hole of the Ti-NdFeB composite ring and everted onto the pins with equal tension around the circumference. The same operation was taken to the other end of the distal PV with another opposite polarity magnetic ring to make sure that the intimal layer of the PV was hung by each pin frmly. The deployments of two ends of allograft vascular with the MCA devices were proceeded simultaneously in the same way. Both proximal and distal anastomoses reconstruction of PV were performed with interposition of allograft in an endto-end fashion using Ti-NdFeB composite MCA devices. The TMS group received continuous sutures using 6-0 Prolene (Ethicon, USA). The two MCA devices of proximal vessel stump and allograft vascular were placed close and attracted to each other, compressed the vessel wall to hold it in place with the pins from one ring penetrating the holes of the other and created a magnetism-based end-to-end non-suture vascular anastomosis. An identical pair of magnets was used to create a similar magnetic anastomosis of distal vessel stump and allograft vascularity. Then, the venous clamps were released to restore perfusion (Fig. 2). The two pairs of magnetic rings compressed the vessel ends outside to make sure intima-tointima contacting contiguously of anastomotic stoma surfaces, and no intraluminal foreign body left with intima or blood. The canine then had a spleen vein cannulated for portal venography later.
The abdominal incisions were closed with 1-0 nonabsorbable sutures (Aipu, China) for the muscle and subcutaneous layers and 2-0 non-absorbable sutures for the cutaneous layer.
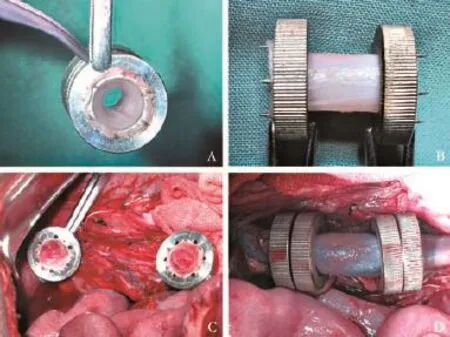
Fig. 2.Surgical application of the MCA device.A&B: Fixed the two ends of allograft vascular with Ti-NdFeB composite MCA devices;C: Excised about 3-cm segment of the portal vein and fixed both the proximal and distal ends of the recipient's portal vein stump;D: Two pairs of Ti-NdFeB composite MCA rings are merged to create PVR. The blood flling was well after the occlusive clamps were released.
Postoperative management
The canines were allowed to wake up and then returned to their cages. The canines received an intramuscular injection of penicillin 800 000 U and a subcutaneous injection of dalteparin sodium 5000 U daily for 3 days.
Imaging examination
X-ray portal venography (PLX7000B High Frequency Mobile C arm System, Nanjing Perlove Medical Equipment Co., Ltd., China) and Duplex ultrasonography (ACUSON X 150 Diagnostic Ultrasound Systerm, Siemens Medical Solutions Inc., USA) were routinely performed to evaluate the patency of PVR anastomotic stoma immediately, 3 days, 1 week, 4 weeks, 12 weeks and 24 weeks after operation.
Examination of anastomoses and sample collection
The canine was anesthetized by intraperitoneal injection of 3% pentobarbital sodium, and portography was performed at 24 weeks postoperatively. Placed in a supine position, the skin of the canine was incised from the midline and the anastomotic stomas of PVR were exposed and evaluated for patency by direct visualization. The abnormalities of the anastomotic stomas such as migration, adhesions, fstulae, or obstruction were examined and recorded. At last the canines were sacrifced and the PV specimens were harvested for histological examination and electron microscope scanning.
Histological analysis
The Ti-NdFeB composite MCA devices were removed from the vessel walls, and the vascular tissue samples were collected and immersed in 10% neutral buffered formalin for histologic examination. Vessels were embedded in paraffn, and 4-µm thick cross sections were longitudinally cut through the anastomoses and both proximal and distal ends adjacent to the anastomoses. The longitudinal sections of the anastomoses were stained with hematoxylineosin and observed under a light microscope.
Electron microscope scanning
The specimens from the canines were fxed in 2.5% glutaraldehyde solution for 2 hours, dehydrated in graded ethanol, and dried at a critical point in liquid carbon dioxide. They were then sputter-coated with goldpalladium and photographed on an electron microscope scanning (JEM-2100, JEOL, Japan) to observe the chang-es of the endovascular surface.
Statistical analysis
Independent samplesttest was used to compare values between the two groups. All data were expressed as mean±standard deviation and aPvalue less than 0.05 was considered statistically signifcant.
Results
Surgical results
The end-to-end anastomosis of PVR was successful in both MCA and TMS canines. All of the canines survived after surgery and recovered without blood leakage. The magnetic device was deployed rapidly and easily. The warm ischemic time was 8.16±1.25 minutes in the MCA group and 36.24±2.17 minutes in the TMS group (P<0.05).
The visceral congestion was relieved immediately after the occlusive clamps were released in the MCA group. The blood fow flled the anastomotic stoma well and no turbulence was observed through the venous wall. The Ti-NdFeB composite MCA device based on strongly magnetic attraction could be applied in large vessels. This non-suture reconstruction is faster, safer and easier than traditional manual suture. Many canines were ambulatory after several hours recovery from the surgery. All canines had normal gait a few days later and throughout the entire study.
Radiographic results
After completion of the anastomosis and reperfusion, contrast medium was injected via the splenic vein, and portal venography showed no interruption in portal venous infow. Duplex ultrasonography and X-ray portal venography were performed at day 3, 7, 28, 84 and 168 after operation.
All vascular anastomotic stomas were patency and the portal infow in the main portal vein at various time points were smooth. No lumen stenosis was seen in all of the canines (Fig. 3).
General examinations
At the end of the experiments, the canines were sacrifced and the anastomoses were examined. We did not fnd obvious abnormity of internal organs. The segment of reestablishment PV and the Ti-NdFeB composite MCA device were encapsulated by the fbrous membrance. The two pairs of Ti-NdFeB composite MCA devices had aligned in straight with no evidence of stenosis, stricture or angulation. There was neither device migration nor proximal or distal transposition. PV specimens were harvested and incised longitudinally after the Ti-NdFeB composite MCA devices were removed. The tissue necrosis of the vessel wall compressed between the pair of Ti-NdFeB MCA devices could be observed. Therewas neither erosion related to the Ti-NdFeB composite MCA devices nor apparent infammatory reaction in the tissue adjacent to the graft. The endovascular surface of the blood vessel anastomotic stoma was covered with layers of intima and appeared to be completely endothelialized at week 24 postoperatively in both groups. The anastomotic stoma surface was smooth and of integrity in the MCA group. The luminal surfaces were uneven in the TMS group, and sutures could be observed in the neointima covered on the anastomotic stoma (Fig. 4).
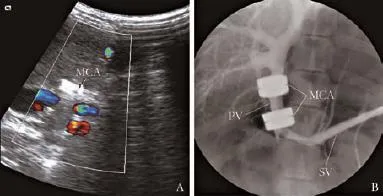
Fig. 3.Image of the MCA devices at week 24 after surgery.A:Duplex ultrasonographic scanning revealed portal inflow in the main portal vein;B: X-ray portal venography showed the patency of portal vein. PV: portal vein; SV: splenic vein; MCA: Ti-NdFeB composite MCA devices.
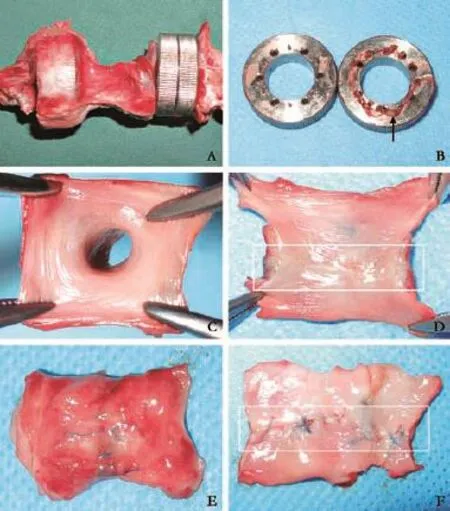
Fig. 4.General observation of the anastomosis site of the MCA and TMS groups at week 24 after surgery.A: The Ti-NdFeB composite MCA device was covered by a thin layer of fibrous tissue capsule;B: The vessel wall necrosis tissue (white arrow) compressed between the pair of Ti-NdFeB MCA devices and no erosion related to the magnetic device;C&D: Normal membrane on the surface of anastomotic stoma in the MCA group;E&F:Sutures and rough anastomotic stoma surface (white rectangle) in the TMS group.
Histological fndings
Histological examination of all the segments of reestablishment PV revealed a continuity of the endothelium of the anastomotic vessel wall and total endothelialization extending from the PV endothelium. The entire neointima was smooth at week 24 and re-endothelialization was complete in both groups. There was no rough surface or obvious fbrin clot on the surface of the blood vessels, and intima-to-intima connection was created in the MCA group. Cells arranged over the anastomotic site corresponding to the whitish and glistening area were lined with layers of endothelial-like cells similar to endothelial cells of the intact site of the PV. Vascular smooth muscle layers arranged regularly under the neointima layers. There were mild infammatory cell infltrations but no necrosis or vessel wall degradation in the MCA group. In the TMS group, the neointima arrangement was integrated but irregular; the vascular smooth muscle layers were distorted (Fig. 5).
Results of electron microscope scanning fndings
The lumen surface of blood vessels was smooth in the MCA group at week 24 after surgery. Cells arranged over the anastomotic site were regular and similar to the endothelial cells of the intact site of the PV. The lumen surface of blood vessels was rough and covered irregularly with vascular endothelial cells in the TMS group (Fig. 6).
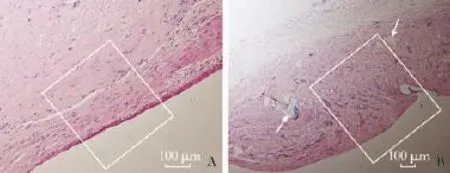
Fig. 5.HE staining of the anastomosis site of the MCA and TMS groups at week 24 postoperatively.A: Layers of endothelial-like cells arranged over the anastomotic sites integrally and regularly (white rectangle);B: Neointima arrangement was irregular with sutures (white arrow) and the vascular smooth muscle layers were distorted (white rectangle).
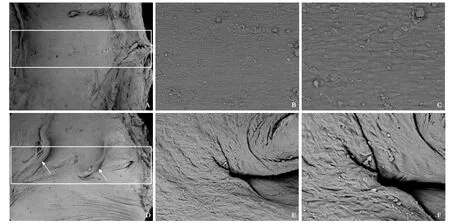
Fig. 6.SEM study of the anastomosis site of the MCA and TMS groups at week 24 postoperatively (white rectangle).A, B&C: Endothelial-like cells arranged over the anastomotic site regularly and smoothly in the MCA group;D, E&F: Rough surface and irregular endothelial cells over the anastomotic sites in the TMS group. White arrows are the sutures.A&D: original magnifcation ×50;B&E: original magnifcation ×500;C&F: original magnifcation ×1000.
Discussion
Large vessel diseases such as coarctation of the aorta, abdominal aortic aneurysm, PV invasion and inferior vena cava thrombus are commonly seen in vascular surgery. Most of these diseases need surgical replacement and require reconstruction after the resection. Aggressive resection of the main tumor combined with PV reconstruction could remove the tumor completely.[10]Yoshitomi et al[2]resected the PV/superior mesenteric vein (SMV) aggressively for 192 patients with pancreatic cancer involving the PV/SMV, and found that the PV/SMV resection and reconstruction are safe and improve the outcome of the patients.
Traditionally, the surgeon needs to suture the graft which is time-consuming and prolongs the warm ischemic time and therefore, deteriorates the liver function. Sometimes the surgeon has to use temporary intraoperative mesentericoportal shunt to shorten warm ischemic time.[11]In addition, the anastomotic bleeding and prolonged clamping of large vessels are often associated with serious postoperative complications that may compromise the surgical outcome. Thus, simplifying vascular anastomosis procedure and shortening the PV block time are key points to decrease deleterious effects on the function of some vital organs. We therefore created a novel MCA method and aimed to simplify the procedure and shorten the ischemic time.
MCA, based on magnetic felds for non-suture vascular anastomoses, was frst reported by Obora et al in 1978.[4]The MCA devices have been wildly used in sutureless vascular anastomosis in recent decades.[12-16]
Previously, we found that a new type of veno-venous bypass device, which comprised one heparinized polyvinylchloride tube and three magnetic rings, was suffcient to shorten the generating time of veno-venous bypass and made theex vivoliver resection possible.[7]
The present study described the Ti-NdFeB composite MCA devices and their application to end-to-end anastomosis of the PVR with allogeneic vein graft. We demonstrated the safety and feasibility of this procedure in live canine model. The operation of PVR anastomosis was signifcantly faster, safer and easier in the MCA group than in the TMS group. Long-term observation showed the good patency of the portal vein reconstructed with MCA. Histological examination revealed mild infammation, but no foreign body in the vessel lumen. Electron microscope scanning showed that the neointima surface was integral, regular and smooth in the MCA group and better than that in the TMS group. Furthermore, Ti-NdFeB composite MCA devices can also be used in vessel reconstruction with autologous veins or artifcial blood vessels, such as inferior vena cava with expanded polytetrafuoroethylene grafts.
Titanium is good biocompatible material, and titanium based devices are commercially available. The example is Refux Management System (Torax Medical, St. Paul, MN, USA). Lipham et al[17]used this system to manage gastroesophageal refux disease. The device was implanted in the body for 4 years and there were no long-term device-related complications such as migration or erosion.
Titanium is biocompatible and safe. The MCA device we designed consists of a pair of Ti-NdFeB composite rings. Each ring contains two parts: the biomedical titanium alloy case and the magnetic core of the NdFeB magnet ring. The titanium alloy case is sealed welding by laser to protect the body from directly contact with NdFeB and to avoid corrosive damage by body fuids as well. The NdFeB core provides a permanent magnetic force to suturelessly anastomose the vascular. Titanium alloy has been extensively used for the construction of femoral head grafts, cardiac pacemaker and dental materials with low noxious properties and good biocompatibility. The new type of Ti-NdFeB composite materials in the human body is safe.[18]This new type of Ti-NdFeB composite MCA device was easy and safe to use in endto-end non-suture anastomosis for PVR. This device signifcantly shortened the time of PVR.
The erosion and safety of the permanent placement of a foreign body such as the Ti-NdFeB composite device in human body is a signifcant concern. The magnetic core of NdFeB magnet ring is sealed in the titanium alloy case. The special structure of a Ti-NdFeB composite device protects the body from directly contact with NdFeB and avoids corrosive damage by body fuids. Titanium alloy has been extensively used as implant materials with good biocompatibility. There were no adverse effects on long-term results throughout the present study nor erosion or apparent infammatory reaction related to the Ti-NdFeB composite MCA device. These fndings were consistent with the previously reported on the magnetic sphincter augmentation device.[19]
In conclusion, the Ti-NdFeB composite MCA device is applicable in large vessel reconstruction after resection. It is easy to manipulate, effcient, fast and secure with low complications. Studies with larger samples and longerterm follow-up are needed to assess the safety of the Ti-NdFeB composite device before its clinical application.
Contributors:LY proposed the study. WSP, YXP, XF, DDH and LY performed research. WSP, ZXF and LY wrote the frst draft. WSP, MF and WHH collected and analyzed the data. All authors contributed to the design and interpretation of the study and to further drafts. LY is the guarantor.
Funding:This study was supported by grants from the NationalNatural Science Foundation of China (30830099 & 81470896 & 81127005), and the Science and Technology Co-ordinating Innovative Engineering Projects of Shaanxi Province (2014KTCQ03-05).Ethical approval:This study was approved by the Ethics Committee of the Animal Experiments of Xi'an Jiaotong University (2010-105).
Competing interest:No benefts in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
1 Fortner JG. Regional resection of cancer of the pancreas: a new surgical approach. Surgery 1973;73:307-320.
2 Yoshitomi H, Kato A, Shimizu H, Ohtsuka M, Furukawa K, Takayashiki T, et al. Tips and tricks of surgical technique for pancreatic cancer: portal vein resection and reconstruction (with videos). J Hepatobiliary Pancreat Sci 2014;21:E69-74.
3 Zeebregts CJ, Heijmen RH, van den Dungen JJ, van Schilfgaarde R. Non-suture methods of vascular anastomosis. Br J Surg 2003;90:261-271.
4 Obora Y, Tamaki N, Matsumoto S. Nonsuture microvascular anastomosis using magnet rings: preliminary report. Surg Neurol 1978;9:117-120.
5 Shi Y, Lv Y, Wang B, Zhang Y, Jiang A, Li JH, et al. Novel magnetic rings for rapid vascular reconstruction in canine liver transplantation model. Transplant Proc 2006;38:3070-3074.
6 Liu SQ, Lei P, Cao ZP, Lv Y, Li JH, Cui XH. Nonsuture anastomosis of arteries and veins using the magnetic pinned-ring device: a histologic and scanning electron microscopic study. Ann Vasc Surg 2012;26:985-995.
7 Lei P, Liu SQ, Cui XH, Lv Y, Zhao G, Li JH. A new veno-venous bypass type for ex-vivo liver resection in dogs. Hepatobiliary Pancreat Dis Int 2013;12:436-439.
8 Liu SQ, Lei P, Cui XH, Lv Y, Li JH, Song YL, et al. Sutureless anastomoses using magnetic rings in canine liver transplantation model. J Surg Res 2013;185:923-933.
9 Yan X, Fan C, Ma J, Li J, Dong D, Wang H, et al. Portacaval shunt established in six dogs using magnetic compression technique. PLoS One 2013;8:e76873.
10 Ravikumar R, Sabin C, Abu Hilal M, Bramhall S, White S, Wigmore S, et al. Portal vein resection in borderline resectable pancreatic cancer: a United Kingdom multicenter study. J Am Coll Surg 2014;218:401-411.
11 Bachellier P, Rosso E, Fuchshuber P, Addeo P, David P, Oussoultzoglou E, et al. Use of a temporary intraoperative mesentericoportal shunt for pancreatic resection for locally advanced pancreatic cancer with portal vein occlusion and portal hypertension. Surgery 2014;155:449-456.
12 Levi DS, Danon S, Gordon B, Virdone N, Vinuela F Jr, Shah S, et al. Creation of transcatheter aortopulmonary and cavopulmonary shunts using magnetic catheters: feasibility study in swine. Pediatr Cardiol 2009;30:397-403.
13 Falk V, Walther T, Stein H, Jacobs S, Walther C, Rastan A, et al. Facilitated endoscopic beating heart coronary artery bypass grafting using a magnetic coupling device. J Thorac Cardiovasc Surg 2003;126:1575-1579.
14 Falk V, Walther T, Jacobs S, Wolf RK, Mohr FW. Facilitated MIDCAB using a magnetic coupling device. Ann Thorac Surg 2005;79:691-693.
15 Erdmann D, Sweis R, Heitmann C, Yasui K, Olbrich KC, Levin LS, et al. Side-to-side sutureless vascular anastomosis with magnets. J Vasc Surg 2004;40:505-511.
16 Heitmann C, Khan FN, Erdmann D, Olbrich KC, Adam Sharkawy A, Klitzman B. Vein graft anastomoses with magnets. J Plast Reconstr Aesthet Surg 2007;60:1296-1301.
17 Lipham JC, DeMeester TR, Ganz RA, Bonavina L, Saino G, Dunn DH, et al. The LINX(R) refux management system:confrmed safety and effcacy now at 4 years. Surg Endosc 2012;26:2944-2949.
18 Ganz RA, Peters JH, Horgan S, Bemelman WA, Dunst CM, Edmundowicz SA, et al. Esophageal sphincter device for gastroesophageal refux disease. N Engl J Med 2013;368:719-727.
19 Ganz RA, Gostout CJ, Grudem J, Swanson W, Berg T, De-Meester TR. Use of a magnetic sphincter for the treatment of GERD: a feasibility study. Gastrointest Endosc 2008;67:287-294.
Received January 3, 2015
Accepted after revision March 25, 2015
Yi Lv, MD, PhD, Department of Hepatobiliary Surgery, First Affliated Hospital, Medical School of Xi'an Jiaotong University, Xi'an 710061, China (Tel: +86-29-85323626; Email: luyi169@126.com)
10.1016/S1499-3872(15)60364-2
AuthorAffliations:Department of Hepatobiliary Surgery, First Affliated Hospital, Medical School of Xi'an Jiaotong University, Xi'an 710061, China (Wang SP, Yan XP, Xue F, Dong DH, Zhang XF and Lv Y); XJTU Research Institute of Advanced Surgical Technology and Engineering); Regenerative Medicine and Surgery Engineering Research Center of Shaanxi Province, Xi'an 710061, China (Wang SP, Yan XP, Xue F, Dong DH, Ma F, Wang HH and Lv Y)
© 2015, Hepatobiliary Pancreat Dis Int. All rights reserved.
杂志排行
Hepatobiliary & Pancreatic Diseases International的其它文章
- Combined right hemicolectomy and pancreaticoduodenectomy for locally advanced right hemicolon cancer
- Histological examination of frozen sections for patients with acute cholecystitis during cholecystectomy
- Omental faps reduces complications after pancreaticoduodenectomy
- Endoscopic ultrasound-guided fne-needle aspiration cytology in pancreaticobiliary carcinomas: diagnostic effcacy of cell-block immunocytochemistry
- High frequency of thrombocytopenia in patients with acute-on-chronic liver failure treated with linezolid
- miR-215 overexpression distinguishes ampullary carcinomas from pancreatic carcinomas
